Abstract
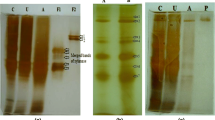
Two endoxylanases were isolated from the xylanolytic enzyme system of the thermophilic actinomycete Microtetraspora flexuosa SIIX, and purified by ammonium sulfate fractionation, DEAE-Sepharose chromatography, gel filtration on Sephacryl S 200 and fast protein liquid chromatography on Q-Sepharose. The molecular masses of xylanase I and II were 26.3 and 16.8 kDa, and isoelectric points were 8.4 and 9.45, respectively. optimal enzyme activities were obtained at 80° C and pH 6.0. The thermostability of both xylanases was greatly diminished during purification but could be restored by preincubation of the purified enzymes in the presence of xylan. The half-lives at 80° C were approximately 25 min. The kinetic constants of xylanases I and II determined with Remazol-brilliant-blue xylan were Vmax of 1537 and 353 μmol·min-1·mg protein-1 and K m values of 2.44 and 1.07 mg·ml-1, respectively. Purified xylanases utilized xylan as well as small oligosaccharides such as xylotriose as substrate. They did not exhibit xylobiase or debranching activities. The predominant products of arabinoxylan hydrolysis were xylobiose and xylotriose, the latter being hydrolysed to xylobiose and xylose upon further incubation. In addition, fragments containing arabinose side chains accumulated. The xylanases did not act on crystalline or amorphous cellulose indicating a possible application in biobleaching processes.
Similar content being viewed by others
References
Bachmann, SL & McCarthy, AJ (1991) Purification and cooperative activity of enzymes constituting the xylan-degrading system of Thermomonospora fusca. Appl. Environ. Microbiol. 55: 1642–1644
Ball, AS & McCarthy, AJ (1989) Production and properties of xylanases from actinomycetes. J. Appl. Bact. 66: 439–444
Biely, P, Mislovicova, D & Toman, R (1988) Remazol-Brilliant-Blue-xylan: a soluble chromogenic substrate for xylanases. In: Wood, WA & Kellogg, ST (Eds) Methods in Enzymology, Vol. 160 (pp 536–541) Academic Press, San Diego
Biely, P, Puls, J & Schneider, H (1985) Acetyl-xylan-esterases in fungal cellulolytic systems. FEBS Lett. 186: 80–86
Elegir, G, Szakas, G & Jeffries, TW (1994) Purification, characterization and substrate specificities of multiple xylanases from Streptomyces sp. strain B-12-2. Appl. Environ. Microbiol. 60: 2609–2615
Éthier, JF, Harpin, S, Girard, C, Beaulieu, C, Déry, C & Brzezinsky, R (1994) Cloning of two xylanase genes from the newly isolated actinomycete Actinomadura sp. strain FC7 and characterization of the gene products. Can. J. Microbiol. 40: 362–368
Fontana, JD, Gebara, M, Blumel, M, Schneider, H, MacKenzie, CR & Johnson, KG (1988) α-4-O-Methyl-D-glucuronidase component of xylanolytic complexes. In: Wood, WA & Kellogg, ST (Eds) Methods in Enzymology, Vol. 160 (pp 560–571). Academic Press, San Diego
Fontes, CMGA, Hall, J, Hirst, BH, Hazlewood, GP & Gilbert, HJ (1995) The resistance of cellulases and xylanases to proteolytic inactivation. Appl. Microbiol. Biotechnol. 43: 52–57
Greiner-Mai, E, Kroppenstedt, RM, Korn-Wendisch, F & Kutzner, HJ (1987) Morphological and biochemical characterization and emended descriptions of thermophilic actinomycetes species. System. Appl. Microbiol. 9: 97–109
Grüninger, H & Fiechter, A (1986) A novel highly thermostable D-xylanase. Enz. Microb. Technol. 8: 309–314
Holtz, C, Kaspari, H & Klemme, JH (1991) Production and properties of xylanases from thermophilic actinomycetes. Antonie van Leeuwenhoek 59: 1–7
Irwin, D, Jung, ED & Wilson, DB (1994) Characterization and sequence of a Thermomonospora fusca xylanase. Appl. Environ. Microbiol. 60: 763–770
Kluepfel, D, Vats-Metha, S, Aumont, F, Shareck, F & Molosoli, R (1990) Purification and characterization of a new xylanase (xylanase B) produced by Streptomyces lividans 66. Biochem. J. 267: 45–50
Kormelink, FJM & Voragen, AGJ (1992) Degradation of different [(glucurono)arabino] xylans by a combination of purified xylan-degrading enzymes. Appl. Microbiol. Biotechnol. 38: 688–695
Lowry, OH, Rosebourgh, NJ, Farr, AL & Randall, RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275
Marui, M, Nakanishi, K & Yasui, T (1985) Purification and properties of three types of xylanases induced by methyl-β-xyloside from Streptomyces sp. Agric. Biol. Chem. 49: 3399–3407
Milagres, AMF & Prade, RA (1994) Production of xylanases from Penicillium janthinellum and its use in the recovery of cellulosic textile fibers. Enz. Microb. Technol. 16: 627–632
Miller, GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428
Nakanishi, K, Marui, M & Yasui, T (1992) Comparison of xylan and methyl β-xyloside-induced xylanases from Streptomyces sp. J. Ferment. Bioeng. 74: 392–394
Nonomura, H & Ohara, Y (1971) Distribution of actinomycetes in soil. J. Ferment. Technol. 49: 904–912
Ristroph, DL & Humphrey, AE (1985) Kinetic characterization of the extracellular xylanases of Thermomonospora sp. Biotechnol. Bioeng. 28: 832–836
Skoog, K & Hahn-Hägerdahl, B (1987) Xylose fermentation. Enz. Microb. Technol. 10: 66–80
Tanaka, T, Shimomura, Y, Himejima, M, Taniguchi, M & Oi, S (1986) Characterization of xylan-utilizing anaerobes from mesophilic and thermophilic methane sludge and their xylan degrading enzymes. Agric. Biol. Chem. 50: 2185–2192
Viikari, L, Kantelinen, A, Sundquist, J & Linko, M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol. Rev. 13: 335–350
Wilkie, K (1979) The hemicelluloses of grasses and cereals. Adv. Carbohydr. Chem. Biochem. 10: 215–262
Wong, KKJ, Tan, LUL & Saddler, JN (1988) Multiplicity of β-1,4-xylanase in microorganisms: functions and applications. Microbiol. Rev. 52: 305–317
Wood, TM (1988) Preparation of crystalline, amorphous, and dyed cellulase substrates. In: Wood, WA & Kellogg, ST (Eds) Methods in Enzymology, Vol. 160 (pp 19–25) Academic Press, San Diego
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berens, S., Kaspari, H. & Klemme, JH. Purification and characterization of two different xylanases from the thermophilic actinomycete Microtetraspora flexuosa SIIX. Antonie van Leeuwenhoek 69, 235–241 (1996). https://doi.org/10.1007/BF00399612
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00399612