Abstract
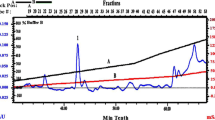
An extracellular thermostable xylanase from a newly isolated thermophilic Actinomadura sp. strain Cpt20 was purified and characterized. Based on matrix-assisted laser desorption–ionization time-of-flight mass spectrometry analysis, the purified enzyme is a monomer with a molecular mass of 20,110.13 Da. The 19 residue N-terminal sequence of the enzyme showed 84% homology with those of actinomycete endoxylanases. The optimum pH and temperature values for xylanase activity were pH 10 and 80 °C, respectively. This xylanase was stable within a pH range of 5–10 and up to a temperature of 90 °C. It showed high thermostability at 60 °C for 5 days and half-life times at 90 °C and 100 °C were 2 and 1 h, respectively. The xylanase was specific for xylans, showing higher specific activity on soluble oat-spelt xylan followed by beechwood xylan. This enzyme obeyed the Michaelis–Menten kinetics, with the K m and k cat values being 1.55 mg soluble oat-spelt xylan/ml and 388 min−1, respectively. While the xylanase from Actinomadura sp. Cpt20 was activated by Mn2+, Ca2+, and Cu2+, it was, strongly inhibited by Hg2+, Zn2+, and Ba2+. These properties make this enzyme a potential candidate for future use in biotechnological applications particularly in the pulp and paper industry.





Similar content being viewed by others
References
Polizeli, M. L., Rizzatti, A. C., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Applied Microbiology and Biotechnology, 67, 577–591.
Wong, K. K. Y., Tan, L. U. L., & Saddler, J. N. (1988). Microbiology Reviews, 52, 305–317.
Collins, T., Gerday, C., & Feller, G. (2005). FEMS Microbiology Reviews, 29, 3–23.
Terrasan, C. R., Temer, B., Duarte, M. C., & Carmona, E. C. (2010). Bioresource Technology, 101, 4139–4143.
Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V., & Henrissat, B. (2009). Nucleic Acids Research, 37, D233–D238.
Sunna, A., & Antranikian, G. (1997). Critical Review in Biotechnology, 17, 39–67.
Ben Romdhane, I. B., Achouri, I. M., & Belghith, H. (2010). Applied Biochemistry and Biotechnology, 162, 1635–1646.
Maalej, I., Belhaj, I., Masmoudi, N. F., & Belghith, H. (2009). Applied Biochemistry and Biotechnology, 158, 200–212.
Chen, X., Xu, S., Zhu, M., Cui, L., Zhu, H., Liang, Y., et al. (2010). Process Biochemistry, 45, 75–80.
Liu, L., Cheng, J., Chen, H., Li, X., Wang, S., Song, A., et al. (2010). Process Biochemistry, 46, 395–398.
Zhou, P., Zhu, H., Yan, Q., Katrolia, P., & Jiang, Z. (2011). Applied Biochemistry and Biotechnology, 164, 944–956.
Shi, Q. Q., Sun, J., Yu, H. L., Li, C. X., Bao, J., & Xu, J. H. (2011). Applied Biochemistry and Biotechnology, 164, 816–830.
Sriyapai, T., Somyoonsap, P., Matsui, K., Kawai, F., & Chansiri, K. (2011). Journal of Bioscience and Bioengineering, 111, 528–536.
Holtz, C., Kaspari, H., & Klemme, J. H. (1991). Antonie Van Leeuwenhoek, 59, 1–7.
Ethier, J. F., Harpin, S., Girard, C., Beaulieu, C., Déry, C. V., & Brzezinski, R. (1994). Canadian Journal of Microbiology, 40, 362–368.
Leskinen, S., Mantyla, A., Fagerstrom, R., Vehmaanpera, J., Lantto, R., Paloheimo, M., et al. (2005). Applied Microbiology and Biotechnology, 67, 495–505.
Berens, S., Kaspari, H., & Klemme, J. H. (1996). Antonie Van Leeuwenhoek, 69, 235–241.
Zhou, J., Shi, P., Zhang, R., Huang, H., Meng, K., Yang, P., et al. (2010). Journal of Industrial Microbiology and Biotechnology, 38, 523–530.
Ninawe, S., Kapoor, M., & Kuhad, R. C. (2008). Bioresource Technology, 99, 1252–1258.
George, S. P., Ahmad, A., & Rao, M. B. (2001). Bioresource Technology, 78, 221–224.
Blanco, J., Coque, J. J., Velasco, J., & Martin, J. F. (1997). Applied Microbiology and Biotechnology, 48, 208–217.
Beg, Q. K., Bhushan, B., Kapoor, M., & Hoondal, G. S. (2000). Enzyme and Microbial Technology, 27, 459–466.
Nanmori, T., Watanabe, T., Shinke, R., Kohno, A., & Kawamura, Y. (1990). Journal of Bacteriology, 172, 6669–6672.
Gurtler, V., & Stanisich, V. A. (1996). Microbiology, 142, 3–16.
Sambrook, J., Fritsch, E., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed., pp. 23–38). Cold Spring Harbor: Cold Spring Harbor Laboratory.
Felsenstein, J. (1985). Evolution, 39, 783–791.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Kubata, B. K., Suzuki, T., Horitsu, H., Kawai, K., & Takamizawa, K. (1994). Applied and Environmental Microbiology, 60, 531–535.
Hewick, R. M., Hunkapiller, M. W., Hood, L. E., & Dreyer, W. J. (1981). The Journal of Biological Chemistry, 256, 7990–7997.
Nawel, B., Said, B., Estelle, C., Hakim, H., & Duchiron, F. (2011). Process Biochemistry, 46, 519–525.
Nascimento, R. P., Coelho, R. R. R., Marques, S., Alves, L., Gírio, F. M., Bon, E. P. S., et al. (2002). Enzyme and Microbial Technology, 31, 549–555.
do Nascimento, R. P., Marques, S., Alves, L., Gírio, F., Amaral-Collaço, M. T., et al. (2003). World Journal of Microbiology and Biotechnology, 19, 879–881.
Techapun, C., Charoenrat, T., Poosaran, N., Watanabe, M., & Sasak, K. (2002). Journal of Bioscience and Bioengineering, 93, 431–433.
Camacho, N. A., & Aguilar, O. G. (2003). Applied Biochemistry and Biotechnology, 104, 159–172.
Fang, H. Y., Chang, S. M., Lan, C. H., & Fang, T. J. (2008). Process Biochemistry, 43, 49–55.
Georis, J., Giannotta, F., Lamotte-Brasseur, J., Devreese, B., Van Beeumen, J., Granier, B., et al. (1999). Gene, 237, 123–133.
Li, X., She, Y., Sun, B., Song, H., Zhu, Y., Lv, Y., et al. (2010). Biochemical Engineering Journal, 52, 71–78.
Lv, Z., Yang, J., & Yuan, H. (2008). Enzyme and Microbial Technology, 43, 343–348.
Kui, H., Luo, H., Shi, P., Bai, Y., Yuan, T., Wang, Y., et al. (2010). Applied Biochemistry and Biotechnology, 162, 953–965.
Vafiadi, C., Christakopoulos, P., & Topakas, E. (2010). Process Biochemistry, 45, 419–424.
Jaouadi, B., Abdelmalek, B., Fodil, D., Ferradji, F. Z., Rekik, H., Zarai, N., et al. (2010). Bioresource Technology, 101, 8361–8369.
Kulkarni, N., Lakshmikumaran, M., & Rao, M. (1999). Biochemical and Biophysical Research Communications, 263, 640–645.
Henrissat, B. (1991). Biochemical Journal, 280, 309–316.
Acknowledgments
This work was funded by the Algerian Ministry of Higher Education and Scientific Research. The authors wish to express their gratitude to Miss Amina Habbeche and Miss Soumaya Haberra for their constructive discussions and valuable help during the preparation of this work. Special thanks are also due to Pr. Anouar Smaoui, from the English department at the Sfax Faculty of Science for carefully proofreading and polishing the language of the present paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated to the memory of our colleague Mrs. Zina Taibi, who passed away on July 20th, 2011. May God forgive her and have mercy on her soul.
Rights and permissions
About this article
Cite this article
Taibi, Z., Saoudi, B., Boudelaa, M. et al. Purification and Biochemical Characterization of a Highly Thermostable Xylanase from Actinomadura sp. Strain Cpt20 Isolated from Poultry Compost. Appl Biochem Biotechnol 166, 663–679 (2012). https://doi.org/10.1007/s12010-011-9457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9457-y