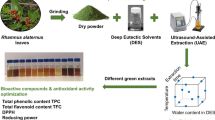
Abstract
An efficient acidic natural deep eutectic solvent (choline chloride-acetic acid, Chcl-AA) was screened out and subsequently combined with ultrasound treatment to recover seabuckthorn leaf phenolics (SLP), and the Plackett–Burman and Box-Behnken designs were used to optimize the process parameters. The optimal parameters were water content of NADES 30%, solvent to solid ratio 32 mL/g, extraction time 14 min, extraction temperature 40°C, ultrasonic power 400 W, and ultrasonic duty cycle 75%. The values of total phenolic content (TPC) and total flavonoid content (TFC) under these conditions were 99.65 ± 2.49 milligram gallic acid equivalent per gram of dry weight (mg GAE/g DW) and 52.02 ± 1.21 milligram rutin equivalent per gram of dry weight (mg RE/g DW), respectively. Twenty-five phenolics were initially identified from SLP by ultra-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry (UPLC-Q-TOF/MS), among which hydrolyzable tannins, isorhamnetin derivatives, and quercetin derivatives are the main constituents. The SLP extract was further evaluated as to its ability to inhibit metabolic enzymes, i.e., α-glucosidase, α-amylase, pancreatic lipase, cholesterol esterase, xanthine oxidase, and angiotensin-converting enzyme. SLP exhibited significant inhibition of α-glucosidase (IC50 = 3.31 μg/mL), pancreatic lipase (IC50 = 57.62 μg/mL), and angiotensin-converting enzyme (IC50 = 63.13 μg/mL). Additionally, the compounds extracted using Chcl-AA exhibited superior bioactivity when compared to those using conventional green solvents, i.e., ethanol and water. These results provide a valuable technical buttress for the efficient extraction of SLP and their further application and research in functional foods.






Similar content being viewed by others
Data Availability
The data of this study are available from the corresponding author upon reasonable request.
References
Airouyuwa, J. O., Mostafa, H., Riaz, A., Stathopoulos, C., & Maqsood, S. (2022). Natural deep eutectic solvents and microwave-assisted green extraction for efficient recovery of bioactive compounds from by-products of date fruit (Phoenix dactylifera L.) processing: Modeling, optimization, and phenolic characterization. Food and Bioprocess Technology, 16(4), 824–843. https://doi.org/10.1007/s11947-022-02960-8.
Ashraf, W., Rehman, A., Hussain, A., Karim, A., Sharif, H. R., Siddiquy, M., & Lianfu, Z. (2023). Optimization of extraction process and estimation of flavonoids from fenugreek using green extracting deep eutectic solvents coupled with ultrasonication. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-023-03170-6
Beyea, M. M., Garg, A. X., & Weir, M. A. (2012). Does orlistat cause acute kidney injury? Therapeutic Advances in Drug Safety, 3(2), 53–57. https://doi.org/10.1177/2042098611429985
Cui, Q., Liu, J., Wang, L., Kang, Y., Meng, Y., Jiao, J., & Fu, Y. (2018). Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from seabuckthorn leaves. Journal of Cleaner Production, 184, 826–835. https://doi.org/10.1016/j.jclepro.2018.02.295
de Almeida Pontes, P. V., Ayumi Shiwaku, I., Maximo, G. J., & Caldas Batista, E. A. (2021). Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chemistry, 352, 129346. https://doi.org/10.1016/j.foodchem.2021.129346.
Dong, K., Binosha Fernando, W. M. A. D., Durham, R., Stockmann, R., & Jayasena, V. (2021). Nutritional value, health-promoting benefits and food application of seabuckthorn. Food Reviews International, 39(4), 2122–2137. https://doi.org/10.1080/87559129.2021.1943429
Farias-Pereira, R., Savarese, J., Yue, Y., Lee, S. H., & Park, Y. (2020). Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Current Research Food Science, 2, 70–76. https://doi.org/10.1016/j.crfs.2019.11.002
Feng, Z., Yang, D., Guo, J., Bo, Y., Zhao, L., & An, M. (2023). Optimization of natural deep eutectic solvents extraction of flavonoids from Xanthoceras sorbifolia Bunge by response surface methodology. Sustainable Chemistry and Pharmacy, 31, 100904. https://doi.org/10.1016/j.scp.2022.100904.
Fu, X., Belwal, T., Cravotto, G., & Luo, Z. (2020). Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrasonics Sonochemistry, 60, 104726. https://doi.org/10.1016/j.ultsonch.2019.104726.
Fu, X., Belwal, T., He, Y., Xu, Y., Li, L., & Luo, Z. (2022). UPLC-Triple-TOF/MS characterization of phenolic constituents and the influence of natural deep eutectic solvents on extraction of Carya cathayensis Sarg. peels: Composition, extraction mechanism and in vitro biological activities. Food Chemistry, 370, 131042. https://doi.org/10.1016/j.foodchem.2021.131042.
Fu, X., Wang, D., Belwal, T., Xu, Y., Li, L., & Luo, Z. (2021). Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chemistry, 355, 129577. https://doi.org/10.1016/j.foodchem.2021.129577
Golovinskaia, O., & Wang, C. (2023). The hypoglycemic potential of phenolics from functional foods and their mechanisms. Food Science and Human Wellness, 12(4), 986–1007. https://doi.org/10.1016/j.fshw.2022.10.020
Han, L., Wang, H., Cao, J., Li, Y., Jin, X., He, C., & Wang, M. (2023). Inhibition mechanism of alpha-glucosidase inhibitors screened from Tartary buckwheat and synergistic effect with acarbose. Food Chemistry, 420, 136102. https://doi.org/10.1016/j.foodchem.2023.136102
Hou, H., Wu, C., Zhou, J., Long, F., Shen, H., Xu, J., Zhou, S., Mao, Q.,Wei, Y., & Li, S. (2023). Accumulation patterns of major bioactive components in two chemotypes of Agastache rugosa during flower development evaluated by GC-QQQ-MS/MS and UPLC-QTOF-MS/MS analyses. Industrial Crops and Products, 191, 115942. https://doi.org/10.1016/j.indcrop.2022.115942
Ismail, B. B., Guo, M., Pu, Y., Wang, W., Ye, X., & Liu, D. (2019). Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrasonics Sonochemistry, 52, 257–267. https://doi.org/10.1016/j.ultsonch.2018.11.023
Ivanović, M., Alañón, M. E., Arráez-Román, D., & Segura-Carretero, A. (2018). Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Research International, 111, 67–76. https://doi.org/10.1016/j.foodres.2018.05.014
Jiang, L., Belwal, T., Huang, H., Ge, Z., Limwachiranon, J., Zhao, Y., Li, L., Ren, G., & Luo, Z. (2019). Extraction and characterization of phenolic compounds from bamboo shoot shell under optimized ultrasonic-assisted conditions: A potential source of nutraceutical compounds. Food and Bioprocess Technology, 12(10), 1741–1755. https://doi.org/10.1007/s11947-019-02321-y
Jovanović, M. S., Krgović, N., Radan, M., Ćujić-Nikolić, N., Mudrić, J., Lazarević, Z., & Šavikin, K. (2023). Natural deep eutectic solvents combined with cyclodextrins: A novel strategy for chokeberry anthocyanins extraction. Food Chemistry, 405(Pt A), 134816. https://doi.org/10.1016/j.foodchem.2022.134816
Jurić, T., Uka, D., Holló, B. B., Jović, B., Kordić, B., & Popović, B. M. (2021). Comprehensive physicochemical evaluation of choline chloride-based natural deep eutectic solvents. Journal of Molecular Liquids, 343, 116968. https://doi.org/10.1016/j.molliq.2021.116968
Lin, Y., Huang, H., & Wang, C. (2022). Effects of high pressure-assisted extraction on yield, antioxidant, antimicrobial, and anti-diabetic properties of chlorogenic acid and caffeine extracted from green coffee beans. Food and Bioprocess Technology, 15(7), 1529–1538. https://doi.org/10.1007/s11947-022-02828-x
Man, G., Xu, L., Wang, Y., Liao, X., & Xu, Z. (2021). Profiling phenolic composition in pomegranate peel from nine selected cultivars using UHPLC-QTOF-MS and UPLC-QQQ-MS. Frontiers in Nutrition, 8, 807447. https://doi.org/10.3389/fnut.2021.807447
Natta, S., Pal, K., Kumar Alam, B., Mondal, D., Kumar Dutta, S., Sahana, N., Mandal, S., Bhowmick, N., Sankar Das, S., Mondal, P., Kumar Pandit, G., Kumar Paul, P., & Choudhury, A. (2023). In-depth evaluation of nutritive, chemical constituents and anti-glycemic properties of jackfruit (Artocarpus heterophyllus Lam) clonal accessions with flake colour diversity from eastern sub-Himalayan plains of India. Food Chemistry, 407, 135098. https://doi.org/10.1016/j.foodchem.2022.135098
Ogihara, W., Aoyama, T., & Ohno, H. (2004). Polarity measurement for ionic liquids containing dissociable protons. Chemistry Letters, 33(11), 1414–1415. https://doi.org/10.1246/cl.2004.1414
Ojeda, G. A., Sgroppo, S. C., Sánchez-Moreno, C., & de Ancos, B. (2022). Mango criollo by-products as a source of polyphenols with antioxidant capacity. Ultrasound assisted extraction evaluated by response surface methodology and HPLC-ESI-QTOF-MS/MS characterization. Food Chemistry, 396, 133738. https://doi.org/10.1016/j.foodchem.2022.133738
Ozturk, B., Parkinson, C., & Gonzalez-Miquel, M. (2018). Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separation and Purification Technology, 206, 1–13. https://doi.org/10.1016/j.seppur.2018.05.052
Pasrija, D., & Anandharamakrishnan, C. (2015). Techniques for extraction of green tea polyphenols: A review. Food and Bioprocess Technology, 8(5), 935–950. https://doi.org/10.1007/s11947-015-1479-y
Patil, S. S., Pathak, A., & Rathod, V. K. (2021). Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrasonics Sonochemistry, 70, 105267. https://doi.org/10.1016/j.ultsonch.2020.105267
Périno-Issartier, S., & Zill e, H., Abert-Vian, M., & Chemat, F. (2010). Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food and Bioprocess Technology, 4(6), 1020–1028. https://doi.org/10.1007/s11947-010-0438-x
Rashid, R., Mohd Wani, S., Manzoor, S., Masoodi, F. A., & Masarat Dar, M. (2023). Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chemistry, 398, 133871. https://doi.org/10.1016/j.foodchem.2022.133871
Rico, X., Gullón, B., & Yáñez, R. (2022). A comparative assessment on the recovery of pectin and phenolic fractions from aqueous and DES extracts obtained from melon peels. Food and Bioprocess Technology, 15(6), 1406–1421. https://doi.org/10.1007/s11947-022-02823-2
Ruesgas-Ramón, M., Figueroa-Espinoza, M. C., & Durand, E. (2017). Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. Journal of Agricultural and Food Chemistry, 65(18), 3591–3601. https://doi.org/10.1021/acs.jafc.7b01054
Sentandreu, E., Cerdán-Calero, M., & Sendra, J. M. (2013). Phenolic profile characterization of pomegranate (Punica granatum) juice by high-performance liquid chromatography with diode array detection coupled to an electrospray ion trap mass analyzer. Journal of Food Composition and Analysis, 30(1), 32–40. https://doi.org/10.1016/j.jfca.2013.01.003
Siegien, J., Buchholz, T., Popowski, D., Granica, S., Osinska, E., Melzig, M. F., & Czerwinska, M. E. (2021). Pancreatic lipase and alpha-amylase inhibitory activity of extracts from selected plant materials after gastrointestinal digestion in vitro. Food Chemistry, 355, 129414. https://doi.org/10.1016/j.foodchem.2021.129414
Silva, D. T. d., Pauletto, R., Cavalheiro, S. d. S., Bochi, V. C., Rodrigues, E., Weber, J., Silva, C. d. B. d., Morisso, F. D. P., Barcia, M. T., & Emanuelli, T. (2020). Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. Journal of Food Composition and Analysis, 89,103470. https://doi.org/10.1016/j.jfca.2020.103470
Stobdan, T., Targais, K., Lamo, D., & Srivastava, R. B. (2013). Judicious use of natural resources: A case study of traditional uses of seabuckthorn (Hippophae rhamnoides L.) in trans-Himalayan Ladakh, India. National Academy Science Letters, 36(6), 609–613. https://doi.org/10.1007/s40009-013-0177-4
Tan, J., Wang, D., Lu, Y., Wang, Y., Tu, Z., Yuan, T., & Zhang, L. (2023). Metabolic enzyme inhibitory abilities, in vivo hypoglycemic ability of palmleaf raspberry fruits extracts and identification of hypoglycemic compounds. Food Science and Human Wellness, 12(4), 1232–1240. https://doi.org/10.1016/j.fshw.2022.10.005
Tkacz, K., Wojdyło, A., Turkiewicz, I. P., & Nowicka, P. (2021). Triterpenoids, phenolic compounds, macro- and microelements in anatomical parts of sea buckthorn (Hippophaë rhamnoides L.) berries, branches and leaves. Journal of Food Composition and Analysis, 103, 104107. https://doi.org/10.1016/j.jfca.2021.104107
Wang, X., Liu, X., Shi, N., Zhang, Z., Chen, Y., Yan, M., & Li, Y. (2023a). Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chemistry, 405(Pt B), 134966. https://doi.org/10.1016/j.foodchem.2022.134966
Wang, Y., Chen, L., Liu, H., Xie, J., Yin, W., Xu, Z., Ma, H., Wu, W., Zheng, M., Liu, M., & Liu, J. (2023b). Characterization of the synergistic inhibitory effect of cyanidin-3-O-glucoside and catechin on pancreatic lipase. Food Chemistry, 404(Pt B), 134672. https://doi.org/10.1016/j.foodchem.2022.134672
Yolmeh, M., & Jafari, S. M. (2017). Applications of response surface methodology in the food industry processes. Food and Bioprocess Technology, 10(3), 413–433. https://doi.org/10.1007/s11947-016-1855-2
Yuca, H., Özbek, H., Demirezer, L. Ö., Sevindik, H. G., Kazaz, C., & Güvenalp, Z. (2021). α-Glucosidase and α-amylase inhibitory potential of main compounds and drug candidates from Elaeagnus rhamnoides (L.) A. Nelson. Chemical Papers, 76(2), 913–922. https://doi.org/10.1007/s11696-021-01904-4
Zengin, G., Cadiz-Gurrea, M. L., Fernandez-Ochoa, A., Leyva-Jimenez, F. J., Carretero, A. S., Momotko, M., Yildiztygay, E., Karatas, R., Jugreet, S., Mahomoodally, M. F., & Boczkaj, G. (2022). Selectivity tuning by natural deep eutectic solvents (NADESs) for extraction of bioactive compounds from Cytinus hypocistis-Studies of antioxidative, enzyme-inhibitive properties and LC-MS Profiles. Molecules, 27(18), Acticle 5788. https://doi.org/10.3390/molecules27185788.
Zhan, A., Niu, D., Li, K., & Li, J. (2023). Characterization of some sucrose-based deep eutectic solvents and their effect on the solubility of piroxicam. Journal of Molecular Liquids, 377, 121556. https://doi.org/10.1016/j.molliq.2023.121556
Zhang, H., & Cheng, Y. (2006). An HPLC/MS method for identifying major constituents in the hypocholesterolemic extracts of Chinese medicine formula ‘Xue-Fu-Zhu-Yu decoction.’ Biomedical Chromatography, 20(8), 821–826. https://doi.org/10.1002/bmc.607
Zhang, Q., Shen, H., Fan, X., Shen, Y., Wang, X., & Song, Y. (2015). Changes of gallic acid mediated by ultrasound in a model extraction solution. Ultrasonics Sonochemistry, 22, 149–154. https://doi.org/10.1016/j.ultsonch.2014.06.010
Zhang, Z., Peng, Y., Meng, W., Pei, L., & Zhang, X. (2022). Browning inhibition of seabuckthorn leaf extract on fresh-cut potato sticks during cold storage. Food Chemistry, 389, 133076. https://doi.org/10.1016/j.foodchem.2022.133076
Zhou, P., Zheng, M., Li, X., Zhou, J., Shang, Y., Li, Z., & Qu, L. (2022). A consecutive extraction of pectin and hesperidin from Citrus aurantium L.: Process optimization, extract mechanism, characterization and bio-activity analysis. Industrial Crops and Products, 182, 114849. https://doi.org/10.1016/j.indcrop.2022.114849
Zouheira, D., Ngnokam, S. L. W., Kamani, S. L. P., Tchegnitegni, B. T., Jouda, J. B., Mba, J. R., Nchouwet, M. L., Nfor, N. G., Nyirimigabo, A. K., Kowa, T. K., & Agbor, G. A. (2022). In vitro antilipidic and antithrombotic activities of Plectranthus glandulosus (Lamiaceae) leaves extracts and fractions. Biomedical Research International, 2022, 4145659. https://doi.org/10.1155/2022/4145659
Funding
This work was supported by the Xinjiang Uygur Autonomous Region Key Research and Development Program (Project No. 2022B02005-4).
Author information
Authors and Affiliations
Contributions
Yuqian Wang conducted the experiments and wrote the first draft. Qi Shan and Yeping Jia assisted in experimentation and revised the manuscript. Tonghua Wu and Jun Zhang revised and polished the manuscript text. Liang Shan guided and supervised the research, and revised thoroughly the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Shan, Q., Jia, Y. et al. Ultrasound-Assisted Acidic Natural Deep Eutectic Solvent as a New Strategy for Extracting Seabuckthorn Leaf Phenolics: Process Optimization, Compositional Identification, and Metabolic Enzyme Inhibition Capacity. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03327-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03327-x