Abstract
Sugar beet pulp (SBP) samples were subjected to a two-step non-isothermal autohydrolysis process in order to obtain mixtures enriched in oligogalacturonides (OGalA) and arabinooligosaccharides (AOS) in separate streams. Operating at a maximum temperature of 130 °C, mixtures containing up to 30.4% oven-dry basis (o.d.b.) of OGalA with an OGalA/AOS ratio of 5.0 g/g were obtained during the first stage. Then, the treated solids were subjected to a second treatment at temperatures in the range 160–175 °C. When those solids were treated up to 175 °C, a mixture mainly made up of AOS (37.5% o.d.b.) with an AOS/OGalA ratio of 3.91 g/g was obtained as an effluent from the reactor. In order to increase their purity, both streams were then subjected to different refining steps. A product enriched in highly methylated and partially acetylated OGalA (42.5% o.d.b., degree of methylation (DM) = 69.2% mol/mol and degree of acetylation (DA) = 36.4% mol/mol), containing 17.2% o.d.b. of non-volatile non-identified compounds, was obtained by membrane filtration of the first-stage liquors, whereas a second one, mainly made up of AOS and galactooligosaccharides (GalOS) (55.0% AOS o.d.b., 13.8% GalOS o.d.b., and 13.3% non-volatile non-identified compounds, o.d.b.), was manufactured after an ion exchange treatment followed by membrane filtration of the second-stage liquors. This strategy was demonstrated to be a suitable and scalable alternative for the separate production of refined mixtures rich in OGalA or neutral pectic-oligosaccharides. Both types of products can result in different effects on the intestinal microbiota: AOS and GalOS show a significant bifidogenic effect and they could be consumed alone or combined with selected probiotic strains of Bifidobacteria for improving an unbalanced microbiota, whereas OGalA has been demonstrated to have a variety of biological properties and can promote the growing of some bacteria such as Faecalibacterium prausnitzii, a butyrate-producing microorganism underrepresented in patients with active IBD and infectious colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The colonic microbiota exerts an important influence in the human health, and it can be modulated through the intake of probiotics and prebiotics. According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), “probiotics” are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host,” whereas a “prebiotic” is a “substrate that is selectively utilized by host microorganisms conferring a health benefit.” Therefore, a prebiotic must affect a limited group of microorganisms in the host rather than the whole microbial ecosystem, in order to meet the criterion of being “selectively utilized” (ISAPP, 2022).
The most commonly studied prebiotics are soluble fibers such as inulin, fructooligosaccharides (FOS), galactooligosaccharides (GalOS), and, more recently, human milk oligosaccharides (ISAPP, 2022). Oligosaccharides are defined as carbohydrates made up of 3–10 sugar units, although some authors also include disaccharides and chains with up to 20 residues (Laurentin & Edwards, 2013) or, when the molecular weight distribution is unknown, the whole set soluble fragments derived from polysaccharide degradation, regardless of their degrees of polymerization (DP) (Gullón et al., 2009). The fermentation of such oligosaccharides in the colon results in the production of a variety of metabolites, mainly short-chain fatty acids (SCFA) that exert several beneficial effects (Fernández et al., 2016; Gullón et al., 2013; Rivière et al., 2016). However, there is an increasing interest on the development of new prebiotics with, potentially, improved and/or additional properties and, in this context, pectin-derived oligosaccharides (POS) have become a promising alternative. Pectin is a very complex and heterogeneous polysaccharide (more information about its structure can be found in B. Gullón et al., 2013) which can be hydrolyzed into a variety of POS including OGalA, GalOS, and AOS or arabinogalactooligosaccharides (AGalOS) which can have different biological effects.
It was observed that the prebiotic effects of pectins and POS depend on their chemical and physicochemical properties. For instance, Onumpai et al. (2011) demonstrated that the structure of oligosaccharides obtained from pectins has a significant impact in the fermentation by human fecal bacteria and that the low molecular weight AOS and GalOS have a significant bifidogenic effect whereas methylated OGalA promotes Faecalibacterium prausnitzii growth. Van Laere et al. (2000) observed that AOS and AGalOS were utilized by individual Bifidobacterium spp. strains, Moon et al. (2015) reported that AOS (DP2-5) have higher bifidogenic effects than arabinan, Al‐Tamimi et al. (2006) published several fermentation patterns when fermented AOS with different molecular weight (Mw), Holck et al. (2011) used AOS with DP 2–14 (with and without feruloyl substituents) concluding that the size of oligomers was more influential on the selective bacterial stimulation than the degree of substitution, and Larsen et al. (2019) assessed the effects of nine structurally diverse pectins from sugar beet and citrus fruit using a TNO in vitro model of the colon (TIM-2) observing general and specific effects on microbiota, suggesting that some characteristics such as the degree of methylation, neutral sugar distribution, and other properties affect the pectin-mediated shifts (Larsen et al., 2019), concluding that the microbial communities can be specifically modulated by different pectins.
Therefore, in order to advance in the use of prebiotics for a given health objective, it is necessary to increase the knowledge of the structure–function interrelationships, making necessary to evaluate the effects of a variety of well-defined oligosaccharides by both in vitro and in vivo assays.
On the other hand, in the most of cases cited above, authors used commercial polymers (galactan, arabinan, or polygalacturonic acid) as raw materials which were broken down into oligomers by acid methods (Onumpai et al., 2011; Miyazawa et al., 2008) or by enzymatic hydrolysis (Al‐Tamimi et al., 2006), but another alternative is feasible: the direct manufacture of such oligomers starting from the native raw materials thereby reducing the operational and equipment costs.
SBP is a pectin-rich agroindustrial byproduct generated in sugar manufacture facilities in huge amounts. For instance, just in the European Union, the annual SBP production is estimated to be in the range 14–20 million tons (in dry matter basis) (Borysiuk et al., 2019; Joanna et al., 2018). SBP is a highly digestible byproduct rich in polysaccharides (cellulose and hemicelluloses besides pectin) (Puligundla & Mok, 2021), but it also contains protein and ferulic and acetic acids (Joanna et al., 2018) as well as some microminerals. Therefore, many efforts have been carried out to develop a variety of alternatives for its valorization as it was summarized by Puligundla and Mok (2021) and Usmani et al. (2022).
In comparison with pectins from orange peels or apple pomace, the SBP one has a lower content of homogalacturonan (HG) and higher proportion of hairy regions (Thibault et al., 1993), mainly rhamnogalacturonan I (RGI) chains, which are rich in arabinan. When SBP is subjected to hydrothermal treatment, mixtures of pectin-derived fragments are produced. In two previous works, Martínez et al. (2009, 2010) obtained mixtures of neutral and acidic POS with an OGalA/AOS ratio of 1.22 g/g. Although these mixtures were assessed for their prebiotic effects in in vitro assays with promising results (Gómez et al., 2016, 2019), when they are fermented, their effects on the intestinal microbiota are more difficult to understand because of their complex composition. Therefore, more defined substrates are preferable to carry out in vitro and in vivo assays in order to obtain more clear conclusions. In this context, preparative chromatography could be used for neutral and acidic oligosaccharide separation although these kinds of alternatives are expensive and difficult to scale up.
In 2010, Martinez et al. observed that galacturonan and arabinan followed different reaction kinetics, characterized by a delay of the maximum concentration of each type of oligomer (Martínez et al., 2009, 2010). Taking all these ideas into account, it was postulated that a two-step hydrothermal process (an environmentally friendly and scalable technology) could be an efficient alternative for a separate production of neutral (mainly AOS) and acidic oligosaccharides (partially acetylated OGalA) from only one agroindustrial byproduct: sugar beet pulp.
The aim of this work was to develop a new processing strategy for a separate production of mixtures enriched in AOS or OGalA using sugar beet pulp as a raw material. To the best of our knowledge, no similar approach has been assessed and reported before.
Materials and Methods
Raw Material
SBP samples (generated during the 2020 season) were provided by a sugar factory (AB Azucarera Iberia SL) located in Jerez (Cádiz, Spain), homogenized in a single lot, and stored in polyethylene bags at − 18 °C until use.
Chemical Characterization of Raw Material and Spent Solids
SBP samples were quantitatively hydrolyzed in two steps using H2SO4 solutions according to the TAPPI T13m method. Aliquots of resulting hydrolysates were assayed for acetic acid and monosaccharide contents using an Agilent HPLC provided with a refractive index (RI) detector and an Aminex HPX-87H column (BioRad). In this case, a 0.005 N H2SO4 solution was used as mobile phase at a flow rate of 0.6 mL/min at 50 °C. Klason lignin was calculated by gravimetry of the solid phase resulting from quantitative acid hydrolysis (QAH) and uronic acids were determined by the method of Blumenkrantz and Asboe-Hansen (Blumenkrantz & Asboe-Hansen, 1973), using galacturonic acid as a standard for quantification. Elemental nitrogen was determined with a Thermo Flash EA 1112 analyzer, using 130 and 100 mL/min of He and 250 mL/min of O2, and oven temperatures of 680 °C (reduction oven) and 900 °C (oxidation oven) being 50 °C the column temperature. Protein content was obtained by multiplying the elemental N by 6.25 (a factor derived from the average N content of proteins which was estimated 16%). Ashes and moisture were determined by using the methods ISO 776 and ISO 638, respectively. Spent solids were also characterized following the same method as for native SBP. All determinations were made in triplicate.
HG and RGI contents were estimated by using the equations reported by Denman and Morris (2015):
where GalA, Rha, Ara, and Gal are the percentages (mol/mol) of galacturonic acid, rhamnose, arabinose, and galactose, respectively, in pectin.
Hydrothermal Treatments
SBP samples were mixed with water at the desired liquor-to-solid ratio (LSR) (12 kg liquid/kg solid, oven-dry basis), and the resulting suspensions were introduced in a 3.75-L stainless steel Parr reactor. Then, the medium was heated until maximum temperatures in the range 130–140 °C (heating times in the range 6.5–13 min, respectively), and once the temperature was achieved, the vessel was cooled with water, following a non-isothermal treatment.
At the end of treatments, liquors and spent solids were separated by centrifugal filtration and both solids and liquids were quantified and analyzed as described in the “Chemical Characterization of Raw Material and Spent Solids” and “Chemical Characterization of Liquors” sections, respectively.
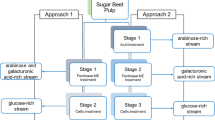
After that, treated solids from the first stage of hydrothermal treatment were subjected to a second step in a 0.6-L Parr reactor under similar conditions except the maximum temperature. In this case, suspensions were heated up to temperatures in the range 160–175 °C (heating times in the interval 25–32 min). Figure 1 shows the processing scheme developed in this work with indication of the selected operational conditions. Under those conditions (130 °C and 175 °C), these experiments were then made in duplicate.
Ion Exchange Processing
Liquors (45 g) from the second stage (stream J in Fig. 1) were mixed with 1.5 g of dry Amberlite IRA910 resin (in OH− form) (LSR = 30 g liquor/g dry resin) and kept at room temperature for 24 h with orbital stirring (150 rpm). At the end, resin and liquors were separated by filtration and liquors analyzed as explained below (“Chemical Characterization of Liquors” section). These experiments were made in triplicate.
Membrane Filtration
Liquors from the first stage of hydrothermal treatment (stream C in Fig. 1) or treated by ion exchange (stream L) were subjected to a two-step membrane filtration using an Amicon setup provided with 5- or 1-kDa cutoff membranes of regenerated cellulose, respectively, which operated at a transmembrane pressure (TMP) = 2 bar (PN2 = 3 bar). During the first step (diafiltration), 50 mL of liquors was mixed with 200 mL of deionized water and filtered throughout the selected membrane (see Fig. 1) up to obtain a permeate volume of 200 mL and a retentate volume of 50 mL. In a second stage (concentration), both retentates were filtered further in order to reduce their volume until achieving a VCR = 1.5–2 mL/mL.
All the streams (feed, permeates, and retentates) were analyzed for their composition using the methodology explained below (see the “Chemical Characterization of Liquors” section). All the experiments were carried out in triplicate.
Chemical Characterization of Liquors
Samples of reaction liquors were filtered through 0.45-μm cellulose acetate membranes and analyzed by high-performance liquid chromatography (HPLC) for monosaccharides and acetic acid (see the “Chemical Characterization of Raw Material and Spent Solids” section for details on HPLC determinations). Simultaneously, another aliquot was subjected to quantitative acid posthydrolysis for determining the neutral oligosaccharide (AOS, glucooligosaccharides or GOS, GalOS, Rhamnosyl moieties or RhaOS) and acetyl groups contents. Non-volatile compounds (NVC) were quantified by oven drying an aliquot at 60 °C until constant weight. The determination of OGalA in autohydrolysis liquors was carried out by enzymatic hydrolysis with a combination of endo-polygalacturonase (Viscozyme L; 0.45 U/mL) and cellulases (Celluclast 1.5 L), and the resulting monomer (galacturonic acid) determined by HPLC. These hydrolyses were carried out at 37 °C for 44 h in Erlenmeyer flasks with orbital agitation (150 rpm) using Na-acetate buffer (50 mM) to keep pH at 5. The contents of oligomers (GOS, OGalA, AOS, GalOS, and RhaOS) were calculated on the basis of the increase in each monomer concentration (glucose, galacturonic acid, arabinose, galactose, and rhamnose, respectively) obtained upon quantitative hydrolysis of liquors. The “other non-identified non-volatile compounds” (ONINVC) content was calculated from the difference between the dry residue of liquors and the sum of the identified compounds including monosaccharides (MS), oligosaccharides, and acetyl groups. This methodology, applied to all the liquid streams of this process, was already used and validated in previous works (Gómez et al., 2016).
The DM of oligomers was measured following the methods reported by Levigne et al. (2002), Ma et al. (2016), Wikiera et al. (2016), Gómez et al. (2016), and Voragen et al. (1986), whereas the DA was determined from the acetyl groups and the galacturonic acid contents in samples quantified as described above.
High-pressure size exclusion chromatography (HPSEC) analyses were carried out using an Agilent chromatograph provided with a RI detector and a tandem of two columns (TSKgel G3000PWxl and TSKgelG2500PWxl) operating at 70 °C with a mobile phase (H2O) flow rate of 0.3 mL/min. The qualitative determination of the DP distribution of oligomers was carried out by high-performance anion exchange chromatography/pulsed amperometric detection (HPAEC-PAD) using an ICS3000 Dionex (Sunnyvale, CA, USA), equipped with a CarboPac PA-1 column (4 mm × 250 mm) and CarboPac PA guard column (4 mm × 50 mm) operating at 30 °C and 0.3 mL/min, following the next elution profile: 0–15 min, 0–5% A; 15–60 min, 70% A; 60–70 min, 100% A; and 70–85 min, 100% B (A = 1 M sodium acetate in 100 mM NaOH and B = NaOH 100 mM).
Results and Discussion
Raw Material Composition
Table 1 shows the composition of the SBP lot used in this work. As can be seen, galacturonan and glucan are the main components of the raw material accounting for 22.55 and 26.36% (o.d.b.), respectively, followed by arabinan (18.03%) and galactan (9.14%). A minor presence of xylan, mannan, and rhamnan was also detected. According to the methodology reported by Denman and Morris (2015), it was estimated that HG and RGI represent up to 35.58% and 64.41% (mol%) of pectin, respectively, confirming that the sugar beet pectin is highly rich in RGI, which (Ara + Gal)/Rha molar ratio (a parameter that measures the average branch length) was 16.5. It is also remarkable the high content of acetyl groups (3.96%), usually linked to O-2 and O-3 of GalA moieties in SBP HG. In this case, a DA = 56.82% (expressed as mol acetyl groups/100 mol GalA) was found in the raw material, indicating that approximately one oxygen (O-2 or O-3) is acetylated (as an average) in each galacturonic acid unit.
Thus, the total polysaccharide content in the raw material was 85.49%, a value close to the upper limit of the previously reported range of 67–80% o.d.b. (Fishman et al., 2013). Similarly, the contents of each fraction observed in this work were close to those published by Fishman et al. (2008, 2013), Zheng et al. (2013), and Huisjes et al. (2012).
Finally, from a quantitative point of view, protein is also an important fraction in SBP, accounting for up to 10.19% (o.d.b.), a value in agreement with that observed by Martínez et al. (2009) and Zheng et al. (2013).
Two-Step Hydrothermal Treatment
In an earlier work, Martínez et al. (2009) optimized the hydrothermal processing of SBP and obtained liquors with an AOS/OGalA ratio of 1.22 g/g, operating under non-isothermal regime up to a maximum temperature of 163 °C. Later, the same authors demonstrated that both fractions were mainly made up of complex mixtures of products with a wide degree of polymerization (Gómez et al., 2016, 2019).
In order to advance in the comprehension of the structure–function interrelationships and, therefore, in the development of targeted prebiotics, it is interesting to obtain well isolated and, if possible, well chemically characterized both types of products. Taking this into account, Liu et al. proposed a process for neutral oligosaccharides production from apple pectin using several stages of chromatography (Liu et al., 2021a) with good results. However, these kinds of technologies are rather expensive and difficult to scale up to industrial level, so that a scalable process based on two-step hydrothermal treatment was proposed and evaluated in this work (see process developed here in Fig. 1). This idea was based on the different kinetic pattern followed by both galacturonan and arabinan components during the hydrothermal treatment of SBP under non-isothermal operation (see Martínez et al., 2009), since the arabinan hydrolysis to AOS was slightly delayed compared to the galacturonan hydrolysis to OGalA. Therefore, it was hypothesized that during the first stage (at lower temperature), a preferential solubilization of galacturonan would be expected (resulting in mixtures with high OGalA/AOS ratios) whereas during the second stage, mixtures with high AOS/OGalA ratios would be generated as consequence of the RGI hydrolysis.
First Stage: OGalA Production
During the hydrothermal processing of SBP, the partial hydrolysis of the biomass components occurs, producing a wide variety of reaction products, including non-volatile (monosaccharides, oligosaccharides, lignin fragments, proteins and/or peptides, and some sugar degradation products) and volatile compounds such as acetic acid. As a result of the treatments carried out in this work, the total content of non-volatile compounds (NVC) in liquors increased with temperature from 1.89 at 130 °C to 2.56 g NVC/100 g liquor at 140 °C (this last value is in agreement with that obtained at 140 °C (≈24 g/kg liquor) by Martínez et al., 2009), demonstrating that a part of the raw SBP can be solubilized during the first stage, even under soft reaction conditions (Martínez et al., 2009).
On the other hand, Fig. 2a shows the composition of the liquors, expressed as mass fraction (g component/100 g NVC o.d.b.). As can be seen, the content of the main pectin-derived products (OGalA and AOS) increases continuously with temperature, with OGalA being the main component of the mixture, reaching values in the range 34.46–35.22 g/100 g NVC, while the AOS content varied from 6.89 at 130 °C to 12.81 g/100 g NVC at 140 °C. In addition, minor amounts of GalOS (2.45–3.74 g/100 g NVC) were also found in liquors.
As a consequence, a product with an OGalA/AOS ratio of 5.0 g/g (i.e., 3.87 mol GalA/mol Ara) was obtained at 130 °C (see Fig. 2b), while this value decreased to 2.75 g/g at 140 °C as a result of a higher arabinan hydrolysis into AOS. This value (5.0 g/g) is significantly higher (7.3 times) than the one obtained by Martínez et al. (2009) (0.82 g OGalA/g AOS) operating in just one stage of autohydrolysis at a Tmax = 163 °C than the GalA/Ara ratio found in SBP (1.15 mol/mol), confirming the hypothesis that is indicated above.
Finally, as can be seen in Fig. 2b, OGalA yields increased with temperature from 7.29 at 130 °C to 11.11 g/100 g of raw dry solid (RDS), corresponding to “galacturonan into OGalA” conversions of 28.05 and 42.76%, respectively. The yields and conversions showed in Fig. 2b are theoretical. This means that they were calculated assuming that all the liquid at the beginning of the treatment is recovered as liquor at the end. Thus, these theoretical values represent the largest conversions and yields that can be obtained under specific conditions. As it was expected, lower yields and conversions were observed for the arabinan fraction.
In terms of concentration, it is necessary to remark that the OGalA and AOS contents varied in the range 6.24–9.01 g/L and 1.40–3.28 g/L, respectively. Moreover, very low concentrations of total monosaccharides (0.27–0.52 g/L) were also found in liquors in comparison with total oligomers (13.84–19.86 g/L) indicating that the breakdown of polymers by the chain extremes was negligible at these temperatures.
In addition to pectic-oligosaccharides, GOS were also found in liquors, being these values significantly higher than the ones observed by Martínez et al. (2009).
On the other hand, it is necessary to remark the significant values of ONINVC in liquors, being 24.23 and 28.35 g/100 g NVC o.d.b. at 130 °C and 135 °C, respectively. This fraction could include protein, ashes, or phenolic compounds, and it should be reduced if a refined functional ingredient for food applications is desired.
All these results confirmed that a fraction of galacturonan can be preferentially dissolved in comparison with RG-I branches, as it was hypothesized, although the explanation is not clear. However, as it was reported, during a thermal treatment, homogalacturonan chains can be broken by acid hydrolysis or by β-elimination resulting in mixtures of saturated and unsaturated OGalA, respectively (Gómez et al., 2016). At high pH, a β-elimination mechanism is more important than acid hydrolysis, including the acid hydrolysis of the lateral chains of arabinan and galactans (Liu et al., 2021b). Moreover, methoxylated pectins, including SBP pectin (Gómez et al., 2016), are more sensible to β-elimination reaction. In fact, Gómez et al. (2016) had demonstrated that unsaturated OGalA with DP2-12 (generated by β-elimination) are obtained by hydrothermal treatment of SBP in a higher proportion in comparison with the saturated ones (30 vs 11%).
Second Stage: Arabinooligosaccharide Production
Solids generated during the first stage were then subjected to a second treatment (see the “Hydrothermal Treatments” section for details) in order to solubilize the remaining arabinan. In this step, the NVC content in liquors increased continuously with the severity of treatments achieving a maximum value (4.00 g NVC/100 g liquor) when solids were sequentially treated at 130 °C (first stage) and 175 °C (second stage). Figure 3a shows the composition of the solutes expressed as mass fractions (g component/100 g NVC o.d.b.).
As can be observed, AOS is the main fraction in these second-stage mixtures, reaching values of 35.4 g/100 g NVC at 135–175 °C and 44.3 g/100 g NVC at 140–165 °C, and its content decreases with the increasing temperature as a consequence of the AOS hydrolysis into arabinose, following the reaction mechanism proposed by Martínez et al. (2009) who demonstrated that AOS hydrolysis mainly increases at temperatures in the range 170–180 °C, a fact which is also in agreement with the observations reported by Sato et al. (2013). Moreover, significant amounts of GalOS were also found in liquors achieving values which varied in the range 7.0–11.2 g/100 g NVC, a fact that was also reported by Sato et al. (2013). It is important to remark that GalOS are defined here as the sum of all the galactose residues present in oligomeric structures, probably including both galactooligosaccharides and arabino(galacto)oligosaccharides.
On the other hand, the OGalA content varied from 22.3 (140–160 °C) to 8.1 g/100 g NVC (135–175 °C) and decreased when the second-stage temperature increases as a consequence of decomposition reactions, as no stoichiometric increase in GalA concentration was observed in liquors. This fact was also observed before by Martínez et al. (2009, 2010) who demonstrated that OGalA can be directly degraded to a variety of decomposition products following a two in series reactions, being this phenomenon more important at T > 160 °C; by Combo et al. (2013) who reported that the decomposition of both native pectin and different de-methoxylated POS starts at 150 °C, temperature where galacturonic acid chains start to undergo extensive thermal degradation; by Sato et al. (2013) who observed an important decrease in uronic acids concentration with increased temperatures (160–180 °C) and reaction times; and by Hamley-Bennett et al. (2016) which demonstrated GalA losses up to 50%, probably by degradation into volatile compounds, when treated SBP with steam at 5 bar for 24 min.
In fact, this is in agreement with the continuous increase in monosaccharides and other non-identified non-volatile compounds content with temperature in all the experiments.
As a consequence, this behavior led to increased AOS/OGalA ratios achieving a maximum value of 4.37 g/g at 135–175 °C (see Fig. 3b), significantly higher than the one achieved (1.22 g/g) by Martínez et al. (2009) in a previous work. Hamley-Bennett et al. (2016) obtained, under selected conditions, mixtures with ratios of 3.3 although starting from a raw material with higher content in arabinan (23.0 vs 17.47%) and lower content in galacturonan (14.4 vs 25.99%) and Sato et al. (2013) produced mixtures with a ratio below 1.35 g/g under selected conditions.
Figure 3b shows data of the theoretical AOS yield and theoretical arabinan to AOS conversions, among other information. As can be seen, a maximum arabinan to AOS conversion of 95.36% was achieved at 130/175 °C. This means that up to 16.66 g/100 g RDS can be obtained in this step. As expected, lower yields and conversions were observed for the galacturonan fraction.
Finally, in terms of concentration, it is necessary to remark that the AOS and OGalA contents varied in the ranges 10.92–14.69 and 2.50–6.56 g/L, respectively. Similar values were observed by Sato et al. (2013) operating at 170 °C for 15 min. As for the first stage, low monosaccharide concentrations were found in liquors except for arabinose, mainly at high temperatures.
Selection of Operational Conditions
The selection of the best operational conditions is not easy in this case owing to the effects of T1 and T2 on the product yields and the process selectivity (product ratios) in both stages. As a consequence, an objective function (O.F.) was defined considering both selectivity and productivity terms, according to the next expression:
where 1 and 2 refer to the first and the second processing stages, respectively. The first terms in brackets are related to the product ratios observed in stages 1 and 2 (the higher the ratio, the higher the selectivity for the target product at each stage), while the second terms are related to the product yields. All the terms are dimensionless and normalized (see Tables S1 and S2 in Supplementary material for more information). Then, O.F. values were correlated with T1 and T2 according to the following second-order model:
Figure 4 shows the dependence of O.F. on both T1 and T2 in a contour graph. As can be seen, the O.F. value increased with decreasing T1 and increasing T2, achieving a maximum (O.F. = 2.36) at T1 = 130 and T2 = 175 °C. Under these conditions (temperature profiles can be seen for each stage in Fig. S1 in Supplementary material), a theoretical OGalA yield of 7.29 g/100 g RDS was achieved in the first step (i.e., 28.05% of galacturonan in RDS) and an AOS yield of 16.66 g/100 g SBP o.d.b. (i.e., 95.36% of arabinan in RDS) in the second one, thereby resulting in a first product enriched in OGalA with an OGalA/AOS ratio of 5 g/g and a second one enriched in AOS with an AOS/OGalA ratio of 3.27 g/g, a ratio higher than the one obtained in previous studies (Hamley-Bennett et al., 2016; Sato et al., 2013).
Downstream Processing of the Autohydrolysis Liquors
As it was mentioned in the previous section, liquors enriched in OGalA and AOS were generated during the first and second stages of hydrothermal treatment, respectively. However, other non-desired products (including other oligomers, protein, non-volatile impurities, or monosaccharides) were also present, so that additional processing steps are needed to obtain refined products.
After selecting the operational conditions, lots of liquors (made up of a mixture of several runs) were manufactured for the study of the refining processes. A more detailed composition of both lots (streams C and J in Fig. 1) can be found in Tables 2 and 3, respectively.
Refining of First-Stage Liquors
The lot of liquors generated during the first stage showed a pH = 4.44 and, as can be seen in Table 2, contained OGalA as main product (5.72 g/L; 30.41 g/100 g NVC o.d.b.) but also low concentrations of AOS (1.25 g/L; 6.63 g/100 g NVC o.d.b.) and GalOS (0.48 g/L; 2.57 g/100 g NVC o.d.b.) besides significant amounts of GOS (25.66 g/100 g NVC o.d.b.) and ONINVC (29.32 g/100 g NVC o.d.b.).
In order to remove low Mw undesirable products and, thereby, to increase the purity of the product for food applications, aliquots of the liquors were subjected to membrane filtration using a 5-kDa cutoff membrane. Table 2 also shows the composition of the retentates (stream E) and permeates (stream F), all of them expressed as mass fraction. As can be observed, the MS and ONINVC contents decreased from 1.24 to 0.23 g/100 g NVC (a removal percentage of 87% over the total amount of MS in stream C) and from 29.32 to 17.20 g/100 g NVC (a removal percentage of 61% over the total amount of ONINVC in stream C), respectively. On the other hand, up to 21% of total oligosaccharide (the lowest DP ones) was lost in permeates (stream F), although no OGalA were found in this stream, confirming that they were completely retained in stream E (retentate). As a consequence of the process, a final product mainly made up of highly methylated and partially acetylated OGalA (42.5 g OGalA/100 g NVC, DM = 69.2% mol/mol), GOS (28.94 g/100 g NVC), and 17.20 g ONINVC/100 g NVC (which includes 2.1 g protein/100 g NVC) was obtained, resulting in an overall OGalA yield of 3.71 g OGalA/100 g RDS).
Moreover, this product showed an OGalA/AOS ratio of 5.65 g/g, a value significantly higher than the obtained (≈1 g/g) by Martínez et al. when treated SBP under non-isothermal regime up to Tmax = 163 °C in one step (Martínez et al., 2009, 2010).
Finally, as a first approach about their structural characteristics, the HPSEC analysis of this stream revealed the existence of a big peak located in the range of retention times 30–37 min (Fig. S2 in supplementary material) that could correspond, according to the data composition shown in Table 2, to high DP GalA chains (they were retained in a 5-kDa membrane) and a second zone in the interval 37–40 min which could be related to residual GalA and low DP OGalA. Finally, it can be inferred that low DP AOS were lost in permeates (by comparing peaks in the interval 60–65 min).
It should be remarked that there are some evidences that this kind of products (methylated, acetylated, low and high DP OGalA) could have interesting biological properties (anticancer, antilipidemic, antibacterial or antioxidant, among others) beyond their prebiotic effects or other healthy effects as it was summarized by Martínez-Gómez et al. (2023).
Processing and Refining of Second-Stage Liquors
After trying several strategies to isolate AOS present in the second-stage liquors which resulted unsatisfactory (data not shown), a new sequence of refining including ion exchange followed by membrane filtration was then assessed. The results are commented in the following paragraphs.
-
(a)
Ion exchange treatment.
Table 3 shows the composition of liquors resulting from ion exchange processing (stream L), using the resin Amberlite IRA910 in OH− form, a resin selected since previous experiments (data not shown). Non-methylated OGalA are weak organic acids that ionize partially so that they can be exchanged by hydroxyl groups (OH-) present in the anion exchange resins, being then retained in their functional groups. As can be seen, a significant reduction of OGalA content (a removal percentage > 67% over the total amount of OGalA in stream J) with minor losses of AOS was achieved in this step, leading to a product with increased AOS content (47.39 vs 37.52 g/100 g NVC) and AOS/OGalA ratios (14.96 vs 3.91 g/g), indicating that OGalA, as it was expected, can be preferentially removed from liquors using this technique. Moreover, this strategy also showed another advantage: it allowed neutralizing the liquors by releasing OH− groups from the resin thereby increasing the pH from 3.92 (a similar value to the observed by Sato et al., 2013) to 6.83. Finally, a significant reduction in the ONINVC content was also observed (from 25.00 to 21.00 g/100 g NVC), indicating that this processing stage resulted in more pure mixtures of neutral oligomers, although a significant presence of monosaccharides can still be found (12.1 g/100 g NVC).
-
(b)
Membrane filtration.
In order to reduce the MS and other impurities (ONINVC) content, samples of liquors treated by ion exchange were then subjected to membrane filtration using 1-kDa membranes of regenerated cellulose following the procedure detailed in the “Membrane Filtration” section, and both permeates and retentates were analyzed for their composition. Table 3 shows the chemical composition of the feed (stream L) as well as the resulting retentates (stream N) and permeates (stream O). As can be seen, remarkable reductions in MS (from 12.1 to 2.3 g/100 g NVC) and ONINVC contents (from 21.0 to 13.3 g/100 g NVC, including up to 6 g protein/100 g NVC) were achieved in this stage, resulting in a final product enriched in AOS (55.01 g/100 g NVC) and GalOS (13.76 g/100 g NVC), as main fractions, with an AOS/OGalA ratio = 10.24 g/g being the overall AOS yield of 7.30 g/100 g RDS.
The experimental arabinan conversion into AOS obtained in this work considering both stages (72.15%) was similar to those achieved by Hamley-Bennett et al. (2016) and by Sato et al. (2013), who reported that near to 80% of arabinan was recovered as AOS after hydrothermal processing of SBP. This value could be increased minimizing the liquor losses during the hydrothermal treatment (the overall theoretical AOS conversion obtained is 105.77%).
These results are also significantly better than the ones previously reported by Gómez et al. (2016) who obtained a product with lower AOS content (36.2 g/100 g NVC) and AOS/OGalA ratio (1.07 g/g) and a slightly higher ONINVC content (14.20 g/100 g NVC), indicating that this new two-step-based approach improved the former strategy based on a one-step hydrothermal treatment.
Regarding the structure of these products, the HPAEC analysis revealed peaks compatible with the presence of arabinose and arabinooligosaccharides (DP2-8) and higher DP (see retention times 32–47 min in Fig. S3 in supplementary material) as well as other peaks indicating the presence of long-chain AOS or arabinogalactooligosaccharides (DP > 9) (see retention times 47–55 min). In this context, it was reported that RGI branches can be made up of up to 50 residues or even more (Kaczmarska et al., 2022), and that, up to 18 kDa arabinan (approx. 136 residues) from sugar beet pulp is also commercially available (Moon et al., 2015), although, as it was commented in the “Raw Material Composition” section, the average branch length in this lot of SBP was estimated to be 16.5.
According to their structure and purity, this product could be of interest as prebiotic. For instance, as it was indicated, Onumpai et al. (2011) demonstrated that the low DP AOS and GalOS have a significant bifidogenic effect, and Van Laere et al. (2000) observed that AOS and AGalOS were utilized by individual Bifidobacterium spp. strains.
Global Mass Balance
After the refining processes, 3.71 kg of highly methylated, partially acetylated OGalA and 9.13 of neutral oligomers (considering the sum of AOS and GalOS) with a high purity were obtained from 100 kg of dry SBP in separate streams. In addition, taking into account both stages, up to 53.32% of SBP was solubilized in the reactor. Sato et al. (2013) achieved values in the range 58–63%, remaining a solid enriched in glucan (44.59 vs 26.36%) and protein (13.56 vs 10.19% o.d.b.) with improved enzymatic digestibility which could be used as substrate for a variety of biotechnological applications including lactic acid fermentation as it was recently purposed by De Oliveira et al. (2020), Berlowska et al. (2018), or Díaz et al. (2020), following the “biorefinery” philosophy. And, in this context, a liquid stream containing residual monosaccharides and oligosaccharides, which is generated during the refining of second-stage liquors, could be used (instead water) for the preparation of the fermentation broths, thereby increasing the carbohydrate availability, according to the process integration concept.
Conclusions
SBP samples were subjected to a two-step non-isothermal autohydrolysis, and the liquids generated were refined following a sequence of stages in order to obtain two refined products enriched in OGalA or AOS in separate streams. A first product mainly containing highly methylated and partially acetylated OGalA (38.17 g OGalA/100 g NVC, 4.27 g acetyl groups/100 g NVC; DM = 69.2%; DA estimated = 36.2% mol/mol) with an ONINVC content of 17.2 g/100 g NVC was obtained after purification of the first-stage liquors, whereas a second concentrate mainly made up of neutral oligomers (55.01 g AOS/100 g NVC, 13.76 g GalOS/100 g NVC, and 13.33 g ONINVC/100 g NVC) was manufactured after refining of the second-stage liquid phases.
This work demonstrated that this strategy could be a suitable alternative for a separate production of acidic oligogalacturonides and neutral arabino(galacto)oligosaccharides, which different properties could give them a variety of functional food applications. Finally, for an integral use of the SBP, a spent solid with increased glucan (44.59% o.d.b.) and protein (13.56% o.d.b.) contents was generated simultaneously being potentially suitable for biotechnological applications.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- AGalOS:
-
Arabinogalactooligosaccharides
- AOS:
-
Arabinooligosaccharides
- DA:
-
Degree of acetylation
- DM:
-
Degree of methylation
- DP:
-
Degrees of polymerization
- GalA:
-
Galacturonic acid
- GalOS:
-
Galactooligosaccharides
- GOS:
-
Glucooligosaccharides
- HG:
-
Homogalacturonan
- HPLC:
-
High-performance liquid chromatography
- MS:
-
Monosaccharides
- Mw:
-
Molecular weight
- NVC:
-
Non-volatile compounds (i.e., dry matter in liquors)
- LSR:
-
Liquor-to-solid ratio
- o.d.b.:
-
Oven-dry basis
- O.F.:
-
Objective function
- OGalA:
-
Oligogalacturonides
- OINVC:
-
Other identified, non-volatile compounds
- ONINVC:
-
Other non-identified, non-volatile compounds
- POS:
-
Pectin-derived oligosaccharides
- QAH:
-
Quantitative acid hydrolysis
- RDS:
-
Raw dry solid
- RGI:
-
Rhamnogalacturonan I
- RhaOS:
-
Rhamnose residues linked to another oligomers
- RI:
-
Refractive index
- SBP:
-
Sugar beet pulp
- SCFA:
-
Short-chain fatty acids
References
Al-Tamimi, M. A. H. M., Palframan, R. J., Cooper, J. M., Gibson, G. R., & Rastall, R. A. (2006). In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. Journal of Applied Microbiology, 100(2), 407–414. https://doi.org/10.1111/j.1365-2672.2005.02780.x
Berlowska, J., Cieciura-Włoch, W., Kalinowska, H., Kregiel, D., Borowski, S., Pawlikowska, E., et al. (2018). Enzymatic conversion of sugar beet pulp: A comparison of simultaneous saccharification and fermentation and separate hydrolysis and fermentation for lactic acid production. Food Technology and Biotechnology, 56(2), 188–196. https://doi.org/10.17113/ftb.56.02.18.5390
Blumenkrantz, N., & Asboe-Hansen, G. (1973). New method for quantitative determination of uronic acids. Analytical Biochemistry, 54(2), 484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Borysiuk, P., Jenczyk-Tolloczko, I., Auriga, R., & Kordzikowski, M. (2019). Sugar beet pulp as raw material for particleboard production. Industrial Crops and Products, 141, 111829. https://doi.org/10.1016/j.indcrop.2019.111829
Combo, A. M. M., Aguedo, M., Quiévy, N., Danthine, S., Goffin, D., Jacquet, N., et al. (2013). Characterization of sugar beet pectic-derived oligosaccharides obtained by enzymatic hydrolysis. International Journal of Biological Macromolecules, 52, 148–156. https://doi.org/10.1016/j.ijbiomac.2012.09.006
Denman, L. J., & Morris, G. A. (2015). An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydrate Polymers, 117, 364–369. https://doi.org/10.1016/j.carbpol.2014.09.081
De Oliveira, R., Schneider, R., Hoss Lunelli, B., Vaz Rossell, C. E., Maciel Filho, R., & Venus, J. (2020). A simple biorefinery concept to produce 2G-lactic acid from sugar beet pulp (SBP): A high-value target approach to valorize a waste stream. Molecules, 25(9). https://doi.org/10.3390/molecules25092113
Díaz, A. B., González, C., Marzo, C., Caro, I., & Blandino, A. (2020). Feasibility of exhausted sugar beet pulp as raw material for lactic acid production. Journal of the Science of Food and Agriculture, 100(7), 3036–3045. https://doi.org/10.1002/jsfa.10334
Fernández, J., Redondo-Blanco, S., Gutiérrez-del-Río, I., Miguélez, E. M., Villar, C. J., & Lombó, F. (2016). Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. Journal of Functional Foods, 25, 511–522. https://doi.org/10.1016/j.jff.2016.06.032
Fishman, M. L., Chau, H. K., Qi, P. X., Hotchkiss, A. T., & Yadav, M. P. (2013). Physico-chemical characterization of protein-associated polysaccharides extracted from sugar beet pulp. Carbohydrate Polymers, 92(2), 2257–2266. https://doi.org/10.1016/j.carbpol.2012.12.001
Fishman, M. L., Chau, H. K., Cooke, P. H., & Hotchkiss, A. T., Jr. (2008). Global structure of microwave-assisted flash-extracted sugar beet pectin. Journal of Agricultural and Food Chemistry, 56(4), 1471–1478. https://doi.org/10.1021/jf072600o
Gómez, B., Gullón, B., Yáñez, R., Schols, H., & Alonso, J. L. (2016). Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. Journal of Functional Foods, 20, 108–121. https://doi.org/10.1016/j.jff.2015.10.029
Gómez, B., Peláez, C., Martínez-Cuesta, M. C., Parajó, J. C., Alonso, J. L., & Requena, T. (2019). Emerging prebiotics obtained from lemon and sugar beet byproducts: Evaluation of their in vitro fermentability by probiotic bacteria. LWT, 109, 17–25. https://doi.org/10.1016/j.lwt.2019.04.008
Gullón, P., Gullón, B., Moure, A., Alonso, J. L., Domínguez, H., & Parajó, J. C. (2009). Manufacture of prebiotics from biomass sources. In D. Charalampopoulos & R. A. Rastall (Eds.), Prebiotics and probiotics science and technology (pp. 535–589). New York, NY: Springer New York. https://doi.org/10.1007/978-0-387-79058-9_14
Gullón, B., Gómez, B., Martínez-Sabajanes, M., Yáñez, R., Parajó, J. C., & Alonso, J. L. (2013). Pectic oligosaccharides: Manufacture and functional properties. Trends in Food Science & Technology, 30(2), 153–161. https://doi.org/10.1016/j.tifs.2013.01.006
Hamley-Bennett, C., Lye, G. J., & Leak, D. J. (2016). Selective fractionation of sugar beet pulp for release of fermentation and chemical feedstocks; optimisation of thermo-chemical pre-treatment. Bioresource Technology, 209, 259–264. https://doi.org/10.1016/j.biortech.2016.02.131
Holck, J., Lorentzen, A., Vigsnaes, L., Licht, T., Mikkelsen, J. D., & Meyer, A. (2011). Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. Journal of Agricultural and Food Chemistry, 59, 6511–6519. https://doi.org/10.1021/jf200996h
Huisjes, E. H., de Hulster, E., van Dam, J. C., Pronk, J. T., & A, van Maris, A. J. A. (2012). Galacturonic acid inhibits the growth of Saccharomyces cerevisiae on galactose, xylose, and arabinose. Applied and Environmental Microbiology, 78(15), 5052–5059. https://doi.org/10.1128/AEM.07617-11
ISAPP (2022). International Scientific Association for Probiotics and Prebiotics (ISAPP). https://isappscience.org/for-scientists/resources/prebiotics/. Accessed 30 March 2023
Joanna, B., Michal, B., Piotr, D., Agnieszka, W., Dorota, K., & Izabela, W. (2018). Chapter 13 - Sugar beet pulp as a source of valuable biotechnological products. In A. M. Holban & A. M. Grumezescu (Eds.), Advances in biotechnology for food industry (pp. 359–392). Academic Press. https://doi.org/10.1016/B978-0-12-811443-8.00013-X
Kaczmarska, A., Pieczywek, P. M., Cybulska, J., & Zdunek, A. (2022). Structure and functionality of Rhamnogalacturonan I in the cell wall and in solution: A review. Carbohydrate Polymers, 278, 118909. https://doi.org/10.1016/j.carbpol.2021.118909
Larsen, N., de Souza, C. B., Krych, L., Cahu, T., Wiese, M., Kot, W., et al. (2019). Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Frontiers in Microbiology, 10. https://doi.org/10.3389/fmicb.2019.00223
Laurentin, A., & Edwards, C. A. (2013). Fiber: Resistant starch and oligosaccharides. In B. Caballero (Ed.), Encyclopedia of human nutrition (third edition) (pp. 246–253). Waltham: Academic Press. https://doi.org/10.1016/B978-0-12-375083-9.00109-4
Levigne, S., Thomas, M., Ralet, M. C., Quemener, B., & Thibault, J. F. (2002). Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocolloids, 16(6), 547–550. https://doi.org/10.1016/S0268-005X(02)00015-2
Liu, H., Wei, X., Zu, S., Lin, X., Zhang, J., Shi, A., et al. (2021a). Separation and identification of neutral oligosaccharides with prebiotic activities from apple pectin. Food Hydrocolloids, 121, 107062. https://doi.org/10.1016/j.foodhyd.2021.107062
Liu, X., Renard, C. M. G. C., Rolland-Sabaté, A., Bureau, S., & Le Bourvellec, C. (2021b). Modification of apple, beet and kiwifruit cell walls by boiling in acid conditions: Common and specific responses. Food Hydrocolloids, 112, 106266. https://doi.org/10.1016/j.foodhyd.2020.106266
Ma, X., Wang, W., Wang, D., Ding, T., Ye, X., & Liu, D. (2016). Degradation kinetics and structural characteristics of pectin under simultaneous sonochemical-enzymatic functions. Carbohydrate Polymers, 154, 176–185. https://doi.org/10.1016/j.carbpol.2016.08.010
Martínez, M., Gullón, B., Yáñez, R., Alonso, J. L., & Parajó, J. C. (2010). Kinetic assessment on the autohydrolysis of pectin-rich by-products. Chemical Engineering Journal, 162(2), 480–486. https://doi.org/10.1016/j.cej.2010.05.048
Martínez, M., Gullón, B., Schols, H. A., Alonso, J. L., & Parajó, J. C. (2009). Assessment of the production of oligomeric compounds from sugar beet pulp. Industrial & Engineering Chemistry Research, 48(10), 4681–4687. https://doi.org/10.1021/ie8017753
Martínez-Gómez, S., Fernández-Bautista, M., Rivas, S., Yáñez, R., & Alonso, J. L. (2023). Recent advances in the production of oligogalacturonides and their biological properties. Food & Function, 14(10), 4507–4521. https://doi.org/10.1039/D3FO00327B
Miyazawa, T., Ohtsu, S., & Funazukuri, T. (2008). Hydrothermal degradation of polysaccharides in a semi-batch reactor: Product distribution as a function of severity parameter. Journal of Materials Science, 43(7), 2447–2451. https://doi.org/10.1007/s10853-007-2014-y
Moon, J. S., Shin, S. Y., Choi, H. S., Joo, W., Cho, S. K., & Li, L. (2015). In vitro digestion and fermentation properties of linear sugar-beet arabinan and its oligosaccharides. Carbohydrate Polymers, 131, 50–56. https://doi.org/10.1016/j.carbpol.2015.05.022
Onumpai, C., Kolida, S., Bonnin, E., & Rastall, R. A. (2011). Microbial utilization and selectivity of pectin fractions with various structures. Applied and Environmental Microbiology, 77(16), 5747–5754. https://doi.org/10.1128/AEM.00179-11
Puligundla, P., & Mok, C. (2021). Valorization of sugar beet pulp through biotechnological approaches: Recent developments. Biotechnology Letters, 43(7), 1253–1263. https://doi.org/10.1007/s10529-021-03146-6
Rivière, A., Selak, M., Lantin, D., Leroy, F., & Vuyst, L. De. (2016). Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.00979
Sato, N., Takano, Y., Mizuno, M., Nozaki, K., Umemura, S., Matsuzawa, T., et al. (2013). Production of feruloylated arabino-oligosaccharides (FA-AOs) from beet fiber by hydrothermal treatment. Journal of Supercritical Fluids, 79, 84–91. https://doi.org/10.1016/j.supflu.2013.01.012
Thibault, J.-F., Renard, C. M. G. C., Axelos, M. A. V, Roger, P., & Crcpeau, M.-J. (1993). Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydrate Research (Vol. 238).
Usmani, Z., Sharma, M., Diwan, D., Tripathi, M., Whale, E., Jayakody, L. N., et al. (2022). Valorization of sugar beet pulp to value-added products: A review. Bioresource Technology, 346, 126580. https://doi.org/10.1016/j.biortech.2021.126580
Van Laere, K. M. J., Hartemink, R., Bosveld, M., Schols, H. A., & Voragen, A. G. J. (2000). Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. Journal of Agricultural and Food Chemistry, 48(5), 1644–1652. https://doi.org/10.1021/jf990519i
Voragen, A. G. J., Schols, H. A., & Pilnik, W. (1986). Determination of the degree of methylation and acetylation of pectins by h.p.l.c. Food Hydrocolloids, 1(1), 65–70. https://doi.org/10.1016/S0268-005X(86)80008-X
Wikiera, A., Mika, M., Starzyńska-Janiszewska, A., & Stodolak, B. (2016). Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydrate Polymers, 142, 199–205. https://doi.org/10.1016/j.carbpol.2016.01.063
Zheng, Y., Lee, C., Yu, C., Cheng, Y. S., Zhang, R., Jenkins, B. M., & VanderGheynst, J. S. (2013). Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Applied Energy, 105, 1–7. https://doi.org/10.1016/j.apenergy.2012.11.070
Acknowledgements
The authors would like to thank the Centro de Apoio Científico-Tecnolóxico á Investigación (C.A.C.T.I.) for performing the HPAEC assays.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Open Access funding provided thanks to the CRUE-CSIC (Universidade de Vigo/CISUG) agreement with Springer Nature. Project PID2020-116717RB-I00 (acronym BIOPLATFUN) funded by MCIN/AEI/ https://doi.org/10.13039/501100011033 and Project GRC-ED431C 2022/08 supported by Xunta de Galicia and by Ministry of Universities. Grants RYC2021-031964-I and PRE2021-098927 funded by MCIN/AEI/ https://doi.org/10.13039/501100011033 and by “ESF + Investing in your future.”
Author information
Authors and Affiliations
Contributions
Conceptualization: José L. Alonso; investigation: Sergio Martínez and José L. Alonso; formal analysis: José L. Alonso; writing — original draft: José L. Alonso; review and editing: Sergio Martínez and Remedios Yáñez; supervision: José L. Alonso and Remedios Yáñez; project administration: Remedios Yáñez and José L. Alonso; funding acquisition: Remedios Yáñez and José L. Alonso.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Gómez, S., Yáñez, R. & Alonso, J.L. A New Strategy for a Separate Manufacture of Arabinooligosaccharides and Oligogalacturonides by Hydrothermal Treatment of Sugar Beet Pulp. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03398-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03398-w