Abstract
Water-based anti-corrosion coatings, which are environmentally-friendly replacements for organic solvent-based coatings, do not perform well enough for use in the most challenging corrosion environments. The high water absorption capacity of water-based latex films may reduce barrier performance by contributing to corrosive reactant/product transport. We seek to understand the coupled effects of water absorption and ion transport in hydrated latex films, and to propose mechanisms explaining these effects. Water absorption and ion transport in films immersed in deionized (DI) water were monitored by mass gain and electrical conductivity measurements, respectively. Despite very similar polymer compositions between films, large differences in water absorption and ion transport rates were observed and explained by percolating networks at latex particle boundaries which facilitate transport. A semi-continuum model with three-component diffusion and convection-like elastic relaxation supported the assumptions of the physical mechanisms governing water absorption and ion transport. The evidence of the coupled processes of water absorption and ion transport in hydrated latex films revealed in this study are useful for designing water-based coatings that provide high levels of corrosion resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coatings are a cost-effective, easily-applied and scalable method of protecting metal surfaces, such as carbon steel, against corrosion. Paint, composed of polymers and other additives dissolved or dispersed in a suitable carrier, is applied to a surface with a wet thickness on the order of 100 µm. The solid film, which forms after evaporation of volatile components, functions as a physical barrier against corrosive reactants, such as water, oxygen, and salts. Much work has focused on the development of water-based anti-corrosion coatings, in which components are initially dispersed or dissolved in water, to reduce the environmental damage caused by the use of solvent-based coatings, which release organic vapor during solvent evaporation.1 Polymers can be dispersed as latex colloids in water-based paint synthesized by emulsion polymerization as in this study. Film formation from a latex dispersion consists of three stages, as categorized by Steward et al.2 First, water evaporation raises the latex solids’ volume fraction until particles come into close contact and occupy a total volume fraction of 60–70%. Second, packed particles deform to fill interparticle voids in the film. Third, polymer chains inter-diffuse across latex particle boundaries, promoting chemical and free-volume homogeneities and reducing the distinction between individual particles. The dried films of practical latex coatings for corrosion protection typically consist of inorganic pigment particles such as titanium dioxide (TiO2) dispersed inside an organic matrix, which consists mostly of polymer along with residual coalescent, surfactant, dispersant, and other minor components.
Because they have some hydrophilicity, latex films often absorb an extraordinary amount of water, sometimes in excess of 100% of their dry mass.3,4,5,6,7,8 The effect of film composition on water absorption has been studied extensively. Indeed, raising film hydrophobicity by increasing the concentration of fluorinated monomers9,10,11,12,13,14,15,16 has been shown effective in reducing water absorption, but fluorinated latexes are limited by high financial cost10,11,13 and difficulty in synthesis due to chemical incompatibility between fluorinated molecules and water.12,15 Raising film hydrophilicity has the opposite effect, as increasing concentration of polar functional groups, such as carboxylic acid,8,17 hydroxyl8,18,19,20 and sulfonic acid,21 raises water absorption. Another important aspect, polymer mechanical properties also influence water absorption. Crosslinking between polymer chains7,8,22 and higher glass transition temperature (Tg)20,23,24 both reduce water absorption. Finally, both covalently and non-covalently bound emulsifiers have a significant effect on water absorption, as increasing emulsifier concentration raises water absorption.10, 16, 25,26,27 Previous studies showed that ion transport in hydrogels is strongly affected by the amount of absorbed water.28,29 When the absorbed water volume fraction in a polyethylene oxide hydrogel was raised from 29 to 81 vol.%, NaCl permeability increased by a factor of 140.28 Faster ion transport may lead to higher corrosion rates of a metal substrate, as higher chloride anion concentration leads to exacerbated pitting corrosion in stainless steel30 and accelerated corrosion of carbon steel.31 Therefore, absorbed water in latex films may have a significant effect on ion transport rate and, hence, anti-corrosion effectiveness of these films. However, ion transport in latex films, and its relationship to water absorption, has not been well studied. Dillon et al. observed ion transport kinetics by measuring electrical conductance and capacitance of a latex film under cyclic applied pressure exposed to different concentrations of Na+(aq) on each side, in addition to tracking the lower concentration side’s Na+(aq) concentration using an ion-selective electrode.32 This study concluded that film mechanical failure under pressure cycling occurred by (1) formation of thin spots with negligible ion permeation, followed by (2) formation of pores leading to significant ion permeation, followed by (3) catastrophic failure. An et al. measured ion equilibrium absorption and diffusivity in latex films with aqueous solutions of different ion concentrations on each side by tracking masses of ions transported through films versus time using electrical conductivity or ion chromatography.33 Ions with larger diameters were found to have lower diffusivity in latex films, while potassium salts showed higher solubility in latex films compared to ammonium salts with the same anion. However, neither study examined a possible correlation between ion transport and water absorption.
To that end, this study investigates the mutual influences of water absorption and ion transport in hydrated latex films using model systems of acrylic latex and TiO2 filler particles. Schematic representations of water absorption into, and ion transport out of, hydrated latex films measured in this study are shown in Fig. 1. Through comparison between water absorption and ion transport measurements supplemented by structural characterization, it was found that (1) absorbed water enhanced ion diffusivity, and (2) both water absorption and ion transport were accelerated by a more hydrophilic percolating latex particle boundary network. It is assumed that the behavior of the ions investigated in this study can be generalized to more corrosive ions such as chloride.
Methodology
Paint composition
Model water-based paints were provided by Dow. Paints with 35 vol.% solids contained acrylic latex particles with one of two surface chemistries—control or a Dow-proprietary surface-functionalization (SF)— but with very similar bulk polymer chemistry and were either non-pigmented (NP) or contained 200 nm-diameter rutile TiO2 particles with 16 vol.% concentration after film formation. Typical procedures for the preparation of the paint are given in US patent 6756459 B2. The glass transition temperature (Tg) of latex particles after emulsion polymerization, but with no further additives, ranges from – 20 to 60°C, with the exact value depending on the monomer composition and experimental procedure. However, since control and SF latex particles were prepared according to very similar recipes, these two types of particles should have very similar values of Tg. Coalescent was added to the paint following emulsion polymerization synthesis of the latex particles at a typical level of 6 wt.%.
Water absorption
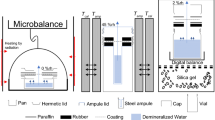
Control-NP, SF-NP, control-TiO2, and SF-TiO2 paints were applied onto non-stick RP-1K release papers (Leneta) using steel drawdown bars (BYK-Gardner, Gardco), film formation occurred at 25°C and 30–40% average relative humidity for 24 h, then free-standing films were carefully peeled from release papers. Water absorption measurements were performed on 5 free-standing samples of each film composition, with 24–35 cm2 area per sample, to minimize random error. The thicknesses of all samples were measured to be 44–64 μm using a CG206 coating thickness gauge (Extech). The average thickness of all samples of each film composition ranged from 50 to 61 μm. Each sample was weighed after film formation using an electronic balance (VWR), then immediately immersed in 250 mL deionized (DI) water in a glass jar. Subsequent weighing occurred every 2 h between 0 and 12 h after immersion, every 12 h between 12 and 324 h after immersion, and every 24 h between 324 and 1000 h after immersion. During subsequent weighing, each sample was removed from its jar, dried with Kimwipes while placed on dry cotton wipes (Texwipe), weighed using the balance, and re-immersed in its glass jar. This process lasted for approximately 1 min per sample. Samples were kept at room temperature between 18°C and 25°C while immersed in DI water. Corrections were applied for small pieces which detached from samples during weighing and handling. Small detached pieces were discarded after detachment. The mass of a small piece from sample X weighing <5 mg which detached at time t was added to the mass of sample X measured after time t. The mass fraction reduction due to a small piece from sample X weighing >5 mg which detached at time t was used to scale the mass of sample X measured after time t.
Weighing with a finer time resolution was performed for SF-NP and SF-TiO2 film compositions. For each film composition, 3 formed film samples with the same preparation procedure as described above were weighed after film formation, and once again after 15, 30, 45, or 60 min immersion.
Leached ion transport
The same free-standing film preparation procedure as the water absorption experiments was performed using control-NP, SF-NP, control-TiO2, and SF-TiO2 paints. 5 free-standing samples each of control-NP, SF-NP, and SF-TiO2, and 4 free-standing samples of control-TiO2, were produced with 24–35 cm2 area per sample and thicknesses between 49 and 82 μm. The average thickness of each film composition ranged from 55 to 63 μm. Each sample was immersed in a glass beaker filled with 75–80 mL DI water, together with an Orion DuraProbe 4-electrode conductivity cell (Thermo Scientific) connected to an Orion Star A212 conductivity benchtop meter (Thermo Scientific). Each beaker was stirred using a stir bar and magnetic stirrer (Thermo Scientific) and capped with a rubber stopper (Grainger) to minimize water evaporation. Electrical conductivity due to leached ions was recorded every 5 min from the start of immersion until 48–144 h after immersion for control-NP, SF-NP, and SF-TiO2, and recorded every 30 min from the start of immersion until 960 h after immersion for control-TiO2. Because a conductivity meter could store a maximum of 2000 conductivity measurements during each experiment, the interval between subsequent control-TiO2 conductivity measurements was longer than those for other films. Samples were maintained at temperatures between 20 and 25°C during measurements. All conductivity measurements were corrected to a reference temperature of 25°C with a temperature compensation coefficient of 2.1%/°C. Each conductivity cell was calibrated with 100 μS/cm, 1413 μS/cm, and 12.9 mS/cm conductivity standards (Thermo Scientific) prior to immersion into a beaker.
Cross-section scanning electron microscopy (SEM)
Control-TiO2 and SF-TiO2 paints were applied to polyimide substrates via drawdown, then formed at 25°C and 50–70% average relative humidity for 1–14 days. The formed film thickness was approximately 100 μm. Formed film-polyimide stacks were roughly cross-sectioned using a razor blade or scissors, after which the cross-section was polished with an Ar ion cross-section polisher (JEOL). Polished, formed film cross-sections were imaged in secondary electron mode using a Zeiss Merlin or Zeiss Ultra-55 SEM (Zeiss).
Structural and chemical characterization
The structures of control-NP and SF-NP free-standing formed films were characterized using a SAXSLAB small-angle X-ray scattering (SAXS) instrument which is capable of probing length scales between 0.3 and 2000 nm in transmission mode. The chemical composition of control-NP and SF-NP films were characterized using Fourier transform infrared (FTIR) spectroscopy, using an IS50R Bench FTIR spectrometer (Thermo Fisher) in attenuated total reflection (ATR) mode, which is capable of measuring infrared absorbance between 4000 and 400 cm−1 frequencies. Prior to ATR-FTIR measurements, control-NP and SF-NP films were dried in a vacuum oven at 100°C for approximately 90 min to minimize residual water in these films.
Results and discussion
SAXS and FTIR results
Results from transmission SAXS of control-NP and SF-NP films are shown in Fig. 2. Two diffraction peaks are visible in each of the diffraction patterns. Two diffraction peaks are visible in each scattering pattern, and the ratio of peak scattering vectors in each pattern suggests that the peaks are (111) and (220) peaks of a face-centered cubic (FCC) latex particle superlattice. The (111) and (220) FCC latex superlattice peaks were also observed in latex films by Konko et al. (2019). The scattering vector is \(q = \frac{{4\pi {\text{sin}}\left( \theta \right)}}{\lambda }\), where \(2\theta\) is the scattering angle and \(\lambda\) is the X-ray wavelength. Interplanar spacing between (hkl) planes may be obtained from the peak scattering vector according to Bragg’s law,37 \(d_{hkl} = \frac{\lambda }{{2{\text{sin}}\left( \theta \right)}} = \frac{2\pi }{q}\). For a cubic crystal structure such as FCC, \(d_{hkl} = \frac{a}{{\sqrt {h^{2} + k^{2} + l^{2} } }}\), where \(a\) is the lattice parameter.37 Assuming that there are four latex particles in each unit cell of the FCC superlattice, latex particle diameter \(d\) in the films is given by the expression \(a^{3} = 4\left( {\frac{\pi }{6}d^{3} } \right)\). Therefore, the latex particles should have a diameter \(d = 110 \;{\text{nm}}\) in control-NP films and a diameter of \(d = 140\; {\text{nm}}\) in SF-NP films, so control-NP film latex particles have only 20% lower diameter compared to SF-NP film particles. Moreover, these measured particle sizes are consistent with the preferred latex particle diameter range of 50–250 nm stated in US patent 6756459 B2.
Infrared absorption spectra of control-NP and SF-NP films measured using ATR-FTIR are shown in Fig. 3. The nearly identical position and intensity of most peaks on control-NP and SF-NP are consistent with the very similar bulk latex particle composition of the films. Major peaks at frequencies greater than 1500 cm−1, outside of the fingerprint region, may be identified with great confidence with the aid of an infrared absorption database. The peak at around 1700 cm−1 may be due C=O bond stretching, the peak at around 2200 cm−1 may be due to \({\text{C}} \equiv {\text{N}}\) stretching, and the peaks between 2800 and 3100 cm−1 may be C–H bond stretching. The most significant difference between control-NP and SF-NP films is the more intense SF-NP peaks in the 900–1100 cm−1 frequency region, which lie in the fingerprint region and are therefore more difficult to identify. In particular, the peaks between 1000 and 1100 cm−1 may be due to S=O bond stretching in sulfoxide groups. Due to the very similar bulk chemistries of control and SF latex particles, the infrared absorption spectra suggest that SF surface chemistry results in a higher concentration of hydrophilic sulfoxide groups than control surface chemistry, and hence that the boundary regions between latex particles in SF latex films are more hydrophilic compared to the corresponding regions in control latex films.
The claim of higher sulfoxide group concentration on SF latex surfaces is strengthened by a study by Chellappa et al. (2015), in which TiO2 particles were dispersed in a dimethyl sulfoxide (DMSO) solvent with high colloidal stability, as a zeta potential of 27 mV was measured for TiO2 particles stabilized in sulfoxide group-rich DMSO. The intention of SF surface chemistry was to improve TiO2 colloidal stability in paint, so it is possible that a sulfoxide-rich chemistry was selected by Dow when formulating the SF surface chemistry.
Water entry mechanism into latex films
Fractional mass gains of latex films during immersion in DI water as a function of time are shown in Fig. 4. A version of Fig. 4 with error bars is shown in Figure S0. The fractional mass gain is approximately proportional to the square root of time during initial water absorption of all dried films, up to at least ~2/3rds peak fractional mass gain. Square-root-of-time dependence of mass gain could result from either Fickian diffusion of water into films or capillary pressure-driven flow of water through interconnected film pores. The former case is derived by solving the diffusion equation along the film thickness dimension, resulting in the following approximate solution at early immersion times38,39:
where \(t\) is time, \(M\left( t \right)\) is fractional mass gain, \(D\) is molecular diffusivity of water, \(l\) is film thickness, and \(M_{\infty }\) is the fractional mass gain at saturation.\(M_{\infty }\) is also termed the water absorption capacity, and experimentally determined as peak fractional mass gain in Fig. 4.
Fractional mass gains of latex films immersed in DI water over time. Each data point is the average of values from 5 repeat samples. The inset more clearly displays water absorption behavior at short timescales along with the results of the linear fitting. Fit lines are truncated near the origin for greater clarity. The table displays water diffusivity and R2 value obtained from fitting for each film composition
The latter case is derived by assuming that water penetration through open dry pores is approximated as penetration through thin parallel tubes to arrive at the Washburn equation40:
where \(L\left( t \right)\) is the length of water penetration along tubes, \(\gamma = 73.08 \;{\text{mN}}/{\text{m}}\) is water surface tension,41 \(r\) is tube radius, \(\varphi\) is contact angle between water and latex film, and \(\eta = 1.002 \;{\text{mPa}}\;{\text{s}}\) is bulk water viscosity.42 Assuming that \(L\left( t \right) = 0\) when \(M\left( t \right) = 0\), \(L\left( t \right) = \frac{l}{2}\) when \(M\left( t \right) = M_{\infty }\), and proportionality between \(L\left( t \right)\) and \(M\left( t \right)\), the Washburn equation relates fractional mass gain to time:
Both equations (1) and (3) assume constant film thickness, which neglects the effect of volume expansion upon water absorption. Assuming that the partial molar volumes of absorbed water and bulk water are identical, the largest fraction volume increase at water absorption capacity—control-TiO2 with dry density of 1.7 g/cm3 and 106 wt.% capacity—is 180%. This volume expansion corresponds to a fraction thickness increase of 41% as the films (unconstrained) are assumed to be isotropic. Note that in order to reduce anisotropy, the films used in this study are not constrained in-plane. They were formed on a non-stick substrate, and then peeled off prior to water immersion. Still, the dried film is probably not perfectly isotropic structurally, because of the evaporation direction during its formation, and therefore the isotropy is a model assumption. This assumption may not apply equally well to actual constrained films.
Since both \(D\) in equation (1) and \(r\) in equation (3) scale with \(l^{2}\) when all other parameters are kept constant, a 41% increase in \(l\) results in a 99% increase in \(D\) and \(r\). That is, \(l\) is 0–41% larger than assumed, and \(D\) and \(r\) are actually 0–99% larger than the values obtained from fitting experimental data. Since equation (3) assumes that \(r\) remains constant during the water absorption process whereas pore shape may change after water absorption, values of \(r\) obtained by fitting equation (3) to experimental data represent the average tube radius during the water ingress process.
To assess the water permeation mechanism, equation (3) was fitted to experimental data in Fig. 4 to extract tube radius by linear fitting of \(M\left( t \right)\) versus \(\sqrt t\) between 0 and 48 h for control-NP, 15–60 min for SF-NP, 0–192 h for control-TiO2, and 15–60 min for SF-TiO2. Estimating \(\cos \left( \varphi \right) = 0.1\) (correct to within an order of magnitude43) yields tube radii ranging from \(2 \times 10^{ - 16} \;{\text{m}}\) for control-TiO2 to \(5 \times 10^{ - 14} \;{\text{m}}\) for SF-NP, many orders of magnitude lower than a typical atom diameter of \(10^{ - 10} \;{\text{m}}\), which is physically unrealistic. However, the viscosity of water with at least one dimension constrained to nanometer or sub-nanometer length scale may be 4–10 orders of magnitude larger than the viscosity of bulk water as measured experimentally.44,45,46,47 \(r\) is directly proportional to \(\eta\) at constant slope of \(M\left( t \right)\) versus \(\sqrt t\), so the lowest tube radius will be raised to \(10^{ - 12} \sim 10^{ - 6} \;{\text{m}}\)—well within a physically realistic range—when confined water viscosity is assumed to be 4–10 orders of magnitudes larger than bulk. However, significant viscosity enhancement is reported only when the length scale of confinement is approximately 1 nm or smaller.46,47 Therefore, it is possible that permeation through pores with a radius of around 1 nm or smaller contributes to water entry into the latex films.
By applying results from the linear fitting of \(M\left( t \right)\) versus \(\sqrt t\) to equation (1), water diffusivity values of \(2 \times 10^{ - 11} \;{\text{cm}}^{2} /{\text{s}}\) for control-NP, \(1 \times 10^{ - 9} \;{\text{cm}}^{2} /{\text{s}}\) for SF-NP, \(5 \times 10^{ - 12} \;{\text{cm}}^{2} /{\text{s}}\) for control-TiO2 and \(2 \times 10^{ - 10} \;{\text{cm}}^{2} /{\text{s}}\) for SF-TiO2 were obtained. Since the bulk polymer chemistries of control and SF latex particles are very similar, the factor-of-70 diffusivity difference between SF-NP and control-NP and factor-of-30 diffusivity difference between SF-TiO2 and control-TiO2 are most likely explained by different latex particle boundary compositions. While latex particle interdiffusion during film formation resulted in films with sufficient mechanical robustness to be peeled from release papers, it is proposed that interdiffusion was insufficient to result in roughly equal atomic density between latex particle boundaries and bulk. That is, latex particle boundaries are assumed to have a higher volume fraction of free volume compared to latex particle interiors, and the resulting higher diffusivity48 at boundaries combined with these boundaries forming a percolating network through the film may cause boundaries to dominate the film’s diffusivity. It is proposed that boundaries in SF polymer-containing film have higher local water absorption capacity compared to control polymer-containing film due to hydrophilic sulfoxide-rich SF groups at boundaries, which leads to higher measured diffusivities in SF polymer-containing films.49 Water whitening50 observed in control-NP and SF-NP films after DI water immersion further suggests that local levels of water absorption in the bulk latex particles and latex boundaries are different. The factor-of-3 diffusivity difference between control-NP and control-TiO2 and the factor-of-6 diffusivity difference between SF-NP and SF-TiO2 is not due to the blocking of water diffusion paths by TiO2 particles. Since TiO2 particles are randomly and mostly individually dispersed within both control-TiO2 and SF-TiO2 films as shown in Figs. 5a and 5b, they reduce water-accessible cross-section area and diffusivity by only 16% relative to NP films as TiO2 fraction is controlled at 16 vol.% in formed pigmented films. Instead, reduced water diffusivity after TiO2 addition may be attributed to TiO2 dispersants which reduce diffusivity in the boundary network by forming stronger film-water bonds, and consequently raising the activation energy for water diffusion.49 That is, water diffusion, partially through a percolating boundary network, is another plausible mechanism for water ingress into films.
Despite higher local water absorption at boundaries in SF polymer-containing films relative to control-polymer-containing films, SF polymer-containing films exhibit lower water absorption capacity as shown in Fig. 4. This apparent contradiction may be explained by the lower surfactant concentration in SF polymer-containing films due to the greater stability of latex particles with hydrophilic SF surface groups in dispersion, and the dominant effect of surfactant concentration on overall water absorption capacity.10
Effect of water absorption on ion leaching
To study ion transport, measurements of conductivity over time due to the leaching of latex film ions into DI water are shown in Fig. 6a. Versions of this figure with error bars are shown in Figs. S3 and S4. Conductivity rise is the complementary measurement to mass gain in Fig. 4: the former experiment measures ion transport out of films into DI water, while the latter measures water absorption into films. The conductivity rise rate in Fig. 6b is equal to the derivative of conductivity with respect to time. For SF-NP and SF-TiO2, the derivative at some time is calculated by the slope of the best fit line through 7 points closest to that time, where data is assumed to be locally linear. For control-NP, the derivative is the slope through the closest 21 points. Despite averaging across multiple samples, significant noise in conductivity measurements appeared after 10, 20, and 60 h during SF-NP, SF-TiO2, and control-NP leaching, respectively. This noise is attributed to the fractured film fragments, generated due to forces induced by stir bars, which temporarily lodge between electrodes in conductivity probes and thereby change the measured conductivity.
(a) Conductivity versus immersion time of DI water baths into which latex films were placed. SF-NP and SF-TiO2 data series are each averaged from five repeat samples at all times shown. Conductivities are normalized by DI water volume and latex film area, and consequently have units of (µS/cm) (mL/cm2). (b) Conductivity rise rate versus immersion time, equal to the time derivative of data series shown in (a). Note that the conductivity rise rate of control-NP has been magnified by a factor of 30. Units of conductivity rise rate are (µS/cm) (mL/cm2) (1/h). (c) Correlation between water diffusion timescale and time of peak ion leaching rate, extracted from (b) and Fig. 4, respectively
As shown in Fig. 6b, ion leaching conductivity rise rates of all films except control-TiO2 (see Figs. S1 and S2) show a peak that precedes a final decrease over time. This behavior is contrary to that expected from Fickian diffusion of mobile ions out of films, which predicts that the rise rate should monotonically decrease over time due to depletion of mobile ion concentration, and hence mobile ion concentration gradient, within films. Figure 6c shows the correlation between water diffusion timescale and time at which rise rate peaks. The water diffusion timescale is defined as \(t = {\raise0.7ex\hbox{${l^{2} }$} \!\mathord{\left/ {\vphantom {{l^{2} } {4D}}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${4D}$}}\). Since water entry by diffusion and capillary flow have the same time-dependence, the water diffusion timescale is a characteristic timescale for both mechanisms of water entry.
To demonstrate the coupled water and ion transport, the time of conductivity rise rate peak was shown to increase monotonically with increasing water diffusion timescale in Fig. 6C. It is proposed that this peak is due to the enhancement of mobile ion diffusivity by rising absorbed water concentration inside films. As the water concentration at mobile ion exit pathways progressively increases due to water absorption, the volume fraction of free volume increases at these pathways,51 leading to higher mobile ion diffusivity. Furthermore, the lack of a noticeable peak for control-TiO2—which has the longest water diffusion timescale of all films—is because ion leaching had proceeded nearly to completion by the time water absorption had a significant impact on mobile ion diffusivity.
Semi-continuum model
A semi-continuum model was developed to explain the experimental results with coupled water absorption and ion transport. In this model, the film was modeled as a series of compartments stacked along the film’s thickness (see Fig. 7a) with water, mobile ions, and coalescent as the mobile species within an immobile matrix of polymer, pigment (if present), and other immobile species. To simplify the model, the mobile species are assumed to reside in a separate volume from the immobile species, which is defined as the hydrophilic volume. Since the model is coarse-grained, the compartment length scale is much larger than the latex particle dimeter, so differences in concentration and transport rates between bulk latex particles and latex boundaries may be ignored. Indeed, since 80 compartments along the thickness of around 60 μm are used, the compartment length scale of 800 nm is much larger than the latex particle diameter of 100 nm.
(a) Schematic of the compartment model used in the present work. The film is assumed to be an infinite surface with a bisecting plane of symmetry. (b) At time t = 0, the film is visualized as a polymer matrix with mobile ions and coalescent inside the hydrophilic volume. At equilibrium, which is also the reference state, mobile ions and coalescent are fully removed; the hydrophilic volume is completely occupied by water. Relative to the reference state, the film is at elastic equilibrium, while initially, the film is under compression
The governing equations of the model are based on the proposed mechanisms from the experimental results. The first experimental conclusion is that water absorption may occur via both random-walk diffusion and pressure-driven flow. Generalizing this conclusion to all mobile species, it is proposed that transport may occur by a combination of diffusion and flow. Therefore, the following mass balance applies within each compartment:
Here, the indices \(j\) and \(k\) refer to the species (water \(w\), mobile ions \(i\) or coalescent \(c\)) and the compartment number, respectively. \(\hat{J}\) is the Fickian diffusion flux and \(\hat{F}\) the convection-like elastic relaxation flux. For brevity, in parts of the subsequent derivation, we drop the index \(j\), indicating that the expression is valid for all species. A quantity with a half-integer compartment number is equal to the average of that quantity in the two nearest compartments. The present elastic compartment model assumes that all expansion due to water absorption is out-of-plane.
The diffusion flux is driven by the concentration gradient within the hydrophilic volume:
Here, \(D\) is the diffusivity, \(l\) the length of the compartment, \(\epsilon\) the hydrophilic volume fraction and \(\hat{n}\) the number of moles of species per unit area.
Absorption of water into a hydrophilic volume52 upon film immersion in water, which lowers the system’s free energy, leads to elastic relaxation (see Fig. 7b) through an elastic relaxation flux:
Here, \(\widehat{k^\prime }\) is a generic proportionality constant, and \(P_{k}\) is the pressure exerted on compartment \(k\) by the polymer matrix. In the present work, the pressure exerted by a fully relaxed film immersed in DI water is taken as the reference pressure. The pressure is evaluated from:
Here, \(E\) is the Young’s modulus of the matrix, and \(l^{{ \ominus }}\) is the equilibrium length, defined as the length of a compartment when immersed for an infinitely long time in DI water. The derivation of the elastic relaxation flux is provided in the supplementary information, and the result is:
Here, \(\hat{V}_{j}\) is the molar volume of species \(j\), \(R_{g}\) the universal gas constant, and \(T\) the temperature.
In the expressions for diffusion flux (equation 5) and elastic relaxation flux (equation 6), the length of a compartment at any time is evaluated from the total amount of species within the compartment:
The equilibrium length (\(l^{{ \ominus }}\)) and the length associated with the matrix part of the film (\(l^{{{\text{matrix}}}}\)) is related by a stretch factor (\(\lambda_{c}\)):
The second experimental conclusion is that ion diffusivity is raised by increasing absorbed water concentration. It was assumed that the dependence of ion diffusivity on absorbed water concentration is described by percolation theory.53 The simplest approximation of percolation theory is used:
Here, \(D^{{ \ominus }}\) is the diffusivity in the reference state; that is, trace amount of mobile ion/coalescent within a relaxed film immersed in DI water. \(\hat{n}_{w,k}^{{ \ominus }}\) is the equilibrium water content, which is evaluated by:
Initial and boundary conditions
Water is initially absent from the film:
Mobile ions and coalescent are initially uniformly distributed in the film:
Here, \(\overline{C}_{j}\) is the initial bulk concentration of species \(j\). Standard boundary conditions are used at the axis of symmetry and at the film/water interface and are listed in Table 1.
The hydrophilic volume fraction is equal to:
Model fitting procedure
The model is solved using 80 compartments in MATLAB and fitted to SF-NP and SF-TiO2 experimental water absorption and ion transport data in Figs. 4 and 6a, respectively, using the in-built MATLAB genetic algorithm with command ga. Known and fitted parameters used in this process are listed in Table 2. The algorithm finds a set of fitted parameters that minimizes an objective function, which is proportional to the square of the difference between experimental data and the model prediction. The formula of the objective function and further details on the fitting procedure are provided in the supplementary information.
Model output and results
The film mass and solution conductivity are evaluated from the simulation output:
Here, \(m_{j}\) is the mass of species \(j\), \(A\) is the area of the immersed film, and \(M_{r,j}\) is the molecular mass of species \(j\). The solution conductivity is evaluated from:
Here, \(z_{i}\) is the charge number of the mobile ions, \(D_{{i,{\text{free}}}}\) is the diffusivity of the mobile ions in DI water, \(F\) is Faraday’s constant, and \(V_{{{\text{bath}}}}\) is the volume of the water bath.
Comparison of model and experiment
To gain a better physical understanding of the film system and the coupled water absorption and ion transport within it, the fitted results were superimposed on the data in Fig. 8. The model is able to capture the trends inherent in the data sets, especially the peak water absorption and non-Fickian ion leaching.
Peak water absorption arises due to the balance between diffusion and elastic relaxation fluxes. Initially, water is drawn into the film primarily by diffusion, leading to a rapid increase in film mass that scales linearly with \(\sqrt t\). Diffusion flux into the film, proportional to osmotic pressure, as shown in equation (S2), decreases with increasing water absorption and loss of mobile ions and coalescent. Elastic relaxation flux out of the film, proportional to film thickness, increases with increasing water absorption. At the peak of water absorption, diffusion and elastic relaxation fluxes are approximately balanced, and beyond the peak, the dominance of elastic relaxation over diffusion results in a slow decrease in film mass. Osmotic pressure arises mostly due to the coalescent, which is an order of magnitude less mobile than water and mobile ions. As shown in Table 2, the diffusivity of water and mobile ions are within the same order of magnitude for both SF-NP and SF-TiO2. The similarity in diffusivity implies that mobile ions do not contribute significantly to osmotic pressure, which requires much lower solute diffusivity compared to water diffusivity. In contrast, the diffusivity of coalescent is lower than that of water or mobile ions, allowing coalescent to contribute much more significantly to osmotic pressure.
As for the conductivity curve (Figs. 8b and 8d), its sigmoidal shape is counterintuitive based on the understanding of Fickian diffusion, which predicts a linear relationship between conductivity and \(\sqrt t\). This effect is attributed to the delay associated with hydrophilic volume wetting and consequent diffusivity enhancement (see equation 12).
Though it predicts much of the transport behavior, the present model is not sufficient to fit the ion transport data of the control-NP film. This failure is probably due to the model’s assumption of perfect phase separation between polymer and absorbed water, whereas the spatial distribution of absorbed water is likely more homogeneous in reality. Experimental results suggest more spatially heterogeneous absorbed water distribution in SF polymer-containing films due to more hydrophilic boundaries, which explains the greater success of the model with these films.
Model validation
An additional mass balance closure experiment was performed to validate the model. Masses of 5 SF-TiO2 film samples were measured immediately before immersion into separate DI water containers. Masses of all the samples were then measured again after 24 h DI water immersion, followed by 24 h drying in ambient conditions. Changes in the water bath conductivity of all samples after 24 h immersion were also measured. The immersion time period was chosen as 24 h because the film has approximately reached equilibrium in that time, as shown by the very small changes in water absorption and ion transport data in Figs. 4 and 6a, respectively, after 24 h.
Using these measured values, the masses of the mobile ions and the coalescent were calculated using sodium chloride and di(propylene glycol) n-butyl ether as the respective model components. The results of validation experiments are summarized in Table 3 and are in good agreement considering the simplicity of the model.
Further validations should be performed, in particular with regards to the stress applied by the polymer matrix on the hydrophilic volume (see equations 6 and 7) and the relationship between diffusion and flow conductance (see equation 19). These key assumptions could be investigated further using in situ synchrotron measurement, which allows real-time observation of polymer expansion and compartment size as a function of time. The resulting data set would provide a more convincing validation of the model, as opposed to the data set we have obtained, which is reliant on measurements averaged over the bulk of the coating.
Conclusions
The mutual influence and mechanisms of water absorption and ion transport in hydrated latex films are investigated using the in situ monitoring techniques of mass gain and conductivity rise upon immersion in DI water, respectively. Mutual influences are deduced by correlating water absorption and ion transport behavior of multiple film compositions. Water ingress into films proceeds either via capillary flow through pores or Fickian diffusion partially through a percolating boundary network. Absorbed water was found to enhance the diffusivity of mobile ions by raising the volume fraction of free volume in films. A semi-continuum model incorporating experimental conclusions is able to reliably fit the water absorption and ion transport data of SF-NP and SF-TiO2 films. This model also explains the mass decrease after peak water absorption, as water diffusion flux into coating is less than the elastic relaxation flux out of the coating, leading to a net mass loss after the peak. These results have significant implications for future high-performance water-based anti-corrosion coating design. Higher water absorption capacity may lead to faster transport of ionic corrosive reactants, assuming these ion mobilities have the same dependence on absorbed water concentration as those in this study. Water absorption capacity, which may also affect delamination rate, may be lowered by stabilizing latex particles in dispersion using hydrophilic surface groups and consequently lowering the surfactant concentration within films.
References
Liu, Y, et al. “Source Profiles of Volatile Organic Compounds (VOCs) Measured in China: Part I.” Atmos. Environ., 42 6247–6260 (2008)
Steward, PA, Hearn, J, Wilkinson, MC, “An Overview of Polymer Latex Film Formation and Properties.” Adv. Colloid Interface Sci., 86 195–267 (2000)
Butler, LN, Fellows, CM, Gilbert, RG, “Effect of Surfactant Systems on the Water Sensitivity of Latex Films.” J. Appl. Polym. Sci., 92 1813–1823 (2004)
Gauthier, C, et al. “Reactive Surfactants in Heterophase Polymerization. XVII. Influence of the Surfactant on the Mechanical Properties and Hydration of the Films.” J. Appl. Polym. Sci., 84 1686–1700 (2002)
Li, J, et al. “Fabrication and Properties of Polysilsesquioxane-Based Trilayer Core-Shell Structure Latex Coatings with Fluorinated Polyacrylate and Silica Nanocomposite as the Shell Layer.” J. Coat. Technol. Res., 15 1077–1088 (2018)
Šňupárek, J, Bidman, A, Hanuš, J, Hájková, B, “Water Absorption in Acrylic Latex Films.” J. Appl. Polym. Sci., 28 1421–1428 (1983)
Teng, G, Soucek, MD, Yang, XF, Tallman, DE, “Effect of the Addition Mode of Cycloaliphatic Diepoxide on the Morphology and Film Properties of Crosslinkable Core-Shell Latex.” J. Appl. Polym. Sci., 88 245–257 (2003)
Wu, S, Jorgensen, JD, Soucek, MD, “Synthesis of Model Acrylic Latexes for Crosslinking with Cycloaliphatic Diepoxides.” Polymer (Guildf)., 41 81–92 (2000)
Xu, W, An, Q, Hao, L, Sun, Z, Zhao, W, “Synthesis and Properties of Cationic Fluorinated Polyacrylate Soap-Free Latex.” J. Macromol. Sci. Part A Pure Appl. Chem., 50 670–677 (2013)
Chen, Y, Cheng, S, Wang, Y, Zhang, C, “Chemical Components and Properties of Core-Shell Acrylate Latex Containing Fluorine in the Shell and Their Films.” J. Appl. Polym. Sci., 99 107–114 (2006)
Xu, W, et al. “Synthesis and Characterization of Novel Fluoroalkyl-Terminated Hyperbranched Polyurethane Latex.” Appl. Surf. Sci., 436 1104–1112 (2018)
Wang, J, Zeng, XR, Li, HQ, “Preparation and Characterization of Soap-free Fluorine-Containing Acrylate Latex.” J. Coat. Technol. Res., 7 469–476 (2010)
Yao, L, Yang, T, Cheng, S, “Synthesis and Characterization of Poly(fluorinated acrylate)/Silica Hybrid Nanocomposites.” J. Appl. Polym. Sci., 115 3500–3507 (2010)
Li, Y, Zhu, J, Gao, X, Yu, Z, “Preparation and Properties of Phosphate-Fluorocarbon Acrylate Anticorrosion Emulsion.” J. Macromol. Sci. Part A Pure Appl. Chem., 50 248–254 (2013)
Chen, S, Yan, W, Chen, L, Chen, Y, Xu, N, “Morphology and Microstructure of Core-Shell Hybrid Latexes Containing Fluoropolymer and Acrylic Copolymer.” Colloid Polym. Sci., 284 413–421 (2006)
Li, G, Li, N, Wang, C, Niu, Y, Gong, X, “Synthesis and Properties of the Cationic Fluorocarbon Emulsifier-Free Latex in a New Micellar System.” Colloid Polym. Sci., 292 123–131 (2014)
Lu, D, et al. “Role of Three Different Carboxylic Monomers in Acrylate Emulsion Copolymerization in the Presence of Reactive Emulsifier.” J. Appl. Polym. Sci., 125 2807–2813 (2012)
Shui, X, Shen, Y, Fei, G, Wang, H, Zhu, K, “The Effect of Nonionic Monomer HAM on Properties of Cationic Surfactant-Free Acrylic/Alkyd Hybrid Emulsion.” J. Appl. Polym. Sci., 132 1–9 (2015)
Ooi, SK, Cook, WD, Simon, GP, Such, CH, “Effects of Composition on the Water Uptake and Hydroplasticisation of the Glass Transition Temperature of Methacrylate Copolymers.” Eur. Polym. J., 38 903–910 (2002)
Liu, D, Ichikawa, H, Cui, F, Fukumori, Y, “Short-Term Delayed-Release Microcapsules Spraycoated with Acrylic Terpolymers.” Int. J. Pharm., 307 300–307 (2006)
Zhong, S, Sun, C, Gao, Y, Cui, X, “Preparation and Characterization of Polymer Electrolyte Membranes Based on Silicon-Containing Core-Shell Structured Nanocomposite Latex Particles.” J. Power Sources, 289 34–40 (2015)
Li, X, et al. “Synthesis and Characterization of Self-Crosslinked Polyurethane/ Polyacrylate Composite Emulsion Based on Carbonyl-Hydrazide Reaction.” J. Polym. Res., 20 1–9 (2013)
Agarwal, N, Farris, RJ, “Water Absorption by Acrylic-Based Latex Blend Films and Its Effect on Their Properties.” J. Appl. Polym. Sci., 72 1407–1419 (1999)
Luo, Z, Huang, H, “Glass-Transition Temperature of a Polyacrylate Latex Film and Its Water Whitening Resistance.” J. Appl. Polym. Sci., 137 1–8 (2020)
Cui, M, Liu, C, Xu, Q, Li, R, “Effect of Hybrid Emulsifier (Reactive Coupling with Anionic) on the Properties of Acrylic Emulsion.” J. Adhes. Sci. Technol., 29 1758–1769 (2015)
Martín-Fabiani, I, et al. “Enhanced Water Barrier Properties of Surfactant-Free Polymer Films Obtained by MacroRAFT-Mediated Emulsion Polymerization.” ACS Appl. Mater. Interfaces, 10 11221–11232 (2018)
Bilgin, S, Tomovska, R, Asua, JM, “Effect of Ionic Monomer Concentration on Latex and Film Properties for Surfactant-Free High Solids Content Polymer Dispersions.” Eur. Polym. J., 93 480–494 (2017)
Ju, H, Sagle, AC, Freeman, BD, Mardel, JI, Hill, AJ, “Characterization of Sodium Chloride and Water Transport in Crosslinked Poly(ethylene oxide) Hydrogels.” J. Memb. Sci., 358 131–141 (2010)
Peng, CC, Chauhan, A, “Ion Transport in Silicone Hydrogel Contact Lenses.” J. Memb. Sci., 399–400 95–105 (2012)
Frankel, GS, “Pitting Corrosion of Metals: A Review of the Critical Factors.” J. Electrochem. Soc., 145 2186–2198 (1998)
Song, Y, Jiang, G, Chen, Y, Zhao, P, Tian, Y, “Effects of Chloride Ions on Corrosion of Ductile Iron and Carbon Steel in Soil Environments.” Sci. Rep., 7 1–13 (2017)
Dillon, JG, Schroeder, LW, “Permeability and Material Characteristics of Vulcanized Latex Film During and Following Cyclic Fatigue in a Saline Environment.” J. Appl. Polym. Sci., 64 553–566 (1997)
An, D, Yang, L, Liu, B, Wang, TJ, Kan, C, “Diffusion Performance of Fertilizer Nutrient through Polymer Latex Film.” J. Agric. Food Chem., 65 10868–10874 (2017)
Taylor, MA, “Synthesis of Polymer Dispersions.” In: Urban, D, Takamura, K (eds.) Polymer Dispersions and Their Industrial Applications, pp. 15–40. Wiley. https://doi.org/10.1002/3527600582.ch2 (2002)
Xu, GH, Dong, J, Severtson, SJ, Houtman, CJ, Gwin, LE, “Modifications of Surfactant Distributions and Surface Morphologies in Latex Films Due to Moisture Exposure.” J. Phys. Chem. B, 113 10189–10195 (2009)
Ziraldo, I, Watts, K, Luk, A, Lagalante, AF, Wolbers, RC, “The Influence of Temperature and Humidity on Swelling and Surfactant Migration in Acrylic Emulsion Paint Films.” Stud. Conserv., 61 209–221 (2016)
Callister, WD, Materials Science and Engineering. Wiley, Berlin (2007)
Amalvy, JI, Unzué, MJ, Schoonbrood, HAS, Asua, JM, “Reactive Surfactants in Heterophase Polymerization: Colloidal Properties, Film-Water Absorption, and Surfactant Exudation.” J. Polym. Sci. Part A Pure Appl. Checm., 40 2994–3000 (2002)
Gatos, KG, Martínez Alcázar, JG, Psarras, GC, Thomann, R, Karger-Kocsis, J, “Polyurethane Latex/Water Dispersible Boehmite Alumina Nanocomposites: Thermal, Mechanical and Dielectrical Properties.” Compos. Sci. Technol., 67 157–167 (2007)
Washburn, EW, “The Dynamics of Capillary Flow.” Phys. Rev., 18 206–209 (1921)
Gittens, GJ, “Variation of Surface Tension of Water with Temperature.” J. Colloid Interface Sci., 30 406–412 (1969)
Korson, L, Drost-Hansen, W, Millero, FJ, “Viscosity of Water at Various Temperatures.” J. Phys. Chem., 73 34–39 (1969)
Tokuda, K, Ogino, T, Kotera, M, Nishino, T, “Simple Method for Lowering Poly (Methyl Methacrylate) Surface Energy with Fluorination.” Polym. J., 47 66–70 (2015)
Zhu, Y, Granick, S, “Viscosity of Interfacial Water.” Phys. Rev. Lett., 87 1–4 (2001)
Ortiz-Young, D, Chiu, HC, Kim, S, Voïtchovsky, K, Riedo, E, “The Interplay Between Apparent Viscosity and Wettability in Nanoconfined Water.” Nat. Commun., 4 1–6 (2013)
Goertz, MP, Houston, JE, Zhu, XY, “Hydrophilicity and the Viscosity of Interfacial Water.” Langmuir, 23 5491–5497 (2007)
Li, T. De, Gao, J, Szoszkiewicz, R, Landman, U, Riedo, E, “Structured and Viscous Water in Subnanometer Gaps.” Phys. Rev. B Condens. Matter Mater. Phys., 75 1–6 (2007)
Cohen, MH, Turnbull, D, “Molecular Transport in Liquids and Glasses.” J. Chem. Phys., 31 1164–1169 (1959)
Yasuda, H, Lamaze, CE, Peterlin, A, “Diffusive and Hydraulic Permeabilities of Water in Water-Swollen Polymer Membranes.” J. Polym. Sci. Part A-2, 9 1117–1131 (1971)
Liu, Y, et al. “Explanations for Water Whitening in Secondary Dispersion and Emulsion Polymer Films.” J. Polym. Sci. Part B Polym. Phys., 54 1658–1674 (2016)
Yasuda, H, Lamaze, CE, Ikenberry, LD, “Permeability of Solutes Through Hydrated Polymer Membranes.” Die Makromol. Chemie, 118 19–35 (1968)
Doi, M, Soft Matter Physics. Oxford University Press (2013)
Sahini, M, Sahimi, M, Applications of Percolation Theory. CRC Press (2003)
Hagan, EWS, Charalambides, MN, Young, CT, Learner, TJS, Hackney, S, “Tensile Properties of Latex Paint Films with TiO2 Pigment.” Mech. Time-Dependent Mater., 13 149–161 (2009)
Vitagliano, V, Lyons, PA, “Diffusion Coefficients for Aqueous Solutions of Sodium Chloride and Barium Chloride.” J. Am. Chem. Soc., 78 1549–1552 (1956)
Cai, J, Perfect, E, Cheng, C, Hu, X, “Generalized Modeling of Spontaneous Imbibition Based on Hagen − Poiseuille Flow in Tortuous Capillaries with Variably Shaped Apertures.” Langmuir, 30 5142–5151 (2014)
Van der Meer-Lerk, LA, Heertjes, PM, “The Influence of Pressure on Blister Growth.” J. Oil Colour Chem. Assoc., 64 30–38 (1981)
Lewis, GN, “The Osmotic Pressure of Concentrated Solutions, and the Laws of the Perfect Solution.” J. Am. Chem. Soc., 30 668–683 (1908)
Acknowledgments
Funding for this project was supplied through a grant from Dow. We thank Drs. Paul Mwasame, Graham Abramo, Zhenwen Fu, and Thomas Kalantar from Dow for helpful discussions and suggestions. This work made use of the MRSEC Shared Experimental Facilities at MIT, supported by the National Science Foundation under Award Number DMR-1419807. This work was performed in part at the Center for Nanoscale Systems (CNS) of Harvard University, which is a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI) supported by the National Science Foundation (NSF) under NSF Award No. ECCS-2025158.
Funding
Open Access funding provided by the MIT Libraries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y.R., Effendy, S., Zhu, J. et al. Coupled effect of water absorption and ion transport in hydrated latex anti-corrosion coatings. J Coat Technol Res 20, 187–200 (2023). https://doi.org/10.1007/s11998-022-00676-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00676-0