Abstract
We propose that microaerobic composting (MC) can be used to decompose vegetal matter with a short turnover time and large carbon (C) recycling potential. We used a novel method for measuring the degree of fragmentation of water-insoluble acid-soluble (WIAS) polysaccharides as a proxy in tracking their relative degree of degradation (i.e., fragmentation endpoint index). Oak leaves and food scrap processed by MC reached a fragmentation end point within 2 weeks. After amending the MC products into soil, the half-life of the polysaccharide residues was ~6–7 times longer (~100–110 days) than that measured during MC. The main products given up during MC were volatile organic acids (VOAs), alcohols and soluble carbohydrates in the compost tea, and CO2. These products accounted for about 2% of the initial carbon in the feedstock. Very small amounts of VOAs, particularly butyric acid, were formed in the amended soil. Based on a residence time of materials in fermentors of 2 weeks, a ~100-m3 capacity MC facility could process 2,000–4,000 metric tons of vegetable matter amended in ten hectares of arable land per year.
Similar content being viewed by others
Introduction
Historically, the concentration of CO2 in the atmosphere has risen from a baseline value of ~260 to ~380 ppm [1–4]. Even though the exchange of carbon (C) between arable lands and the atmosphere is the most dynamic component of the global C cycle (~120 Gt year−1) [5, 6], and there is a positive correlation between atmospheric CO2 concentration and C-fixation in plants [7, 8], since the beginning of mechanized agriculture, arable land has lost ~44–78 Gt of C worldwide [2–6]. Causes of this disequilibrium can be traced to unsustainable agricultural practices such as excess tilling, overharvesting of crops, and overreliance on chemical fertilization. The importance of retaining C in soil in the form of stable soil organic carbon (SOC) is also underestimated. One of the best ways to increase the stable SOC is to use C-rich fertilizers that have low turnover after introduction into soil [2, 3].
Better strategies for improving the organic C content of soil involving recycling of organic C could help offset the loss of C from arable lands. But directly amending soils with vegetal matter, especially matter rich in lignocellulose, is inefficient [9]. The solution to this problem is to compost such materials prior to their introduction into soil. Most composting technologies (including forced air, thermophilic and manure-augmented composting) are however time-, space-, and energy-consuming, and they have large greenhouse gas (GHG) footprints, and poor C sequestration efficiency. When using these methods, >50% of the C in the starting feedstock is lost [10–13]. In this study, we analyzed the C cycling efficiency while processing vegetal matter (oak leaves and discarded urban food scrap) using an alternative method called microaerobic composting (MC). We verified the dominance of microbes from the genus Clostridium to the microbial community during MC, and furthermore tracked the turnover of processed materials in soil over a period of several months.
Materials and Methods
3,5-Dintrosalicyclic (DNS) acid was obtained from Sigma, and 89% phenol was from Mallinckrodt. MC fermentors (210 L capacity) were obtained from Bokashicycle, LLC. Atmospheric gas chromatography (gc) columns (3′-silica and 6′-13X molecular sieves), used for measuring changes in CO2, O2, and CH4, were obtained from SRI Instruments. Stabilwax-DA and RtQPLOT 30 m × 0.32 mm ID capillary gc columns, for separation of volatile organic acids (VOAs), alcohols, and ketone metabolites, respectively, were obtained from Restek. Standards for VOAs analyses (acetic, propanoic, butyric, isovaleric, valeric, and caproic acid), alcohols, and ketones (ethanol, acetone, acetylacetone, methanol, propanol, butanol, and pentanol) were obtained from Aldrich, Sigma, and J.T. Baker. All other chemicals were reagent grade or better.
Polysaccharides were measured in glucose equivalents using the phenol–sulfuric acid (PSA) carbohydrate assay [14–16]. Polysaccharide glycosyl end group DNS reactive aldehyde-reducing activity (also expressed in glucose equivalents) was measured by an adaptation of Miller's colorimetric assay to microtiter plates [17, 18]. Changes in the fragmentation state of water-insoluble acid-soluble (WIAS) polysaccharides were tracked using the endpoint fragmentation index (EPF index) assay (summarized in Fig. 1) [18], which is a broad indicator of the progression of the hydrolysis of WIAS polysaccharides.
Soil amended with MC-treated feedstocks was a Helvetia silt loam. Field site tests were done at a farm outside Portland (New Earth Farm, Hillsboro, OR).
Oak leaves were prepared as aqueous slurries and dispensed into 70-ml capacity air tight serum bottles. Each bottle received 10 g of oak leaves blended with 30 ml of 1 g/L glucose solution and 1 ml of wheat bran slurry filtrate. This slurry was prepared by suspending 5 g of wheat bran rich in Clostridium sp. spores in 50-ml water followed by passing the slurry after overnight incubation at room temperature (~21 °C) through a stainless steel sieve (0.5 mm). Leaf slurries were added in equal amounts to each of a series of bottles sufficient in number to allow for triplicate harvests at different time intervals during the experiment. The bottles were capped with 1-cm-thick butyl rubber stoppers, sealed with aluminum caps, and incubated at 30 °C. Atmospheric gases were sampled over the course of the MC phase of the experiment. The pressure in the gas space was measured using a Thermo Fisher Scientific Traceable pressure meter.
Earlier observations indicated that the MC microbial communities were dominated by Gram-positive clostridia. To verify this dominance, we isolated organotrophs by plating serial diluted suspensions of compost tea on glucose–cellulose–agar plates (30 g/L tryptic soy broth; 10 g/L d-glucose; 15 g/L agar; and 2 g/L cellulose (Sigma, C6288); adjusted to pH 6.5). Inoculated media were incubated at 30 °C under aerobic conditions (~21% O2) and also under microaerophilic conditions (~1.6% O2 in N2). Fifty clones were picked up randomly and saved in a library. The isolated clones were grown for 2 days in the same culture medium (minus agar and cellulose), cells were separated by centrifugation (13,000 rpm, 2 min at 2 °C), and the genomic DNA (gDNA) was extracted by direct boiling for 5 min in 1× TE [19, 20]. After centrifugation (13,000 rpm, 10 min at 2 °C), ~70% of the supernatant was recovered, and the dsDNA content was quantified using a NanoDrop 1000 instrument. The PCR amplification mixtures contained each primer at 5 μM concentration, ~3.6 ng/μl gDNA, and 1% DMSO to minimize inhibition by melanin. All clones were verified with the 16S rDNA universal primers 8F 5′-AGAGTTTGATCCTGGCTCAG and 1492R 5′-GGTTACCTTGTTACGACTT [20], and with the 16S rDNA Clostridium-specific primers Chis150F 5′-AAAGGRAGATTAATACCGCATAA and ClostIR 5′-TTCTTCCTAATCTCTACGCA [21, 22]. For PCR amplification, we used an Applied Biosystems GeneAmp 2700 instrument and the following conditions: denaturing at 95 °C for 2 min; 40 cycles of 95 °C for 30 s, 46 °C for 30 s, and 72 °C for 2 min; and final extension at 72 °C for 5 min. Amplicon sizes were verified by electrophoresis in 0.7% agarose gels stained with ethidium bromide. The strains showing amplification with the Chis150F–ClostIR primer pair were considered to be phylogenetically related to Clostridium.
Microbial activity was monitored by following changes in O2 and CO2 content in the headspaces and formation of VOAs, alcohols, and ketones. For the analysis of atmospheric gases, gas was drawn through the stopper by means of a needle guidance sampler and separated on an SRI 31C GC analyzer equipped with thermal conductivity detector. VOAs, alcohols, and ketones were analyzed using a Shimadzu GC-2010 equipped with a splitter (50:1 setting) and flame ionization detector, and using the following temperature profile setup: injector at 240 °C, iso 60 °C for 10 min., ramp 10 °C per min to 240 °C, and detector at 260 °C.
For field experiments, urban food scrap was obtained by New Earth Farm, (Hillsboro, OR) from restaurant kitchens and serving delis. This food scrap was made up mostly of discarded produce, restaurant leftovers, coffee grounds, and paper filters. These materials were premixed with a small amount of wheat bran inoculum (~0.015 w/w ratio), blended through a wood chipper, and processed in 210-L capacity MC-fermentors (airtight plastic containers equipped with a spigot for draining the compost tea). Fermentors also had a one-way gas vent for CO2 release. Following ~1–1.5-week incubation at ambient temperatures ranging on day/night cycles of ~10–18 °C, the fermentors were drained. Residue remaining in the fermentors was amended by shovel and tractor spreaders into swaths of soil to a depth of not more than ~20 cm at varying loads (kg m−2) as shown in “Results” section.
Soil samples (~100 g per site) taken from the top ~10 cm layer for analysis were dried in an oven for 48 h at 65 °C, and then ground to uniformity using a mortar and pestle before analysis. Control soil (soil not having received processed food scrap) was retained to obtain baseline polysaccharide levels, EPF index values, and organic volatile content.
WIAS polysaccharides and the aldehyde reactive group content of soil samples were expressed in gram glucose per gram DW soil. The soil's pH was measured using a pH meter calibrated to the nearest 0.01 pH unit between pH 4.00 and 10.00 on aqueous slurries of soil made up in 1:25 (w/w) ratios with deionized H2O (dH2O).
Results
Microbial isolation and then characterization based on staining, optical microscopy, production of extracellular cellulases, and formation of butyrate during fermentation of glucose and screening of isolates by PCR amplification with 16S rDNA Clostridium-specific primers showed that the wheat bran slurries used to inoculate the MC fermentors are dominated (~60%) by cells from the genus Clostridium. During laboratory MC experiments using oak leaves inoculated with these wheat bran slurries, the EPF index dropped sharply through about the 10th day, reaching an end point at ~0.5 EPF (Fig. 2). After amending the MC leaves into soil, the EPF index fell slowly with an estimated polysaccharide half-life of about 100 days.
Progressive fragmentation of polysaccharides in MC fermentors (circles), and after, MC-treated materials were amended in soil (triangles). The progressive decrease in the EPF index values indicates ongoing hydrolysis and breakdown of WIAS polysaccharides into smaller size fragments. Each data point in the amended soil is a single determination taken at the time shown in the plot. Error bars are ±2 SD of triplicate assays
While leaves were in MC fermentors, O2 in the headspace fell sharply, bottoming out between ~0.4% and 2% by the third day. CO2 rose steadily peaking by the 10th day at 740 ± 42 μmol (1SD), (=25 ± 1% CO2) gas content in the headspace. The amount of C given up as CO2 in the headspace during 2 weeks of MC fermentation was ~1% of the initial biomass. In MC experiments occurring for longer time intervals (up to 5 weeks; results not shown), CO2 production was much larger, up to 92% in the gas phase per week. The ratio of O2 respiration to CO2 production ranged between 1.25:1 and 4.6:1. Assuming that sugars were the main energy source, the total CO2 produced during MC exceeded that expected based on aerobic respiration alone, therefore indicating that fermentation is the dominant CO2-releasing process during MC. No CH4 was detected; this is likely because of (1) the acidic pH caused by accumulation of VOAs; and (2) sufficient O2 (~1–2%) retained in the headspace of the fermentors. These conditions are known to inhibit the activity of methanogens [23–25].
The pH of compost tea drawn from the MC fermentor containing leaf feedstock began at ~5.5, bottomed out at 3.69 by the 10th day, and over the next 20 days remained remarkably stable (i.e., dropped by only an additional 0.16 pH units). VOAs (principally acetic, propanoic, butyric, and to a lesser extent isovaleric and valeric acid) and alcohols (principally ethanol, and in lesser amounts propanol) reached steady states within the first ~10 days (data not shown). Similar results regarding pH, the EPF, VOAs, and alcohols were seen using food scrap in place of oak leaves in 20- and 210-L capacity fermentors.
In 210-L fermentors, the average pH of the compost tea derived from MC food scraps was remarkably similar to that seen in tea derived from MC leaves and consistent at 3.65 ± 0.06 (n = 6, ±1 SD) by ~7 days after composting was started. Glucose equivalents (based on the PSA assay) averaged 9.2 ± 1.8 mg ml−1 (n = 6, ±1 SD), and the ethanol content was 0.80 ± 0.14% w/v (n = 6, ±1 SD). Approximately 10–15 L of tea was produced per week by each 210-L fermentor containing food scrap. About 2% of the initial C was recovered in the form of glucose, alcohols, and VOAs.
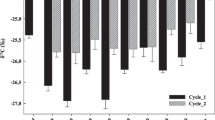
Processed residue from 210-L fermentors was amended into soil to a depth of ~5–20 cm in ~100-m2 swaths at varying loads ranging from 10 to 70 kg m−2. Tea was set aside for further studies and testing since it appeared too acidic (~3.6 pH) and rich in by-products (VOAs and alcohols) to be applied directly to the soil. The pH of soil amended with processed residue showed a biphasic shift in pH relative to the date of its amendment in soil, starting slightly higher than that of the tea (~4.5), and shifting into the neutral pH range within 10 days, and thereafter slowly climbing upward toward that of control soil over a period of approximately 5 months (Fig. 3). Control (unamended) soil had a pH in the range 7.5–8.0. The transient and rapid upward shift in soil pH seen during the first 10 days after amendment of processed food scrap into soil was confirmed by re-examining the pH of three sites showing an initial low soil pH. Follow-up testing made on drawing a second set of soil samples from the same sites 2 weeks later showed that they had shifted upward in pH into the neutral range (>6.5).
The half-life of the MC food scrap amended in soil was about 100–110 days (Fig. 4). Changes in the EPF index correlated with changes in the residual polysaccharide content (Fig. 4). There was however an upper limit (6–9%) in polysaccharide content achieved with progressive increasing loads of processed food scrap amended into the soil (Fig. 5). The upper limit observed in this study was not the result of nonlinearity or poor recovery. Serial dilutions of polysaccharide-spiked soil samples showed that the PSA assay was responsive and linear in excess of the 9% threshold.
Effect of cumulative load of MC-processed food scrap amended into soil on the soil polysaccharide content at subsequent sampling. Each data point represents a different field site we examined. Processed residue amended in soil is the cumulative load of MC-processed food scrap added to the specific site tested and calculated from the area covered and amount of material added independent of amendment date within a 6-month interval since amendment commenced
VOA analysis run on fresh soil samples drawn from field sites previously amended with processed food scrap ~2 months out showed the presence of three distinct VOAs—acetic, propanoic, and butyric acid (Fig. 6). Butyric acid was the dominant VOA present (~0.08% w/ww). The concentration of acetic and propanoic acid in the same soil samples was far less, approximately 1/100th of that of butyric acid. Although alcohols were present in the MC product, no alcohols were detected in the soil samples, most likely due to fast oxidation.
Discussion
A significant amount of C (~40–80 Gt) has been lost from soil in the last 200 years due to deforestation and poor management of arable soils [3, 4]. Because restoration of SOC improves soil quality and promotes further C sequestration from the atmosphere via C-fixation by plants, there is much interest in finding how best to recapture and restore the SOC content of soil. Aerated composting (AC), one of the most common routes for recycling organic C into soil, has undeniable economical and ecological advantages, but also has some notable shortcomings. Its C recycling potential is 50% or less, the entire process is long (≥6 months), and its GHG footprint is very large [10–13]. The main gas produced during AC is CO2, but CH4 (~25 times more potent as a GHG than CO2) is also a notable by-product. Along with C mineralization, N is also released, mostly as amines, heterocyclic compounds, ammonia, nitrite, and nitrate. Suboxic, acidic, and organic rich conditions can lead to incomplete denitrification with the formation of N2O as well [26, 27]. The amount of N2O emitted is small relative to CO2, yet N2O is ~300 times more powerful as a GHG than CO2 [28]. There is furthermore clear evidence that unchecked N2O produced during the turnover of C residues amended in soil can contribute GHG equivalents to the atmosphere which more than offset C savings due to AC [29].
The purpose of this work was to increase the C recycling potential and to minimize CH4 emission during MC of leaves and food scrap. MC uses microbial communities dominated by aerotolerant fermenters and microaerophiles (cellulolytic Gram-positive bacteria and yeasts). As practiced in this study, MC is less labor-intensive than AC; forced aeration and physically turning over the compost biomass, for example, are not required. MC also requires less time (about 2 weeks) to reach an end point. Since the combined loss of C at the end point is small (≤2%), most of the C processed ends up in soil. Furthermore, in MC fermentors, the pH shift toward acidic conditions, and the continuous presence of small amounts of O2 blocks methanogens. MC therefore appears to be a more efficient means of recycling C into soil than AC. Because MC can operate as a close circuit system, it is feasible to control (scavenge, restrict) soluble nitrogen compounds and thus limit the emission of N2O.
The progressive falloff in EPF index values after amendment of the composted materials into soil shows that degradation continued, albeit at a slower rate. Based upon our measurements, within 1 year, ~10% of the amended polysaccharides will remain in soil. Amending MC processed food scrap into soil caused the soil to become briefly more acidic, over a period of few days to a little more than a week, after which the pH rose steadily back to the baseline of controls within 5 months. Our results indicate that the turnover of polysaccharides and pH is linked.
The relationship between soil polysaccharide content relative to the load of processed food scrap amended back into the soil is complex. An upper threshold of soil polysaccharide content peaking in the range of 6–9% (DW) occurred as MC processed food scrap was added to soil. Variability in the polysaccharide content relative to different load levels was also seen. Some of this variation could be due to a priming effect. This effect assumes that C added back to soil induces the growth of microbial populations, causing a rise in the capacity of soil to fix more C [30].
Based on VOAs, the processing of organic C continued months after addition of MC-treated feedstock to soil. Momma et al. [31] proposed a biological soil disinfestation strategy (i.e., killing certain soil-borne diseases) based on amending organic materials such as wheat bran in soil subsequently flooded with water to induce anaerobic generation of acetic and butyric acid, which they identified as effective suppressants in blocking the growth of Fusarium oxysporoum f.sp. lycopersici and Ralstonia solanacearum. Browning et al. [32] showed that butyric acid amended in sand at a concentration of 0.88 mg g−1 markedly suppressed survival of plant parasitic and fungivorous nematodes. Since the concentration of butyric acid detected in soil amended with MC-processed food scrap 2 months out was 0.8 mg g−1 soil, adding MC-processed vegetal matter to soil warrants further study as a strategy for suppressing the growth of plant pathogens and parasites.
Lastly, one potential application of combining MC with soil amending is in the processing of urban food scrap and vegetal debris. Considering mid-range loads amended into soil in this study (~20–40 kg m−2), and assuming a 1-year turnover, we calculate that one hectare of soil has the capacity to assimilate 200–400 metric tons of processed food scrap and/or vegetal debris yearly. A MC facility with a capacity of ~100 m3 and a residence time of materials in fermentors of 2 weeks would require ten hectares of land to process 2,000–4,000 metric tons of food scrap per year.
References
Angulo-Brown, F., Sanchez-Salas, N., Barranco-Jimenez, M. A., & Rosales, M. A. (2009). Renewable Energy, 34, 2344–2352.
Zeng, N. (2008). Carbon Balance and Management, 3, 1–12.
Mullen, R. W., Thomason, W. E., & Raun, W. R. (1999). Communications in Soil Science and Plant Analysis, 30, 1713–1719.
Buringh, P. (1984). The role of terrestrial vegetation in the global carbon cycle: Measurement by remote sensing, Chapter 3: Organic carbon in soils of the world. In: G. M. Woodwell (Ed.). (pp. 91–109). Wiley
Houghton, R. A. (2005). The contemporary carbon cycle. In W. H. Schlesinger (Ed.), Biogeochemistry (pp. 473–513). Amsterdam: Elsevier Science.
Schlesinger, W. H. (1995). In R. Lal, J. Kimble, E. Levine, & B. A. Stewart (Eds.), An overview of the carbon cycle, in soils and global change (pp. 9–25). Boca Raton, FL: CRC/Lewis Publishers.
Houghton, R. A. (2007). Annual Review of Earth and Planetary Sciences, 35, 313–347.
Janzen, H. H. (2004). Agriculture, Ecosystems & Environment, 104, 399–417.
Lal, R., Griffin, M., Apt, J., Lave, L., & Morgan, M. G. (2004). Managing Soil Carbon. Science, 304, 393.
Xiao, Y., Zeng, G. M., Yang, Z. H., Shi, W. J., Huang, C., Fan, C. Z., et al. (2009). Bioresource Technology, 100, 4807–4813.
Andersen, J. K., Boldrin, A., Samuelsson, J., Christensen, T. H., & Scheutz, C. (2010). Journal of Environmental Quality, 39, 713–724.
Sanchez, C. (2009). Biotechnology Advances, 27, 185–194.
Hao, X., Chang, C., Larney, F. J., & Travis, G. R. (2001). Journal of Environmental Quality, 30, 376–386.
Safarik, I., & Santruckova, H. (1992). Plant and Soil, 143, 109–114.
Dubois, M., Giles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Analytical Chemistry, 28, 350–356.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Green, T.R. and Popa, R. (2010) Journal of Polymers and the Environment, online http://www.springerlink.com/content/h2m554v2h154887j/.
Green, T.R. and Popa, R. (2010) Applied Biochemistry and Technology, online http://www.citeulike.org/article/7821425.
Emerson, D., & Moyer, C. (1997). Applied and Environmental Microbiology, 63, 4784–4792.
Cook, A. E., & Meyers, P. R. (2003). International Journal of Systematic and Evolutionary Microbiology, 53, 1907–1915.
Lane, D. J. (1991). Nucleic acid techniques in bacterial systematics (pp. 115–175). Chichester: Wiley.
Hung, C. H., Cheng, C. H., Cheng, L. H., Liang, C. M., & Lin, C. Y. (2008). International Journal of Hydrogen Energy, 33, 1586–1592.
Jones, W. J., Nagle, D. P., Jr., & Whitman, W. B. (1987). Microbiological Reviews, 51, 135–177.
Grahame, D. A., & Stadtman, T. C. (1993). In J. G. Ferry (Ed.), Methanogenesis, ecology, physiology, biochemistry, and genetics (pp. 335–359). London: Chapman & Hall Inc.
Segers, R. (1998). Biogeochemistry, 41, 23–51.
Otte, S., Grobben, N. G., Robertson, L. A., Jetten, M. S. M., & Kuenen, J. G. (1996). Applied and Environmental Microbiology, 62, 2421–2426.
Jiang, Q., & Bakken, L. R. (1999). Appl. Environmental Microbiology, 65, 2679–2684.
Intergovernmental Panel on Climate Change, in Climate Change (2007). The Physical Science Basis (2007), p. 212.
Li, C., Frolking, S., & Butterbach-Bahl, K. (2005). Climatic Change, 72, 321–338.
Fontaine, S., Bardoux, G., Benest, D., Verdier, B., Mariotti, A., & Abbadie, L. (2004). Soil Science Society of America Journal, 68, 125–131.
Momma, N., Yamamoto, K., Simandi, P., & Shishido, M. (2006). Journal of General Plant Pathology, 72, 247–252.
Browning, M., Dawson, C., Aim, S. R., Gorres, J. H., & Amador, J. A. (2004). Applied Soil Ecology, 27, 47–54.
Acknowledgments
This work was supported by a grant from the Center for Sustainable Processes and Practices at Portland State University and by a faculty development award from the Office of Research and Sponsored Projects. We also want to acknowledge Scott Olsen from New Earth Farm (Hillsboro, OR).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Green, T.R., Popa, R. Turnover of Carbohydrate-Rich Vegetal Matter During Microaerobic Composting and After Amendment in Soil. Appl Biochem Biotechnol 165, 270–278 (2011). https://doi.org/10.1007/s12010-011-9249-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9249-4