Abstract
Background
Total hip arthroplasty (THA) is often performed in patients who are older and may take bisphosphonates to treat a variety of conditions, most commonly osteoporosis. However, the clinical effects of bisphosphonate use on patients who have undergone THA are not well described.
Questions/purposes
(1) Is bisphosphonate use in patients with osteoarthritis undergoing primary THA associated with a change in the risk of all-cause revision, aseptic revision, or periprosthetic fracture compared with patients not treated with bisphosphonates? (2) Does the risk of bisphosphonate use and revision and periprosthetic fracture vary by patient bone mineral density and age?
Methods
A retrospective cohort study of 12,878 THA recipients for the diagnosis of osteoarthritis was conducted; 17.8% of patients were bisphosphonate users. Data sources for this study included a joint replacement registry (93% voluntary participation) and electronic health records and an osteoporosis screening database with complete capture of cases as part of the Kaiser Permanente integrated healthcare system. The endpoints for this study were revision surgery for any cause, aseptic revision, and periprosthetic fracture. The exposure of interest was bisphosphonate use; patients were considered users if prescriptions were continuously refilled for a period equal to or longer than 6 months. Bone quality (based on dual-energy x-ray absorptiometery ordered based on the National Osteoporosis Foundation’s clinical guidelines taken within 5 years of the THA) and patient age (< 65 versus ≥ 65 years) were evaluated as effect modifiers. Patient, surgeon, and hospital factors were evaluated as confounders. Cox proportional hazards models were used. Hazard ratios (HRs) and 95% confidence intervals (CIs) were determined.
Results
Age- and sex-adjusted risks of all-cause (HR, 0.50; 95% CI, 0.33–0.74; p < 0.001) and aseptic revision (HR, 0.53; 95% CI, 0.34–0.81; p = 0.004) was lower in bisphosphonate users than in nonusers. The adjusted risk of periprosthetic fractures in patients on bisphosphonates was higher than in patients not on bisphosphonates (HR, 1.92; 95% CI, 1.13–3.27; p = 0.016). Lower risks of all-cause revision and aseptic revision were observed in patients with osteopenia (HR, 0.49; 95% CI, 0.29–0.84; and HR, 0.53; 95% CI, 0.29–0.99, respectively) and osteoporosis (HR, 0.22; 95% CI, 0.08–0.62; and HR, 0.33; 95% CI, 0.11–0.99, respectively).
Conclusions
Patients considered bisphosphonate users who underwent THA had a lower risk for revision surgery. Bisphosphonate use was associated with a higher risk of periprosthetic fractures in younger patients with normal bone quantity. Evaluation of bone quality and bisphosphonate use for the diagnosis of osteoporosis is encouraged in patients with osteoarthritis who are candidates for primary THA. Further research is required to determine the optimal duration of therapy because long-term bisphosphonate use has been associated with atypical femur fractures.
Level of Evidence
Level III, therapeutic study.
Similar content being viewed by others
Introduction
Failure of implant fixation after THA can lead to pain, morbidity, and revision surgery. Inadequate quantity or quality of the supporting bone can contribute to THA failure, including aseptic loosening and periprosthetic fracture [27, 28]. Multiple studies have evaluated the effect of bisphosphonate use on bone mineral density (BMD) surrounding THA devices [1, 2, 5, 12, 15, 16, 29, 32, 37, 38]. All the studies focused on the short-term effect of a single antiosteoporotic agent on the BMD surrounding the implant. Most studies showed a positive effect with less bone loss compared with a placebo.
To our knowledge, three studies have examined fracture risk and revision arthroplasty after the use of bisphosphonates in patients who had undergone THA and TKA [27, 28, 31]. Although the studies revealed a lower risk of fracture and revision surgeries, it may be difficult to generalize these results because the samples were relatively homogenous populations and none of the studies quantified the degree of preoperative osteoporosis in the populations studied.
The purpose of our investigation was to determine if bisphosphonate use in patients undergoing primary THA was associated with a change in the risk of all-cause revision, aseptic revision, or periprosthetic fracture compared with patients not treated with bisphosphonates. Furthermore, we studied the risk of revision and periprosthetic fracture stratified by patient BMD and age.
Patients and Methods
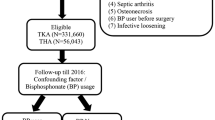
A retrospective study, from April 2001 to December 2010, was conducted. Three data sources from Kaiser Permanente (KP) Southern California (KPSC), a large integrated healthcare system, were used to conduct the study.
All patients who underwent primary elective THA and were aged > 40 years [28] with the primary diagnosis of osteoarthritis treated between April 2001 and December 2010 in the Southern California region of KP were included in the study (N = 12,878). During the course of the study, membership in the region was approximately three million people.
The main exposure of interest in our study was the use of bisphosphonate medication. Bisphosphonate use was determined similarly to the criteria reported by Prieto-Alhambra et al. [28]. Patients were considered bisphosphonate users if they (1) had not had a revision procedure and had filled at least two medication prescriptions (each with three refills) at any time during the study period, indicating a minimum of 6 months of treatment with high adherence; and (2) had a revision and had been prescribed the medication for at least 6 months (two continuous prescriptions each with three refills) with high adherence 6 months before the revision. High adherence was defined as medication possession more than 80% of the time (proportion of days between first and last prescription). Nonusers were patients who never had a prescription (from 2001 to 2010) or had a prescription only after their revision procedure or were partial users (had less than two prescriptions and less than 6 months of treatment or medication possession less than 80% of the time as previously defined).
The main endpoint of our study was revision for any reason after the index THA. A revision procedure was defined as any procedure in which at least one component was replaced. Secondary outcomes of our study were aseptic revision and periprosthetic fracture. Aseptic revision was defined as a revision for any reason other than infection. Periprosthetic fracture was defined as an ipsilateral femur fracture, which could have been treated operatively or nonoperatively. Periprosthetic fracture information was available from 2005 to 2010 for the study and therefore became the denominator of the subanalysis (n = 9505).
Patient bone quantity within 5 years of the index operation and age at the time of the index operation (< 65 years old versus ≥ 65 years old) were investigated as possible effect modifiers [9]. This age categorization was chosen because the National Osteoporosis Foundation’s clinical guidelines recommend dual-energy x-ray absorptiometry (DEXA) scan on all women aged > 65 years and for men aged 70 years or more [22]. Age, therefore, became the indication for measuring bone quantity and we elected to include this in our analysis. Bone mineral density was measured by means of a DEXA scan obtained at any time within 5 years of the primary THA and categorized by T scores (World Health Organization criteria for osteoporosis: normal, osteopenia, osteoporosis) [36].
Patient characteristics investigated as possible confounders included sex, age, body mass index, American Society of Anesthesiologists score, race, and diabetic status. Other factors evaluated as possible confounders included type of implant fixation (cemented versus uncemented versus hybrid), surgeon (< 10 versus 10–49 versus 50+ cases performed/year), and hospital (< 100 versus 100–200 versus 200+ cases/year) annual mean volume and surgeon arthroplasty fellowship training.
Data from the three KPSC data sources were linked using unique patient identifiers that the organization assigns to its members.
The Kaiser Permanente Total Joint Replacement Registry (KPTJRR) was used to identify our study sample and implant longevity (all-cause and aseptic revisions) [23]. Data collection procedures, coverage, participation rate, and data and tools available from the KPTJRR have been published [23, 25, 26]. Briefly, the KPTJRR collects intraoperative information on all arthroplasty surgeries from the surgeon. In addition, patient-, surgeon-, and medical center-specific information is collected by the KPTJRR using other data sources within KP (eg, electronic medical records [EMRs], Diabetes Registry, Geographically Enriched Member Sociodemographics, institutional membership and mortality, administrative claims data). Arthroplasty outcomes (ie, revision procedures, surgical site infections, and thromboembolic events) are prospectively ascertained by the KPTJRR using electronic screening algorithms of the EMR and administrative claims data in combination with a chart review of cases to confirm the event. The registry covers eight US geographic regions and reported a 93% participation rate for patients who underwent THA in 2010 [26]. Loss to followup in our cohort was 10%. Most outside (non-KP) hospital admissions will be repatriated to a KP facility before surgical intervention, thereby assuring full capture of revision surgery and periprosthetic fracture management in patients who are current members.
The second source for our study was the KP EMR. The pharmacy module, which captures all medications prescribed and dispensed within KP, was used to determine bisphosphonate use of patients within the cohort. We identified all medication orders for risedronate sodium, alendronate sodium, ibandornate sodium, alendronate-sodium-cholecalciferol, etidronate disodium, and zoledronic acid written and dispensed during the study period. The inpatient and outpatient modules of the EMR were used to identify periprosthetic fractures (using the International Classification of Diseases, 9th Revision codes 820-821, 996.44, and 733.10, 733.14, 733.15, 733.19, 733.96, 733.9).
The KPSC “Healthy Bones Database,” which tracks periprosthetic fractures and DEXA scan results of members of the KPSC healthcare system, was the third source used for our study. Details on this data source and its processes have been published [9, 10, 14].
Means, SDs, medians, interquartile ranges, frequencies, and proportions were used to describe the study sample. Cumulative crude incidence of all-cause revision, aseptic revision, and periprosthetic fractures was also calculated by bisphosphonate user status. Survival estimates were obtained from life tables. Cox proportional hazards models were used to estimate the risk of all-cause revision, aseptic revision, and periprosthetic fractures along with the use of bisphosphonates. Proportional hazard assumptions were evaluated using survival function compared with survival time graphs. Hazard ratios (HRs) and 95% confidence intervals (CIs) were determined as were Wald chi square test p values. Regression adjustment was used and variables (ie, age, gender) were considered confounders if they changed the risk estimation by at least 20% and were significantly associated with the outcome. Collinearity was evaluated and tolerance values less than 0.1 were set as the threshold. Models for each of the outcomes were created along with models stratified by patient age category (< 65 versus ≥ 65 years old) and DEXA scan status (no DEXA, normal DEXA, DEXA with osteopenia, DEXA with osteoporosis) per a priori hypotheses. Sensitivity analyses, using propensity score stratification adjustment, were conducted to determine if confounding was properly addressed using chosen models (data not shown). Missing data were handled using multiple imputation with 10 imputed data sets; the imputation model consisted of all exposure variables in the particular analysis model being used, additional covariates that included the event indicator for revision, and the Nelson-Aalen estimator of the cumulative baseline hazard at the event or censored survival time for each individual [21, 35]. The variable with highest proportion of missing data was race (8%; Table 1). SAS (Version 9.2; SAS Institute, Cary, NC, USA) was used to analyze the data and α = 0.05 was set as the threshold for statistical significance.
A sample of 12,878 patients who underwent primary THA fit our study criteria. The majority of patients were women (57.6%, n = 7416), older than 65 years (58.3%, n = 7510), and white (68.5%, n = 8817). Of the sample, 17.8% (n = 2292) patients met criteria for bisphosphonate users and the majority of the cohort (61.1%, n = 7873) had a DEXA scan within 5 years of their primary THA. There were different age, sex, race, body mass index, American Society of Anesthesiologists, diabetes, fixation, and DEXA scan use distributions between patients considered bisphosphonate users and those who were not users (Table 1).
During the study period, 10% of patients (1288) were lost to followup, 1153 (11% out of 10,586) of the nonbisphosphonate group and 135 of the (6% out of 2292) the bisphosphonate users. Of the total cohort (N = 12,878), 814 patients (6%) died, 6% (n = 660 of 10,586) of the nonbisphosphonate group and 7% (n = 154 of 2292) of the bisphosphonate users.
Results
Association of Bisphosphanate Use With Revision and Periprosthetic Fracture
Age- and sex-adjusted risks of all-cause revision (HR, 0.50; 95% CI, 0.33–0.74; p < 0.001) and aseptic revision (HR, 0.53; 95% CI, 0.34–0.81; p = 0.004) were lower in bisphosphonate users than in nonusers. The adjusted risk of periprosthetic fractures in patients on bisphosphonates was higher than in patients not on bisphosphonates (HR, 1.92; 95% CI, 1.13–3.27; p = 0.016; Table 2). At 8 years post-THA, the overall survival probability (all-cause revision) was 97% (95% CI, 96%–97%) and aseptic revision survival was 97% (95% CI, 97%–98%). At 5.7 years post-THA, the periprosthetic fracture survival-free rate was 99% (95% CI, 98%–99%; Table 3).
BMD and Age-specific Association of Bisphosphanate Use With Revision and Periprosthetic Fracture
Overall, age- and sex-adjusted risks of all-cause revision were only observed in patients aged 65 years and older when stratified by age (HR, 0.48; 95% CI, 0.31–0.74). In patients without DEXA scans or with normal scans, risk of all-cause revision was not different between bisphosphonate users and nonusers. For older patients with a diagnosis of osteopenia who were on bisphosphonates, HR for all-cause revision was 0.51 (95% CI, 0.27–0.95), the same HR in patients who did not take bisphosphonates. In patients with osteoporosis, risk of all-cause revision in bisphosphonate users was 0.11 (95% CI, 0.01–0.88); that of nonbisphosphonate users younger than age 65 years; and 0.24 (95% CI, 0.08–0.76) in patients 65 years old and older compared with nonbisphosphonate users (Table 2).
Overall, adjusted aseptic risk of revision was also lower in bisphosphonate users in the 65-year-old and older group (HR, 0.47; 95% CI, 0.29–0.76). In patients with osteopenia, risk of aseptic revision was slightly lower in bisphosphonate users (HR, 0.53; 95% CI, 0.29–0.99). For patients with osteoporosis, risk of aseptic revision was slightly lower for patients on bisphosphonates (HR, 0.33; 95% CI, 0.11–0.99). In patients with either osteopenia or osteoporosis, the hazard of events was not different by age groups for patients on bisphosphonates or not (Table 2).
Overall, in patients < 65 years old, bisphosphonate users had a slightly higher adjusted risk of periprosthetic fracture (HR, 4.55; 95% CI, 1.05–19.60) compared with nonusers, but this was not different in patients 65 years old and older (HR, 1.65; 95% CI, 0.98–2.78). In patients with normal DEXA scans, the only difference was observed in patients aged < 65 years old on bisphosphonates who had an HR of 32.69 (95% CI, 2.65–403.7) of fracture compared with same-aged patients not on bisphosphonates. No differences were observed in risk of fracture for patients with osteopenia or osteoporosis treated with bisphosphonates.
All risk estimations were consistent with those from models using propensity score stratification.
Discussion
Patients who are candidates for primary THA for the diagnosis of osteoarthritis are commonly older with comorbid conditions including osteoporosis. Bisphosphonate therapy has been used successfully in the management of osteoporosis but has not been extensively studied for its effects on patients undergoing primary THA including revision and periprosthetic fracture risk. Bisphosphonate use was associated with a lower risk for all-cause and aseptic revision in patients undergoing primary THA for osteoarthritis. However, the use of bisphosphonates was associated with a higher risk of periprosthetic fractures in younger patients with normal BMD.
Limitations of our study included the lack of indication for bisphosphonate use in patients with normal BMD or in patients who did not receive a DEXA scan. There were 305 patients without a DEXA scan and 272 patients with normal BMD who received bisphosphonate therapy in this cohort. It is common at our institutions to initiate bisphosphonates in patients receiving long-term steroids, regardless of DEXA score. Loss to followup in our cohort (10%) was another limitation. Patients lost to followup could have been revised or sustained a fracture. This concern was mitigated by the number of patients in the bisphosphonate user group, whose attrition was lower than that in the nonuser group (5.9% versus 10.9%), making our estimations of risk of revision likely more conservative. Conversely, we may have overestimated risk of periprosthetic fracture in patients who were not on bisphosphonates. However, the proportion of cases is small and it is very unlikely this proportion would have had an effect on our estimations. Of note, the data on periprosthetic fracture outcomes were based on a smaller cohort of patients; fewer events occurred and therefore uncertainty (ie, wide CIs) surrounds our estimates. We acknowledge that there may have been changes in patient BMD from the time of the DEXA scan to actual THA; however, this time period has been previously used and our methodology was thus consistent with published data [28]. There may be a further change in BMD after initiation of a bisphosphonate but that change tends to be only 3% to 5%, even after 3 years of bisphosphonate treatment, and not likely to affect DEXA scores significantly [8]. The definition of bisphosphonate user included high adherence and use for a minimum of 6 months, which did not fully account for dose, duration, and patient compliance to bisphosphonate use in all patients. The definition, however, was consistent in patients who underwent revision or sustained a fracture and those who did not. The definition was used to replicate the methodology of a previously published study to allow for comparison [28]. Additionally, although our analysis evaluated and adjusted for available confounders, there were other potential confounders such as smoking and steroid use that we were not able to investigate. We tried, partially, to address the lack of steroid use information by excluding patients with any surgical indication other than osteoarthritis. Finally, multiple individual bisphosphonate agents were grouped as a class of drug while each agent has its own side effects. We did not evaluate the other possible side effects of these individual agents.
Aseptic loosening has been described as a major cause for revision after THA at long-term followup [4, 24] Aseptic loosening of THA includes multiple pathways, including osteolysis and mechanical failure of fixation [7]. Osteolysis is a result of macrophage-induced activation of osteoclast production and inhibition of osteoblast formation [34]. Stiffness mismatch between implant and host bone can also lead to stress shielding, which results in disuse osteopenia and can lead to fracture or implant loosening [7]. Finally, THAs are performed, in large part, on older patients and female patients [24], patient groups also at higher risk for primary osteopenia and osteoporosis [10].
Antiosteoporotic agents have been introduced to reduce bone mineral loss [6]. Bisphosphonates have been evaluated with regard to prevention of bone loss surrounding THA implants in multiple studies [1, 2, 12, 15, 16, 37, 38]. The ability to help prevent bone loss surrounding THA implants suggests a clinically relevant tool for prevention of osteolysis and stress shielding around these devices and, ultimately, implant failure and revision surgery.
Prieto-Alhambra et al. evaluated patients undergoing primary THA and TKA in the United Kingdom and found bisphosphonate use to have a strongly protective effect on implant survival [28]. The effect was more pronounced in TKA than THA and was more pronounced in patients with the diagnosis of osteoarthritis. In a Danish cohort study, Thilleman et al. evaluated patients undergoing THA with a diagnosis of osteoporosis or prior osteoporotic fracture [31] and reported a reduction in risk of all-cause revision when bisphosphonates were used long term (more than 240 days). However, they also reported an increased risk of deep infection with bisphosphonate use. Their study included patients with the diagnosis of rheumatoid arthritis. Neither Prieto-Alhambra et al. nor Thillerman et al. evaluated the effect of bisphosphonates on periprosthetic fractures that did not lead to revision surgery [31].
Lower risk of all-cause revision and aseptic revision in patients using bisphosphonates was observed in our study, consistent with earlier studies [28, 31]. Our study stratified patients of normal bone density, osteopenia, and osteoporosis as well as age, revealing that the benefits of bisphosphonate use were even more pronounced in patients with initially lower BMD. Furthermore, after stratifying by bone density, older patients were found to benefit more than younger patients from the use of bisphosphonates.
Patient, implant, surgeon, and facility/hospital factors were also evaluated as potential confounders. A pertinent negative finding was the lack of influence of implant fixation type on revision surgery. We investigated whether cement was a potential confounder of the association of bisphosphonate use and risk of revision and periprosthetic fracture using the somewhat standard/conservative procedure of adding it to the survival model and determining whether it changed the risk estimations (of bisphosphonate and the outcomes evaluated) by at least 20% and was significantly associated with the outcome (p < 0.05). Although cemented fixation of the femoral stem has been shown to reduce revision rates and periprosthetic fractures in elderly patients in other studies, our analysis revealed that cement fixation did not affect the relationship of bisphosphonate use and risk of all cause revision, aseptic revision, or periprosthetic fracture [19, 30, 33].
Periprosthetic fracture rates in primary THA recipients have been reported as high as 1.1% in a large US cohort [3] and 0.64% at 10 years by the Swedish hip register [18], which are comparable to our cohort estimates (0.6%). We have not found studies that specifically evaluated the risk of periprosthetic fracture in bisphosphonate users compared with nonusers, although osteoporosis has been postulated as an explanation for this mode of failure [18]. Risk of periprosthetic fracture was higher in patients undergoing THA who had normal BMD and used bisphosphonates. In patients with either osteopenia or osteoporosis, bisphosphonate use was not found to be associated with a higher risk of fracture.
Bisphosphonates are known to accumulate in the bone and have effects for years [11], leading to lack of bone turnover through osteoclastic and osteoblastic activity inhibition [20]. Clinically, the loss of bone turnover and subsequent increased risk of femur fracture have been noted in prolonged bisphosphonate users and in younger, more active patients as atypical femur fractures [11, 13]. As such patients comprised a small group in our study, and our estimations had a great deal of uncertainty (wide CIs); further investigation is warranted.
A strength of our study was its large and representative patient sample [17]. Additionally, our ability to combine clinical, pharmacologic, and radiographic data for such a large sample was unique, and the capacity to link this information in our integrated healthcare system, where all patients have unique patient identifiers, minimized the bias involved with collecting and merging data from several sources. In summary, we found that bisphosphonate use was associated with a lower risk for all-cause and aseptic revision in patients undergoing primary THA for osteoarthritis. This lower risk was most pronounced in older and more osteoporotic patients. Bisphosphonate use was associated with a higher risk of periprosthetic fractures in younger patients with normal BMD, although this association had wide CIs and the indication for bisphosphonate use in these young patients with normal BMD was unknown. Evaluation of patients for prospective THA with DEXA scan and appropriate treatment of osteoporosis with bisphosphonates may be associated with a lower risk of revision surgery. The duration of bisphosphonate treatment remains to be determined. Care must be taken in prescribing bisphosphonates to younger patients (< 65 years old) with normal BMD as a result of the possible higher risk of fracture. Further research is required to determine optimal duration of therapy because long-term bisphosphonate use has been associated with atypical femur fractures.
References
Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J Orthop Res. 2009;27:183–188.
Arabmotlagh M, Rittmeister M, Hennigs T. Alendronate prevents femoral periprosthetic bone loss following total hip arthroplasty: prospective randomized double-blind study. J Orthop Res. 2006;24:1336–1341.
Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30:183–190.
Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177.
Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schunemann H, Einhorn TA. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2005;87:293–301.
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
Canale T, Beaty JH. Campbell’s Operative Orthopaedics. 12th ed. Philadelphia, PA, USA: Elsevier; 2013.
Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi J, Shea B, Tugwell P, Guyatt G. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–516.
Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, Zhou H, Burchette RJ, Ott SM. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–2550.
Dell RM, Greene D, Anderson D, Williams K. Osteoporosis disease management: What every orthopaedic surgeon should know. J Bone Joint Surg Am. 2009;91(Suppl 6):79–86.
Erviti J, Alonso A, Oliva B, Gorricho J, Lopez A, Timoner J, Huerta C, Gil M, De Abajo F. Oral bisphosphonates are associated with increased risk of subtrochanteric and diaphyseal fractures in elderly women: a nested case-control study. BMJ Open. 2013;3. pii: e002091.
Fokter SK, Komadina R, Repse-Fokter A. Effect of etidronate in preventing periprosthetic bone loss following cemented hip arthroplasty: a randomized, double blind, controlled trial. Wien Klin Wochenschr. 2006;118(Suppl 2):23–28.
Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349–353.
Greene D, Dell RM. Outcomes of an osteoporosis disease-management program managed by nurse practitioners. J Am Acad Nurse Pract. 2010;22:326–329.
Iwamoto N, Inaba Y, Kobayashi N, Ishida T, Yukizawa Y, Saito T. A comparison of the effects of alendronate and alfacalcidol on bone mineral density around the femoral implant and in the lumbar spine after total hip arthroplasty. J Bone Joint Surg Am. 2011;93:1203–1209.
Kinov P, Tivchev P, Doukova P, Leithner A. Effect of risedronate on bone metabolism after total hip arthroplasty: a prospective randomised study. Acta Orthop Belg. 2006;72:44–50.
Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, Chen W, Jacobsen SJ. Sociodemographic Characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41.
Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury. 2007;38:651–654.
Mäkelä KT, Matilainen M, Pulkkinen P, Fenstad AM, Havelin L, Engesaeter L, Furnes O, Pedersen AB, Overgaard S, Kärrholm J, Malchau H, Garellick G, Ranstam J, Eskelinen A. Failure rate of cemented and uncemented total hip replacements: register study of combined Nordic database of four nations. BMJ. 2013;348:f7592.
Miller PD. Optimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapy. Clin Ther. 2005;27:361–376.
Moons KG, Donders RA, Stijnen T, Harrell FE, Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101.
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: http://nof.org/files/nof/public/content/resource/913/files/580.pdf. Accessed November 20, 2013.
Paxton E, Inacio M, Slipchenko T, Fithian D. The Kaiser Permanente National Total Joint Replacement Registry. Perm J. 2008;12:12–16.
Paxton E, Namba R, Maletis G, Khatod M, Yue E, Davies M, Low R, Wyatt R, Inacio M, Funahashi T. A prospective study of 80,000 total joint and 5,000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg Am. 2010;92(Suppl 2):117–132.
Paxton EW, Inacio MC, Khatod M, Yue EJ, Namba RS. Kaiser Permanente National Total Joint Replacement Registry: aligning operations with information technology. Clin Orthop Relat Res. 2010;468:2646–2663.
Paxton EW, Inacio MCS, Kiley ML. The Kaiser Permanente implant registries: effect on patient safety, quality improvement, cost effectiveness, and research opportunities. Perm J. 2012;16:33–40.
Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Kiran A, de Vries F, Cooper C, Arden NK. Fracture risk before and after total hip replacement in patients with osteoarthritis: potential benefits of bisphosphonate use. Arthritis Rheum. 2011;63:992–1001.
Prieto-Alhambra D, Javaid MK, Judge A, Murray D, Carr A, Cooper C, Arden NK. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:d7222.
Skoldenberg OG, Salemyr MO, Boden HS, Ahl TE, Adolphson PY. The effect of weekly risedronate on periprosthetic bone resorption following total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am. 2011;93:1857–1864.
Stea S, Comfort T, Sedrakyan A, Havelin L, Marinelli M, Barber T, Paxton E, Banerjee S, Isaacs AJ, Graves S. Multinational comprehensive evaluation of the fixation method used in hip replacement: interaction with age in context. J Bone Joint Surg Am. 2014;96(Suppl 1):42–51.
Thillemann TM, Pedersen AB, Mehnert F, Johnsen SP, Soballe K. Postoperative use of bisphosphonates and risk of revision after primary total hip arthroplasty: a nationwide population-based study. Bone. 2010;46:946–951.
Trevisan C, Ortolani S, Romano P, Isaia G, Agnese L, Dallari D, Grappiolo G, Cherubini R, Massari L, Bianchi G. Decreased periprosthetic bone loss in patients treated with clodronate: a 1-year randomized controlled study. Calcif Tissue Int. 2010;86:436–446.
Troelsen A, Malchau E, Sillesen N, Malchau H. A review of current fixation use and registry outcomes in total hip arthroplasty: the uncemented paradox. Clin Orthop Relat Res. 2013;471:2052–2059.
Tuan RS, Lee FY, T Konttinen Y, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16(Suppl 1):S42–48.
White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998.
WHO. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis. Geneva, Switzerland: World Health Organization; 1994.
Yamaguchi K, Masuhara K, Yamasaki S, Nakai T, Fuji T. Cyclic therapy with etidronate has a therapeutic effect against local osteoporosis after cementless total hip arthroplasty. Bone. 2003;33:144–149.
Yamasaki S, Masuhara K, Yamaguchi K, Nakai T, Fuji T, Seino Y. Risedronate reduces postoperative bone resorption after cementless total hip arthroplasty. Osteoporos Int. 2007;18:1009–1015.
Acknowledgments
We thank all Kaiser Permanente orthopaedic surgeons and the staff of the Department of Surgical Outcomes and Analysis who have contributed to the success of the National Total Joint Replacement Registry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Khatod, M., Inacio, M.C.S., Dell, R.M. et al. Association of Bisphosphonate Use and Risk of Revision After THA: Outcomes From a US Total Joint Replacement Registry. Clin Orthop Relat Res 473, 3412–3420 (2015). https://doi.org/10.1007/s11999-015-4263-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4263-4