Abstract
This study proposes a process based on the application of high-voltage pulsed electric field (PEF) in combination with pH shift for protein and solute release from fresh edible seaweed from Gracilaria sp.. The PEF was administered using a custom-made generator within and electroporation cell with sliding electrodes. The applied PEF parameters comprised 1000 V applied voltage, 50 µs pulse duration, 200 pulses delivered at 3 Hz frequency, and an inter-electrode gap ranging from 3.9 to 4.4 mm. The pH shift extraction was performed through a combination of fractions extracted sequentially at pH-shift steps spanning the pH range of 1 to 12. Both PEF and pH played pivotal roles in the fractionation of Gracilaria sp., enhancing protein release compared to water extraction, PEF pre-treatment, or pH shift alone. The combination of PEF and sequential pH washes (pH 1 to 12) resulted in a total solute release of 9.61 ± 0.04% and protein release of 28.02 ± 0.2% with essential amino acid (EAA) content of 63.6 ± 5.3%, and branched amino acids (BCAA) content of 3.47 ± 0.06%. It is noteworthy that PEF significantly increased the EAA and BCAA content of the protein extracts, irrespective of pH shifts. These findings underscore the promising potential of PEF pre-treatment in extracting proteins from edible seaweeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alternative proteins play a pivotal role in achieving the UN Sustainable Development Goals (SDGs) by addressing concerns related to climate change and food insecurity (Fanzo, 2019). Conventional animal–based protein production is unsustainable due to its adverse environmental impact, low plant-protein to animal-protein conversion efficiency, and high ecological footprints (Geada et al., 2021). While plant-based alternatives are more environmentally friendly, they still impose strain on freshwater and agricultural land resources (De Boer & Aiking, 2011). Edible seaweeds present a promising solution to these challenges, as they can be cultivated in seawater, not requiring freshwater or land, and yield valuable biomass with various prospects, especially as alternative protein sources (Hua et al., 2019; Øverland et al., 2019). Among seaweeds, red macroalgae, including certain Gracilaria sp., have gained attention due to their relatively high protein content, with reported content ranging from 2.7 to 47% (Rawiwan et al., 2022). However, commercializing these proteins remains challenging due to variability of chemical composition, lack in suitable harvesting technologies and in efficient and environmentally responsible extraction processes. Innovative approaches are necessary to unlock the full potential of edible seaweeds, such as Gracilaria sp. as sustainable protein sources.
The production of proteins from macroalgae involves four main steps: cultivation, harvesting, extraction, and purification. Harvesting and extraction, in particular, are costly and pose significant obstacles. Various techniques, such as ultrasonication, microwave-assisted extraction, pH-shift methods, and high-pressure processing, have been explored for protein extraction from Gracilaria sp. However, the presence of agar in its cell walls makes protein extraction challenging in this species, as it reduces protein release yield (Juul et al., 2021; Kazir et al., 2019; Magnusson et al., 2019; O’Connor et al., 2020).
One promising technology for value-added compound extraction from biomass is pulsed electric fields (PEF) (Golberg et al., 2016).
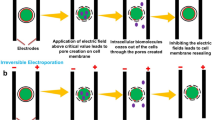
PEF is a non-thermal technique applied across diverse sectors such as medicine, food processing, and environment. PEF is based on the electroporation phenomenon, where short pulses of high voltage induce damage to cell membrane, increasing its permeability. Previous research, including studies by (Töpfl, 2006), Kranjc et al. (2016), and (Postma, 2016) (Kranjc et al., 2016; Postma, 2016; Töpfl, 2006), demonstrated the various applications of PEF for biomass processing and beyond. For extraction processes, PEF treatment involves applying voltage pulses across biological membranes, leading to membrane permeabilization which enables extraction of biomolecules from cells (Pereira et al., 2022, 2023).
Specifically, investigations into seaweeds (including several performed by our group) have shown PEF to be an energy-efficient process compared to other extraction methods, as highlighted in literature (Kashyap et al., 2022; Polikovsky et al., 2016; Robin et al., 2018a, b). In prior studies focusing on Gracilaria and Ulva sp., it was found that energy consumption using a PEF device ranged from 2.94 ± 0.91 kJ/kg to 4.06 ± 0.48 kJ/kg of fresh weight (FW) biomass (Kashyap et al., 2022) in the case of Gracilaria sp.. However, energy consumption varies depending on PEF parameters, necessitating fine-tuning to maximize permeabilization effect per energy usage. Furthermore, combining PEF with water reduces solvent usage, rendering it an environmentally friendly method for processing algal biomass. This combination offers several advantages over physical processes, including lower solvent requirements, decreased energy consumption, and cost-effectiveness, as evidenced by previous studies (Costa et al., 2021; Kashyap et al., 2022; Steinbruch et al., 2023).
Studies have also shown that PEF treatment can be effectively used to extract water-soluble proteins from Gracilaria sp. in a continuous system (Kashyap et al., 2022). Specifically, research on Gracilaria sp. using a continuous PEF device developed in our lab obtained extraction of 4.75% protein per dry weight (dw) from an initial 8.4% protein per dw (Kashyap et al., 2022). Moreover, combining PEF with enzymatic treatment has shown promising results in Ulva sp.. Steinbruch et al. (2023) demonstrated that the combined effect of PEF with enzymatic treatment resulted in a 102% increase in protein extraction content compared to the enzymatic process alone, showing 81% increase in protein content (Steinbruch et al., 2023).
We recently reported the use of a continuous PEF device to extract water-soluble proteins from Gracilaria sp. biomass (Kashyap et al., 2022). The current study aims to further enhance protein extraction by combining PEF with pH shift. pH shift is a promising technology for seaweed protein extraction, previously used on Porphyra umbilicalis, Ulva lactuca, and Saccharina latissimi (Harrysson et al., 2018), and some other species like Ulva fenestrate (Juul et al., 2021), but not on Gracilaria sp. While previous studies explored protein extraction from various Gracilaria species (Table 4) (Bozdemir et al., 2022; Fleurence et al., 1995; Kazir et al., 2019), none have utilized PEF pretreatment methods except for our previous study on Gracilaria sp. using a continuous PEF device, (Kashyap et al., 2022), in which the combination with pH shift was not explored. The present study is the first report of combining PEF and pH shift to improve total solute and protein release from Gracilaria sp. The objective of the current study was to investigate the combined effect of PEF and pH-shift methods on protein release from Gracilaria sp. This study marks the first attempt to synergize PEF with pH-shift methods to enhance protein release from macroalgae, (e.g. Gracilaria sp.) and to investigate the effects on release yield and composition of Gracilaria sp. proteins for their potential application as food ingredients. We show how process parameters significantly affect the release yield and composition of Gracilaria sp. proteins, with regard to their potential food application.
Materials and Methods
Gracilaria sp. Biomass Production and Harvesting.
Gracilaria sp. biomass was grown in a 700-L polyvinylchloride (PVC) tank with running seawater and continuous aeration at Israel Oceanographic and Limnological Research (IOLR), Haifa, Israel, in 2021. Twice a week, 0.1 mM PO4 and 1.0 mM NH4 were added to the seaweed tank. The biomass utilized as a stock was 1.5 kg FW m−3 and harvested after it grew to 3–4 kg FW m−3 in around 5 weeks. The harvested biomass was cleansed in a 20-L tank filled with clean tap water. A batch centrifuge was used to remove surface water, and the “fresh weight” (FW) biomass of Gracilaria sp. was obtained.
Description of the High-Voltage PEF System
The electrical circuit of the high-voltage pulse generator (HVPG) used in this investigation is shown in Fig. 1a and was described in detail in our previous publications (Levkov et al., 2020; Prabhu et al., 2019, 2020). The sliding press-electrode device (Fig. 1b) was created especially for handling biomass while applying pressure during PEF-induced electroporation. To exert this pressure, one electrode is allowed to move freely and vertically while being driven by the weight placed on the load-receiving platform.
The sliding press-electrode device consists of an electroporation cell (EPC), an electroporation cell retainer (EPCR), a load-receiving platform, a node of the moving electrode, a holder, and a sensor for detecting the inter-electrode gap. Guides at the base of the holder ensure precise alignment of the installed EPCR. The EPC (Fig. 1c), which consists of a cylinder with one electrode at the bottom and another at the lower end of the sliding electrode assembly’s rod, acts as the working volume for electroporation and pressing. The liquid fraction can exit the cylinder through slit-like apertures in the side walls, and a ring-shaped groove renders it convenient to drain the liquid component into a storage container. The high-voltage input of the HVPG is attached to the operational electrode of the EPC via a male connector.
Experimental Design
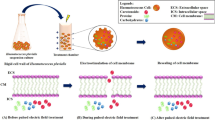
Using a spinner, 20 g of FW Gracilaria sp. biomass was dewatered until less than 1 g of surface water remained. The PEF apparatus described above was then used to treat 20 g of FW biomass in three replicates using the following parameters: frequency = 3 Hz; pulse duration = 50 µs; number of pulses = 200; pulse voltage = 1000 V; inter-electrode gap was varied in the range of 3.9 to 4.4 mm; the 20 g of biomass was treated with 10 PEF cycles by feeding 2 g/cycle (step 1 in Fig. 2). The selection of experimental conditions for PEF treatment was based on preliminary tests. This study not only explores the impact of pH-shifts but also investigates the combination of PEF with pH-shift in extracting solubilized proteins from Gracilaria sp. Indeed, PEF constitutes just one step in the experimental setup; however, this step is where the treatment of experimental samples diverges from that of the control samples. That is, two distinct experiments were conducted: one involved PEF followed by aqueous and pH-shift extractions while the other (the control), did not use PEF, only aqueous and pH-shift extractions. Consequently, PEF treatment stands out as the major point in the experimental design where the biomass from Gracilaria sp. underwent different treatments.
The PEF treatment steps were not used in the control group. Following PEF, the treated biomass was suspended in 100 mL of distilled water, agitated at 32 °C and 150 RPM overnight in a shaking incubator (Benchmark Scientific, Incu-shaker small, USA), and then separated with a batch centrifuge (Yingtai Instruments, TGL 18, China) at 3750 g for 10 min (steps 2 and 3 in Fig. 2). The biomass (solids 3b, Fig. 2), was further treated with different pH treatments: (1) sequential pH 12 to 1 extraction and (2) sequential pH 1 to 12 extraction (Table 1; steps 4 and 5 in Fig. 2). The compositions of buffers used appear in Table 2. To establish the total solutes (dry matter) released and protein released, all the liquid fractions from the sequential extractions were combined. The residual biomass was dried in an oven at 40 °C (step 6, Fig. 2).
Protein Quantification
The protein content in the initial biomass was quantified after cell wall and membrane disruption by bead-beating under alkaline conditions, and after drying in an oven at 40 °C until reaching a constant weight. Gracilaria sp. dry biomass (15 mg) and 1.5 mL of 2 M NaOH were mixed. Zirconium beads (2 mm, Sarstedt) were added to one-third of the bead-beater tubes containing a mixture of biomass and 2 M NaOH. The tubes containing the mixture were then put into a Mini Bead-Beater-16, Model 607 EUR, Biospec, OK, and subjected to three cycles of 60 s each, separated by 10-min intervals, at 25 °C. After bead beating, the tubes were centrifuged at 19,500 g for 20 min in an Eppendorf 5424 centrifuge (Germany), and protein was quantified in the supernatant (Prabhu et al., 2020).
The protein content of the various liquid fractions collected throughout the investigation was determined using Lowry’s technique. To create the linear calibration curve, several dilutions corresponding to concentrations ranging from 0 to 500 µg/mL were prepared, using a bovine serum albumin (BSA) stock solution of 1 mg/mL (Lowry et al., 1951). To measure protein concentration, 100 µL of samples or standard (BSA, 0–500 g/mL) were mixed with 200 µL of Biuret reagent (500 µL of 1% cupric sulfate, 500 µL of 2% sodium potassium tartrate, in 50 mL of 2% sodium carbonate in 0.1 N NaOH). After thoroughly blending, the mixture was equilibrated at room temperature for 15 min. 20 µL of 1 N Folin and Ciocalteu’s reagent (Sigma-Aldrich, St. Louis, USA) were added to the reaction mixture, and it was incubated for 30 min at room temperature. After the incubation, absorbance at 650 nm was measured using a microplate reader (TECAN Infinite 200-Pro, Tecan, Switzerland).
Amino Acid Profiling
The protein was extracted from the PEF-treated samples and control from step 3a) and step 5a) (Fig. 2) in aqueous solution and following sequential pH changes from 1 to 12 and from 12 to 1. The protein-rich sediment was precipitated using 35% and 85% ammonium sulfate salting out processes (Duong-Ly & Gabelli, 2014). The precipitated proteins were then dialyzed using a SnakeSkin™ dialysis bag (3.5 kDa, ThermoFisher Scientific, USA). The dialyzed proteins were then lyophilized and analyzed for amino acid profile through high-pressure anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) as follows. One milligram of biomass was added to 1 mL of 6 M HCl and heated at 112 °C for 16 h in a dry bath (Bio-Base, China). The samples were then dried using nitrogen. The dried samples were suspended in 1 mL of ultrapure ion chromatography grade water and left at rest for 1 h (Kazir et al., 2019). The samples were then filtered using a 0.22-μm syringe filter. The filtered samples were separated through HPAED-PAD Dionex ICS-5000 (Dionex, Thermo Fischer Scientific, MA, USA). Amino-pack 10 analytical grade column was used for amino acid separation. Signal detection was done using a gold AAA electrochemical detector with an AgCl reference electrode. Separation was achieved with gradient elution of the following parameters: 0.250 mL/min, column temperature 30 °C, auto-sampler temperature 5 °C, and a 25 μL injection loop volume. An amino acid standard mix (AAS18, Sigma-Aldrich, MO, USA) with the following dilutions 1/5, 1/10, 1/15, 1/20, and 1/25 was used to validate the separation program. The following amino acids were detected in the amino acid mix: alanine, arginine, aspartate, cysteine, glutamate, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, and valine. The calibration curve formed from these dilutions (R2 > 99% for each amino acid) was then incorporated into the program to quantify the amino acids in the unknown samples.
Release Yield and Content Calculations
Solute (dry matter) release yield (DMRY %) was calculated according to the following Eq 1:
where the dry matter (DM(g)) of the initial biomass was measured by drying in the oven at 40 °C to constant weight and the DM (g) of the extract was measured by drying all the extracts combined for each of the conditions in Table 1 in the oven at 40 °C to constant weight.
Protein release yield (PRY%) was calculated using the following Eq 2:
where protein (g) in the extracts (Proteinextract) was measured by Lowry and protein in the initial biomass (Proteininitial_biomass) was measured by Lowy after bead beading in alkaline solution. PRY% of the pH shift experiments is the combined yield of water and all pH extracts (1 to 12) in the specific experiment.
Essential amino acid content (EAA %) was calculated using the following Eq 3:
where YEAA (g) is the content of essential amino acids detected with HPIC in the Proteinextract and YTAA are the total content of amino acids detected in the Proteinextract. EAA determined were histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, and total amino acids (TAA) determined were alanine, arginine, aspartate, cysteine, glutamate, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tyrosine, and valine.
Branched amino acid content (BCAA%) was calculated using the following Eq 4:
where Y (g) is the content of amino acids detected with HPIC in the Pextract. BCAA determined were leucine, isoleucine, and valine.
Statistical Analysis
Student’s t-test and one-way ANOVA were performed to analyze the significance of the difference between data sets obtained in the study. The p-values were calculated using the Student’s t-test in a paired manner with 0.05 as the maximal p value indicating statistical significance. ANOVA was also performed wherever needed, and the confidence level was set to 95% for the analysis.
Results
Gracilaria sp. Biomass Fractionation Using PEF and pH Shifts
The wet Gracilaria sp. biomass was treated under different conditions and fractionated into soluble and insoluble fractions (Table 1). The control and PEF-treated biomass samples were further subjected to water-mediated extraction or to sequential pH extraction to estimate the amount of dry matter and protein released. In addition, the essential amino acid content was determined and the branched amino acid content (BCAA) of the protein fraction was calculated. The mass balance analysis of each condition described in Fig. 1 and Table 1 appears in supplementary Tables S1to S6 and is discussed below.
Water-Mediated Extraction Process of PEF Treated Biomass
The raw biomass had an initial protein content of 15.58 ± 0.25%DW. The water-mediated extraction alone yielded 7.5 ± 0.04% DMRY and 7.22 ± 0.3% of PRY (Table S1), with 4.4 ± 2.4% EAA, and 1.4 ± 0.5% BCAA content. PEF treatment before the water-mediated extraction resulted in 7.5 ± 0.04% DMRY and 8.1 ± 0.1% of PRY, with 54.5 ± 5.9% EAA, and 2.8 ± 1.3% BCAA content (Table S2). PEF pre-treatment did not significantly impact DMRY (p-value = 1), and BCAA content (p-value = 0.29), but significantly increased the PRY (p-value = 0.009) and the fraction of EAA (p-value = 0.009).
Gracilaria Fractionation Process Using PEF Pre-treatment and pH shift
Sequential pH-shift extraction (12 to 1) led to 9.62 ± 0.04% DMRY and 14.45 ± 0.1% of PRY (Table S3) with 14.12 ± 1.4% EAA and 0.06 ± 0.03% of BCAA content. Sequential pH extraction (12 to 1), which followed PEF, led to 9.64 ± 0.02% DMRY and 24.1 ± 0.03% of PRY (Table S4), with 62.1 ± 5.5% EAA and 3.1 ± 0.7% BCAA content in the extract. PEF pre-treatment did not change the DMRY (p-value = 0.68) but significantly increased the PRY (1.7 fold, p-value = 3 × 10−8) and the content of EAA (4.4-fold, p-value = 0.007) and branched amino acids (51.6-fold, p-value = 0.03).
Sequential pH-shift extraction (1 to 12) led to 9.61 ± 0.02% DMRY and 17.33 ± 0.8% of PRY (Table S5), with 31.1 ± 2.2% EAA and 0.75 ± 0.09% BCAA content. Sequential pH-shift extraction with PEF (1 to 12) led to of 9.61 ± 0.04% DMRY, and 28.02 ± 0.2% of PRY (Table S6), 63.6 ± 5.3% EAA and 3.47 ± 0.06% BCAA (Table S7). PEF pre-treatment did not change the DMRY (p-value = 0.99) but significantly increased the PRY (1.6-fold, p-value = 3 × 10−5) and the content of EAA (twofold, p-value = 0.02) and BCAA (4.6-fold, p-value = 8 × 10−4).
In experiments without PEF, although the order of pH shift sequence (1 to12 vs. 12 to 1) did not impact the DMRY, (p-value = 0.42), the order significantly affected the PRY (1.19-fold p-value = 0.004), EAA content (2.2-fold change, p-value = 0.01), and BCAA content (12.5-fold change, p-value = 0.009).
The order of pH shifts (1 to 12 vs. 12 to 1) following PEF, did not impact the DMRY (p-value = 0.25), EAA content (p-value = 0.85), and BAA content (p-value = 0.5). However, the PRY was impacted significantly (1.16-fold change, p-value = 9 × 10−6) when PEF pre-treated samples further treated with pH 1 to 12, and pH 12 to 1 were compared.
Thus, regardless of PEF pretreatment, the PRY was impacted by the order of the pH shifts. In both cases, the samples treated with pH 1 to 12 showed higher PRY compared to those treated with pH 12 to 1.
Determination of the Impact of Process Parameters on Gracilaria sp. Biomass Fractionation
The Impact of pH on Solute and Protein Release Yield and Content of Essential and Branched Amino Acids of the Extracts
We investigated how pH shifting impacts the Gracilaria fractionation efficiency and quality of the products without PEF pre-treatment. The addition of pH shifts in comparison to water-mediated extraction (without pH shifts), impacted the DMRY (Fig. 3a; fold change 1.28, p-value = 7.7 × 10−6) and PRY (Fig. 3b; 1.64-fold change, p-value = 4.8 × 10−6). However, it did not significantly affect EAA, and BCAA content (Fig. 3c and d; p-value = 0.28, and 0.73) of the extracts.
The Impact of PEF Pre-treatment on Solute and Protein Release Yield, and on Essential and Branched Amino Acid Contents of the Extracts
We also investigated if addition of PEF pre-treatment increases the Gracilaria biomass fractionation efficiency and quality of the products. There was no significant difference in the DMRY between PEF treated and untreated samples (Fig. 4a; p-value = 0.99). However, the addition of PEF significantly increased the PRY% (Fig. 4b; 1.54-fold change, p-value = 0.03), EAA fraction (Fig. 4c; 3.62-fold change, p-value = 8.0 × 10−5) and BCAA fraction (Fig. 4d; 4.27-fold change, p-value = 1.3 × 10−4).
The impact of PEF pre-processing step on a solute (dry matter) release yield (DMRY%), b protein release yield (PRY%), c essential amino acid content (EAA%) of the extract, and d branched amino acid content (BCAA%) of the extract. **p-values are indicated in the figure between the compared groups (n = 3)
Combined Effect of pH and PEF Pre-treatment on Solute and Protein Release Yields and on Essential and Branched Amino Acid Contents of the Extracts
Next, we tested whether the combined effect of pH and PEF significantly improves the Gracilaria sp. biomass fractionation. Combined PEF and pH-shift treatments did not improve the DMRY in comparison with pH shifts alone (Fig. 5a; p-value = 0.44), but showed higher DMRY in comparison with PEF pre-treatment alone (Fig. 5a; 1.28-fold change, p-value = 2.8 × 10−7). A combined PEF and pH-shift treatment led to higher protein release yield, higher EAA and BCAA contents than PEF or pH-shift alone (Fig. 5b; p-value = 0.00017, and 0.0003; Fig. 5c, p-value = 0.02, and 0.04; Fig. 5d, p-value = 0.0077, and 0.05, respectively) with a fold change of 3.23, and 1.97 in protein release yield, 1.15, and 2.71 in EAA, 1.18, and 13.12 in BCAA, respectively. The %DMRY, %PRY, %EAA, and %BCAA under different conditions used in the study are summarized in Table 3.
Discussion
Protein solubility and release from the algal biomass strongly depend on pH (which determines protein surface charge), on membrane and cell wall integrity, and on protein interactions with the algal polysaccharides, some of which make the cell walls. In our current study, we explored both the impact of PEF, and of sequentially shifting pH through a range of pH values during pH-shift experiments to increase protein extraction yield.
A recent study on Gracilaria dura sheds light on how some of these factors impact the behavior of proteins (Bozdemir et al., 2022). Notably, when re-dissolved in water, the “powdered” Gracilaria dura exhibited a negative surface charge (zeta potential), likely attributed to its cell wall composition primarily comprising negatively charged polysaccharides (Bozdemir et al., 2022). The minimal solubility of Gracilaria dura proteins occurred at pH 4.0, and from that point it increased with increasing pH, at least up to pH 13 (Bozdemir et al., 2022).
Recent investigations into microalgal species have revealed intriguing patterns of protein solubility. Notably, one study demonstrated an impressive ~ 84% solubility within a pH range of 2–12 (Grossmann et al., 2019). In contrast, research on Tetraselmis spp. highlighted a unique behavior: protein solubility remained unaffected by the ionic strength of the solution, even up to 0.5 M (Schwenzfeier et al., 2011). Remarkably, proteins from Tetraselmis spp. exhibited complete solubility above pH ≥ 5.5. Our current study underscores the importance of broad pH exploration during protein extraction. By encompassing a wide pH range, we aimed to enhance overall protein release yield, a critical consideration for optimizing protein extraction protocols.
In some earlier articles (Harnedy & FitzGerald, 2013; Kumar et al., 2014), the topic of protein isolation using pH alterations in red macroalgae was discussed. A study on Kappaphycus alvarezii show that the minimal solubility of nitrogenous compounds was found at pH 4, and the authors suggested that this pH may be the isoelectric point of the protein concentrate of Kappaphycus alvarezii (Kumar et al., 2014). Proteins acquire net positive and net negative charge, below and above their pI, respectively, that lead to repulsion between the molecules and enhance protein solubility (Seena & Sridhar, 2005). The attractive interactions between positively charged proteins and polyanions can lower the apparent pI, by shifting the pH of minimal solubility of the protein to a lower pH (Zimet & Livney, 2009), where charge-neutralized complexes form with the polyanions (Williams, 2009). The mechanisms underlying protein release yield under various pH and pre-treatment conditions depends on this insight.
The pH shift approach is based on the fact that each protein has a unique isoelectric pH, at which it is least soluble, hence collecting the soluble proteins during each step of the pH shift process and combining them, should significantly increase the protein extraction yield (compared to collecting the soluble proteins at a certain pH where some of the proteins have zero zeta potential, and are hence insoluble). It should also help avoiding loss of protein due to complex formation with the anionic polysaccharides, which, as discussed above, also has a strong pH dependence.
We treated the biomass in two ways: (1) starting with the highest pH 12 and progressively adjusting to each subsequent pH level until reaching pH 1 and (2) starting with the lowest pH 1 and progressively adjusting to each subsequent pH level until reaching pH 12. We found that the second way, i.e. when pH is gradually increased, was more effective in increasing protein release (Table 3) leading to a higher PRY 17.33 ± 0.8 vs. 14.45 ± 0.1 in the first way. A likely reason for this is the presence of the anionic cell wall polysaccharides, like agar, which are uncharged at very low pH (much below the pka of its carboxylic groups), which is around 5 (Carisma et al., 2020). As pH rises, and cell wall polysaccharides become more negatively charged, they bind positively charged proteins (up to the pI of each protein, or slightly above it, if local positive patches exist) and form electrostatic complexes with them. This complexation decreases protein release. Above the pI, solubility of the proteins and their consequent release increase, as they become more negatively charged, and repulsed from the polysaccharides. When starting at high pH, the cell wall polysaccharides are very negative during most of the process, binding much proteins below their pIs, and decreasing their release. In contrast, when starting at low pH, as the cell wall polysaccharides are uncharged, most of the proteins are released in the beginning, as they are very positively charged, and the uncharged cell wall polysaccharides interfere less with their release.
The scientific literature highlights the impact of pulsed electric field (PEF) treatment on macroalgal cells, increasing membrane permeability/porosity, thereby leading to loss of turgor pressure within the cells, dehydration, and release of salts and small proteins (Polikovsky et al., 2016; Prabhu et al., 2019; Robin et al., 2018a, b).
In our current study, we improved protein extraction by combining PEF with pH shifts. This combination synergizes (1) the increase membrane porosity; (2) the pH-dependent protein release, partly hampered by cell-wall polysaccharides; and (3) the chemical potential gradient between the cell interior and its surrounding medium. Consequently, at each pH step (pH solutions, as detailed in Table 2), there exists a motive force for the diffusive release of distinct proteins, which is facilitated by the loss of membrane-barrier efficacy by PEF, and escaping cell-wall polysaccharide complexation by shifting the pH. PEF both increases the permeability of the cell membrane and removes salts, which increases protein release, thereby leading to higher protein release yield (Robin et al., 2018a, b).
A combination of PEF and sequential pH-shifting from 1 to 12 led to the highest fractionation increasing the DMRY by 28.3% (from 7.5 ± 0.04% to 9.61 ± 0.04%), PRY by 288.1% (from 7.22 ± 0.3% to 28.02 ± 0.2%), and EAA content by 1282.6% (from 4.6 ± 2.4% to 63.6 ± 5.3%), and BCAA content by 147.9% (from 1.4 ± 0.5% to 3.47 ± 0.06%w) in comparison to aqueous extraction alone. These results for the protein release are higher than previous reports on Gracilaria protein extraction (Table 2), probably due to the hurdle effects and the synergy of PEF and pH-shifting, where PEF pre-treatment facilitates the following protein release yield at various pH conditions. We observed that the combination of PEF and pH shift method can provide much better yields than enzymatic methods, ultrasonication, chemical methods, and PEF alone (Table 4). The increase in protein and amino acid content indicates that these components were released from the solid matrix into the surrounding solution. This release could be due to various factors, such as breakdown of cellular structures or enhanced diffusion resulting from the PEF treatment, which makes the cell membranes more porosive. While proteins and amino acids from the cytosol are released into the solution, the major other non-proteinaceous components, predominantly carbohydrates, are attached to the cell wall of the seaweed biomass, hence are not significantly released by the PEF treatment. Therefore, the observed change in DMRY (28.3%) was much smaller than the change in PRY (288.1%).
Our findings demonstrate that PEF treatment alone and in conjunction with pH shifting substantially enhances protein release and contributes to the augmentation of essential and branched-chain amino acids release, either as free AA or in peptides/proteins. In this study, the total amino acids were estimated, and then, essential and branched amino acids were determined. These results suggest a direct correlation between the increased release of proteins induced by PEF (alone or with pH-shift treatments) and the subsequent elevation in essential and branched amino acid levels.
Understanding the amino acid content of extracted protein fractions is essential for their applications in human food or animal feed. The successive pH treatments in the control and PEF-pretreated biomass resulted in higher total released amino acids content. Application of these fractions in human nutrition depends on the contents of essential amino acids (EAA) and the total amino acids present. The availability of EAA in the diet is critical because the human body cannot synthesize them (Nollet & Toldrá, 2012). Our study demonstrates that the compositions of extracted amino acids, and particularly essential AA content, depends not only on their contents in the raw material (macroalgae, in this case) but also on the method utilized for protein extraction. For instance, we did not find leucine in the Gracilaria sp. biomass without PEF treatment (control), only in the PEF pretreated samples. This was in accord with our earlier study on Gracilaria sp. biomass utilizing a continuous PEF device (Kashyap et al., 2022). The most common essential amino acid (EAA) in Gracilaria sp., according to the current study, was found to be threonine, with concentrations that were noticeably higher in PEF-treated samples (31.78 to 42.13%) than in control samples (0.49 to 0.93%). Following threonine, lysine was likewise significantly enhanced by PEF, with quantities ranging from 8.33 to 17.74% in PEF-treated samples compared to 1.84 to 8.89% in the control. Given that plant-based products frequently have insufficient lysine content, the presence of this EAA in the protein fractions from Gracilaria sp. is especially important for its application in human nutrition (Young & Pellett, 1994).
The effect of protein extraction procedure on AA composition observed in the current study agrees with- and complements earlier studies (Kashyap et al., 2022; Kazir et al., 2019; Norziah & Ching, 2000). Indeed, various protein extraction methods can provide varied amino acid compositions. Additionally, not all the proteins found in the biomass of Gracilaria sp. were isolated in this study, which may have resulted in additional variations in amino acid profiles. With fresh information regarding the amino acid content of Gracilaria sp. and prospective implications for human nutrition and food uses, these findings provide a significant contribution to the field of edible seaweed research. The most interesting impact of the PEF pre-treatment is the enhancement in the EAA and BCAA in the extracts obtained from PEF pre-treated biomass. The PEF pre-treated samples followed by aqueous extraction had highest enhancement in EAA 54.5 ± 5.9% (p-value = 0.02), and BCAA 2.8 ± 1.3% (p-value = 0.009), respectively, compared to samples treated only with sequential pH (pH 1 to 12) that had 31.1 ± 2.2%, and 0.75 ± 0.09% of EAA, and BCAA respectively. This is a new and interesting observation, which is in line with our previous works on Ulva sp. biomass, in which we reported selective protein extraction by PEF in comparison to aqueous extraction alone (Polikovsky et al., 2016). This observed enhancement in the EAA, and BCAA extraction in PEF-treated biomass with or without pH shifts, suggests that the PEF treatment aids in the preferential release of EAA and BCAA rich proteins or peptides (or even free amino acids). The mechanisms of such selective extraction are still unknown, and motivate further study.
Conclusions
Both PEF and pH play crucial roles in the fractionation of Gracilaria sp. biomass. Utilizing a combination of PEF and sequential pH shifts (pH 1 to 12) resulted in notable outcomes, including a solute (dry matter) release yield of 9.61 ± 0.04%, protein release yield of 28.02 ± 0.2%, EAA content of 63.6 ± 5.3%, and BCAA content of 3.47 ± 0.06%. It is noteworthy that PEF significantly increased EAA and BCAA extraction efficiency, as demonstrated by samples treated with PEF alone, which exhibited 54.5 ± 5.9% EAA and 2.8 ± 1.3% BCAA. In contrast, samples treated solely with pH shifts (pH 1 to 12) and without PEF showed EAA and BCAA levels of 31.1 ± 2.2% and 0.75 ± 0.09%, respectively. This underscores the cooperative effect of combining PEF and pH-shift treatments (particularly when gradually raising pH from 1 to 12), highlighting their cooperative impact in amplifying protein release yield and particularly essential amino acids and branched amino acids in Gracilaria sp. biomass extracts.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bozdemir, A., Şensu, E., Okudan, E. Ş, Özçelik, B., & Yücetepe, A. (2022). Ultrasound-assisted enzymatic extraction of proteins from Gracilaria dura: Investigation of antioxidant activity and techno-functional properties. Journal of Food Processing and Preservation, 46(8), e16803.
Carisma, N. A. S., Gonzales, R. Y. E., & Lazaro-Llanos, N. (2020). An investigation on zinc biosorption with agar extraction waste from Gracilaria tenuistipitata. Kimika, 31(2), 11–26. https://doi.org/10.26534/kimika.v31i2.11-26
Costa, M., Pio, L., Bule, P., Cardoso, V., Alfaia, C. M., Coelho, D., Brás, J., Fontes, C. M., & Prates, J. A. (2021). An individual alginate lyase is effective in the disruption of Laminaria digitata recalcitrant cell wall. Scientific Reports, 11(1), 9706.
De Boer, J., & Aiking, H. (2011). On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecological Economics, 70(7), 1259–1265.
Duong-Ly, K. C., & Gabelli, S. B. (2014). Salting out of proteins using ammonium sulfate precipitation. In: Methods in enzymology (Vol. 541, pp. 85–94). Elsevier.
Fanzo, J. (2019). Healthy and sustainable diets and food systems: The key to achieving sustainable development goal 2? Food Ethics, 4(2), 159–174. https://doi.org/10.1007/s41055-019-00052-6
Fleurence, J., Le Coeur, C., Mabeau, S., Maurice, M., & Landrein, A. (1995). Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. Journal of Applied Phycology, 7, 577–582.
Geada, P., Moreira, C., Silva, M., Nunes, R., Madureira, L., Rocha, C. M., Pereira, R. N., Vicente, A. A., & Teixeira, J. A. (2021). Algal proteins: Production strategies and nutritional and functional properties. Bioresource Technology, 332, 125125.
Golberg, A., Sack, M., Teissie, J., Pataro, G., Pliquett, U., Saulis, G., Stefan, T., Miklavcic, D., Vorobiev, E., & Frey, W. (2016). Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnology for Biofuels, 9, 1–22.
Grossmann, L., Hinrichs, J., & Weiss, J. (2019). Solubility and aggregation behavior of protein fractions from the heterotrophically cultivated microalga Chlorella protothecoides. Food Research International, 116, 283–290.
Harnedy, P. A., & FitzGerald, R. J. (2013). Extraction of protein from the macroalga Palmaria palmata. LWT-Food Science and Technology, 51(1), 375–382.
Harrysson, H., Hayes, M., Eimer, F., Carlsson, N.-G., Toth, G. B., & Undeland, I. (2018). Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) JV Lamouroux using three different methods. Journal of Applied Phycology, 30, 3565–3580.
Hua, K., Cobcroft, J. M., Cole, A., Condon, K., Jerry, D. R., Mangott, A., Praeger, C., Vucko, M. J., Zeng, C., & Zenger, K. (2019). The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth, 1(3), 316–329.
Juul, L., Danielsen, M., Nebel, C., Steinhagen, S., Bruhn, A., Jensen, S., Undeland, I., & Dalsgaard, T. (2021). Ulva fenestrata protein—Comparison of three extraction methods with respect to protein yield and protein quality. Algal Research, 60, 102496.
Kashyap, M., Ghosh, S., Steinbruch, E., Levkov, K., Israel, A. I., Bala, K., Livney, Y., & Golberg, A. (2022). Extracting water-soluble proteins from the red macroalgae Gracilaria sp. with pulsed electric field in a continuous process. ACS Food Science & Technology, 3(4), 562–575.
Kazir, M., Abuhassira, Y., Robin, A., Nahor, O., Luo, J., Israel, A., Golberg, A., & Livney, Y. D. (2019). Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocolloids, 87, 194–203.
Kranjc, M., Bajd, F., Serša, I., de Boevere, M., & Miklavčič, D. (2016). Electric field distribution in relation to cell membrane electroporation in potato tuber tissue studied by magnetic resonance techniques. Innovative Food Science & Emerging Technologies, 37, 384–390.
Kumar, K. S., Ganesan, K., Selvaraj, K., & Rao, P. S. (2014). Studies on the functional properties of protein concentrate of Kappaphycus alvarezii (Doty) Doty–An edible seaweed. Food Chemistry, 153, 353–360.
Levkov, K., Linzon, Y., Mercadal, B., Ivorra, A., González, C. A., & Golberg, A. (2020). High-voltage pulsed electric field laboratory device with asymmetric voltage multiplier for marine macroalgae electroporation. Innovative Food Science & Emerging Technologies, 60, 102288.
Lowry, O., Rosebrough, N., Farr, A. L., & Randall, R. (1951). Protein measurement with the folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Magnusson, M., Glasson, C. R., Vucko, M. J., Angell, A., Neoh, T. L., & de Nys, R. (2019). Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Research, 41, 101555.
Nollet, L. M., & Toldrá, F. (2012). Handbook of analysis of active compounds in functional foods. CRC Press.
Norziah, M. H., & Ching, C. Y. (2000). Nutritional composition of edible seaweed Gracilaria changgi. Food Chemistry, 68(1), 69–76.
O’Connor, J., Meaney, S., Williams, G. A., & Hayes, M. (2020). Extraction of protein from four different seaweeds using three different physical pre-treatment strategies. Molecules, 25(8), 2005.
Øverland, M., Mydland, L. T., & Skrede, A. (2019). Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture, 99(1), 13–24.
Pereira R. N., Avelar Z., Pereira S. G., Rocha C. M., Teixeira, J. A. (2022). Pulsed electric fields for the extraction of proteins and carbohydrates from marine resources. In: Innovative and Emerging Technologies in the Bio-marine Food Sector (vol 173–195). Elsevier.
Pereira, S. G., Pereira, R. N., Rocha C. M., Teixeira, J. A. (2023). Electric fields as a promising technology for the recovery of valuable bio compounds from algae: Novel and sustainable approaches. Bioresource Technology Reports, 101420.
Polikovsky, M., Fernand, F., Sack, M., Frey, W., Müller, G., & Golberg, A. (2016). Towards marine biorefineries: Selective proteins extractions from marine macroalgae Ulva with pulsed electric fields. Innovative Food Science & Emerging Technologies, 37, 194–200.
Postma, R. (2016). Mild disintegration of green microalgae and macroalgae. PhD thesis, Wageningen University, Wageningen, NL (2016) https://doi.org/10.18174/391430
Prabhu, M. S., Israel, A., Palatnik, R. R., Zilberman, D., & Golberg, A. (2020). Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. Journal of Applied Phycology, 32, 2271–2282.
Prabhu, M. S., Levkov, K., Livney, Y. D., Israel, A., & Golberg, A. (2019). High-voltage pulsed electric field preprocessing enhances extraction of starch, proteins, and ash from marine macroalgae Ulva ohnoi. ACS Sustainable Chemistry & Engineering, 7(20), 17453–17463.
Rawiwan, P., Peng, Y., Paramayuda, I. G. P. B., & Quek, S. Y. (2022). Red seaweed: A promising alternative protein source for global food sustainability. Trends in Food Science & Technology, 123, 37–56.
Robin, A., Kazir, M., Sack, M., Israel, A., Frey, W., Mueller, G., Livney, Y. D., & Golberg, A. (2018b). Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS sustainable chemistry & engineering, 6(11), 13696–13705.
Robin, A., Sack, M., Israel, A., Frey, W., Müller, G., & Golberg, A. (2018a). Deashing macroalgae biomass by pulsed electric field treatment. Bioresource Technology, 255, 131–139.
Schwenzfeier, A., Wierenga, P. A., & Gruppen, H. (2011). Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresource Technology, 102(19), 9121–9127.
Seena, S., & Sridhar, K. (2005). Physiochemical, functional and cooking properties of Canavalia. Journal of Food Chemistry, 32, 406–412.
Steinbruch, E., Wise, J., Levkov, K., Chemodanov, A., Israel, Á., Livney, Y. D., & Golberg, A. (2023). Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innovative Food Science & Emerging Technologies, 84, 103231.
Töpfl, S. (2006). Pulsed electric fields (PEF) for permeabilization of cell membranes in food-and bioprocessing—applications, process and equipment design and cost analysis.
Williams, P. A. (2009). Molecular interactions of plant and algal polysaccharides. Structural Chemistry, 20(2), 299–308. https://doi.org/10.1007/s11224-009-9420-5
Young, V. R., & Pellett, P. L. (1994). Plant proteins in relation to human protein and amino acid nutrition. The American Journal of Clinical Nutrition, 59(5), 1203S-1212S.
Zimet, P., & Livney, Y. D. (2009). Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocolloids, 23(4), 1120–1126. https://doi.org/10.1016/j.foodhyd.2008.10.008
Funding
Open access funding provided by Tel Aviv University. The study in the present manuscript was funded by the Good Food Institute, Israel, and Israel Ministry of Health.
Author information
Authors and Affiliations
Contributions
Mrinal Kashyap. Writing original manuscript draft, execution of experiments, data interpretation and analysis. Supratim Ghosh. Supportive role in execution of experiments, Klimentiy Levkov. Hardware, software development, experiment. Yoav D Livney. Review and editing of the original manuscript. Alvaro Israel. Review and editing of the original manuscript. Alexander Golberg. Conceptualization Data analysis, editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
AG and KL have patent applications on devices and use of PEF technologies for seaweed processing with Ramot, Tel Aviv University. AG has interest in Genesea Advanced Technologies Ltd, which focuses on seaweed protein production.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kashyap, M., Ghosh, S., Levkov, K. et al. High-Voltage Pulsed Electric Fields and pH Shift Process for Protein and Solute Release from Gracilaria sp., Red Edible Seaweed. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03432-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03432-x