Abstract
Many probiotic products, with properly selected microorganisms, may not be effective for the intended purpose due to the low tolerance of microorganisms to gastrointestinal digestion. The microencapsulation seems to be one of the most promising techniques to protect probiotics against adverse environmental conditions. Therefore, the aim of this work was the design of soy protein isolate-alginate microcapsules for the encapsulation of probiotics for the poultry industry by the water-in-oil emulsion technique. To this end, the strain Ligilactobacillus salivarius CRL2217, with the ability to bind wheat germ agglutinin (WGA) on its surface and protect intestinal epithelial cells from the cytotoxicity of the glycoprotein, was used as model microorganism. Several parameters were varied in order to find the better conditions for microencapsulation: oil source and nature, SPI and sodium alginate concentration, stirring equipment and time for emulsion formation, CaCl2 concentration, and absence or presence of stirring after the addition of the CaCl2 solution. The survival of entrapped cells to a simulated gastric digestion and their survival and release during simulated intestinal digestion were also investigated. The obtained particles effectively protected L. salivarius CRL2217 from the proteolytic activity and low pH present in the gastric environment. Besides, their content was released in contact with a simulated intestinal juice, as viable counts and binding of WGA after a simulated intestinal digestion revealed. This work paves the way for the design of probiotic supplements for poultry including gastrointestinal digestion-susceptible bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The poultry industry provides a significant fraction of components to the human diet, especially meats with proteins of very good nutritional quality and of low cost for the consumer. Except for a decrease because of SARS-CoV-2 pandemic (Attia et al., 2022), the production of poultry meat has increased steadily globally in recent years, and this trend is expected to continue due to the increase in the human population and their preference to eat lean meats such as from poultry (Kadykalo et al., 2018).
Maize is the main energy source in diets for poultry, while wheat may be combined with it in feeds when available (Akter et al., 2017; de Keyser et al., 2016). However, the consumption of wheat may not be recommendable due to the presence of some antinutritional factors (Verni et al., 2019), such as wheat germ agglutinin (WGA), a lectin with cytotoxicity on intestinal epithelial cells from poultry (Babot et al., 2017).
In previous studies, probiotic bacteria with the ability to bind dietary lectins on their surface were used as a new approach to avoid the toxicity of these glycoproteins (Babot et al., 2016, 2017, 2018, 2021). Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host in a safe and efficacious manner (Hill et al., 2014). Many probiotic products, with correctly selected microorganisms, may not be effective for the intended purpose due to the low tolerance of microorganisms to gastrointestinal digestion. In this sense, microencapsulation seems to be one of the most promising techniques to protect probiotics against adverse environmental conditions by entrapment within a matrix of biopolymeric material (Reque & Brandelli, 2021).
Currently, the most common probiotic encapsulation methods are emulsion, extrusion, coacervation, lyophilization, and spray drying (Mahmoud et al., 2020). In the emulsion method, a small volume of a polymer suspension containing microorganisms (discontinuous phase) is added to a larger volume of vegetable oil (continuous phase). Using an emulsifier, the mixture is homogenized and, after formation of the emulsion, the water-soluble polymer can be insolubilized to produce gel capsules (Gheorghita et al., 2021). Contrary to other microencapsulation techniques, the emulsion method can be easily scaled up and the diameter of produced beads is considerably smaller (Huq et al., 2013).
Calcium alginate, owing to it being non-toxic, biocompatible, cheap, and simple to use, is the preferred material for the encapsulation of probiotic cells. Nevertheless, the use of alginate as the encapsulating material has certain disadvantages as well. The main challenge posed is that the alginate beads are sensitive to the acidic environment (Sarao & Arora, 2017). However, these problems can be overcome by using a mixture of alginate and some other polymer compound such as proteins (Krasaekoopt et al., 2003). Soy proteins have potential as encapsulating material due to their renewability and low costs in comparison with proteins from animal and dairy sources, in addition to well-recognized health effects (Tang & Li, 2013). During gastrointestinal digestion, proteins act as buffering agents and polysaccharides provide a physical barrier which protects encapsulated cells from acid and bile (Liu et al., 2019). Besides, interactions between protein and polysaccharide, such as hydrogen bonds, further protect cells during gastrointestinal digestion by strengthening the structure of microcapsules (Liu et al., 2016). Thus, the encapsulation of bacteria using a combination of protein and polysaccharide may protect both viability and superficial structures responsible for binding lectins during gastrointestinal digestion.
Therefore, the aim of this work was the design of soy protein isolate (SPI)-alginate microcapsules for the encapsulation of probiotics for the poultry industry by the water-in-oil emulsion technique. To this end, the strain Ligilactobacillus salivarius CRL2217, with the ability to bind WGA on its surface and protect intestinal epithelial cells from the cytotoxicity of the glycoprotein (Babot et al., 2017), was used as model microorganism.
Materials and Methods
Bacterial Strain and Culture Conditions
The strain L. salivarius CRL2217, previously isolated from the intestine of a healthy chick (Babot et al., 2014), was used in this study. This strain is deposited in the CRL Culture Collection (CERELA-CONICET, Tucumán, Argentina). It was stored at − 70 °C in 10% reconstituted non-fat milk supplemented with 0.5% yeast extract and 15% glycerol. Before use, it was activated by three successive transfers for 24 h at 37 °C in MRS broth (De Man et al., 1960) in a chamber gassed with 10% CO2 (Nuaire Co., Plymouth, MN, USA).
Materials
Soybean of the DM8002 variety was provided by Don Mario Semillas (Chacabuco, Buenos Aires, Argentina). Soybean protein isolate (98% protein, Neix Supplements, Virrey del Pino, Buenos Aires, Argentina) derived from non-GMO soybean (Glycine max) was used in the present study. Food grade alginic acid sodium salt from brown algae was provided by Alginatos Chile S.A. (Providencia, Región Metropolitana, Chile). Other chemicals were purchased from Sigma-Aldrich (Buenos Aires, Argentina).
Selection of Soybean Protein Source
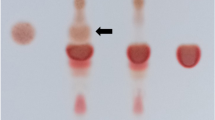
Soybean seeds and commercial SPI (CSPI) were compared as sources of soybean protein. To obtain lab-prepared SPI (LSPI), soybean seeds were grounded (Thermomix TM-31, Vorwerk, Wuppertal, Germany) and the globular proteins were isolated using the method described by Teng et al. (2012), with some modifications. Briefly, 160 g of flour was suspended in 3 L of distilled water, the pH was adjusted to 8.0 using 1 N NAOH, and the suspension was stirred for 1 h at 10,000 rpm. The soluble fraction was recovered by centrifugation (14,000 rpm, 30 min, 4 °C), the pH was adjusted to 4.5 with 1 N HCl, and it was stirred overnight at 1000 rpm. The pellet was recovered (14,000 rpm, 30 min, 4 °C), dissolved in 30 mL of distilled water, pH was adjusted to 7.0 with 1 N NaOH, and it was stored at 4 °C until use. On the other hand, the CSPI was dissolved in distilled water. The protein profiles of both LSPI and CSPI were compared by SDS-PAGE according to López et al. (2015).
Soy Protein Isolate-Sodium Alginate Microcapsule Formation
The microcapsules were produced by the water-in-oil emulsion technique described by Wang et al. (2014), with some modifications. Briefly, 100 mL of vegetable oil containing 0.2% Tween 80 was placed into a glass flask. Ten milliliters of bacterial suspension with denatured SPI [30 min, 85 °C, pH 12.0, according to Zhang et al. (2012)] and sodium alginate were added drop by drop with the aid of a syringe with a 0.8-mm diameter needle while stirring. After stirring, 100 mL of ice-cold CaCl2 solution was added to break the emulsion. The suspension was transferred to a separatory funnel where the oil phase was discharged. Finally, the microcapsules were recovered by centrifugation (2000 × g, 10 min, 4 °C), washed three times (2000 × g, 10 min, 4 °C) with 1% Tween 80, and stored at 4 °C.
Optimization of the Microencapsulation of L. salivarius CRL2217
Overnight cultures of L. salivarius CRL2217 were washed twice (10,000 rpm, 10 min, 4 °C) with sterile saline, resuspended in SPI solution, and alginate was slowly added while stirring. After the complete dissolution of the polysaccharide, the microencapsulation of bacteria proceeded according to the protocol already described. As shown in Table 1, the following parameters were varied in order to find the better conditions for microencapsulation: oil source (soybean oil vs. olive oil) and nature [commercial or raw (obtained using a UG OW500s cold oilpress, Ölwerk)], SPI concentration (0, 30, 60, and 100 mg/mL), sodium alginate concentration (0, 0.2, and 1%), stirring equipment [magnetic stirrer (MS20, Zeltec, Argentina) vs. overhead stirrer (BDC250, Caframo, Canada)], stirring time for emulsion formation (15, 30, and 60 min), CaCl2 concentration (0.01, 0.1, and 0.5 M), and absence or presence of stirring after the addition of the CaCl2 solution.
In each case, the morphology of the microcapsules was evaluated using an optic microscope (Axio Scope A1, Carl Zeiss, Germany) and their diameter was assessed by measuring at least 200 capsules (Gómez-Mascaraque et al., 2019) in different microscopic fields by using the AxioVision Release 4.8 software (Carl Zeiss Imaging Systems, Jena, Thuringia, Germany).
To assess the encapsulation efficiency [EE(%)] of each condition, the suspensions of microcapsules were filtered with 8-µm pore membranes, and counts in MRS agar of these filtered suspensions (N) and those of the initial bacterial suspensions (N0) were introduced in the following expression: \(EE \left(\%\right)=\frac{N0-N}{N0}\times 100\) (El Kadri et al., 2015).
Once the microencapsulation conditions were optimized, the structure of the microcapsules containing L. salivarius CRL2217 was analyzed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) by using Zeiss Supra 55-VP (Carl Zeiss, Oberkochen, Germany) and Zeiss Libra 120 (Carl Zeiss, Oberkochen, Germany) microscopes, respectively, at CIME (Centro Integral de Microscopía Electrónica) CONICET-UNT-Tucumán.
Optimized Microencapsulation Protocol
The final protocol for the encapsulation of L. salivarius CRL2217 with denatured SPI and sodium alginate was as follows: 100 mL of soybean oil containing 0.2% Tween 80 was placed into a glass flask. Ten milliliters of bacterial solution with 60 mg/mL commercial SPI and 0.2% sodium alginate was added drop by drop with the aid of a syringe with a 0.8-mm diameter needle while agitating with an overhead stirrer (1000 rpm). After 30 min stirring, 100 mL of ice-cold 0.1 M CaCl2 solution was added to break the emulsion and the agitation continued for 30 min. Then, the suspension was transferred to a separatory funnel where the oil phase was discharged. Finally, the microcapsules were recovered by centrifugation (2000 × g, 10 min, 4 °C), washed three times (2000 × g, 10 min, 4 °C) with 1% Tween 80, and stored at 4 °C.
Survival of Free and Encapsulated L. salivarius to a Simulated Gastric Digestion
The viability of microencapsulated L. salivarius CRL2217, as compared to free cells, was assessed after a treatment with simulated gastric juice, according to Babot et al. (2014). Briefly, an overnight culture of L. salivarius CRL2217 was adjusted to approximately 8.5 log CFU/mL, an aliquot was stored at 4 °C, and the remaining volume was microencapsulated following the optimized protocol. One milliliter of microcapsule suspension and free cells was transferred to different sterile tubes, washed twice with sterile phosphate-buffered saline (PBS) pH 7.0, and resuspended in PBS pH 7.0 to the original volume. One milliliter of simulated gastric juice (125 mM NaCl, 7 mM KCl, 45 mM NaHCO3, 3 g/L pepsin, pH 2.0) was added to each tube, pH was adjusted to 3.0 with concentrated HCl, and they were incubated for 1 h at 41.5 ± 0.5 °C (mean retention time in proventriculus plus gizzard and temperature of the gastrointestinal tract of poultry). Then, the suspensions were centrifuged (10,000 × g, 10 min, 4 °C) and the pellets resuspended in 1 mL of sterile PBS pH 7.0. The initial and treated microcapsule suspensions were sonicated (three cycles of 30 s on and 30 s off, 35% amplitude) to release the entrapped bacteria. Finally, the initial (free and microencapsulated bacteria) and treated (free and microencapsulated bacteria) suspensions were properly diluted, plated onto MRS agar plates, and incubated for 48 h at 37 °C in a chamber gassed with 10% CO2 (Nuaire Co., MN, USA) to obtain CFU/mL counts. Before sonication, an aliquot of microcapsule suspension was separated for SEM visualization using a Zeiss Libra 120 (Carl Zeiss, Oberkochen, Germany) microscope at CIME (Centro Integral de Microscopía Electrónica) CONICET-UNT-Tucumán.
Release and Survival of L. salivarius in Simulated Intestinal Juice
This assay was performed according to Wang et al. (2014), with some modifications. Briefly, an overnight culture of L. salivarius CRL2217 was adjusted to approximately 8.5 log CFU/mL, an aliquot was stored at 4 °C, and the remaining volume was microencapsulated following the optimized protocol. Half of the microcapsules obtained were washed twice and resuspended in a volume of PBS pH 7.0 equal to that of simulated intestinal juice employed after, and counts of microcapsules/mL were assessed using a Neubauer chamber. Then, free and remaining encapsulated bacteria were washed twice with sterile PBS pH 7.0, resuspended separately in simulated intestinal juice [0.3% (p/v) bile salts, 0.8 mg/ml pancreatin, pH 8.00] (Babot et al., 2014), and incubated at 41.5 ± 0.5 °C with agitation. Samples were taken at 0, 10, 60, 120, and 180 min. They were properly diluted, plated onto MRS agar plates, and incubated for 48 h at 37 °C in a chamber gassed with 10% CO2 (Nuaire Co., MN, USA) to obtain CFU/mL counts. At 120 min, an aliquot of the microcapsule suspension was separated for SEM visualization using a Zeiss Libra 120 (Carl Zeiss, Oberkochen, Germany) microscope at CIME (Centro Integral de Microscopía Electrónica) CONICET-UNT-Tucumán. At the same time, other aliquot was separated for WGA binding assay.
Binding of Wheat Germ Agglutinin by Bacteria Released from Microcapsules
The capture of WGA by bacteria released from microcapsules after the simulated intestinal digestion was assessed according to Babot et al. (2017). To this end, bacteria were washed three times with a lectin buffer (60.57 g/L Tris, 87 g/L NaCl, 1.11 g/L CaCl2, pH 7.6) described by Leathem and Brooks (1997), suspended in an equal volume of the same buffer containing 20 µg/mL of FITC-labeled WGA, and incubated 1 h at 25 °C. Cell suspensions were washed 4 times (10,000 × g, 10 min, 4 °C), suspended in an equal volume of lectin buffer, and observed in a conventional fluorescence microscope (Carl Zeiss Axio Scope A1, Gottingen, Germany) with an appropriate filter.
Results and Discussion
Selection of Soybean Protein Source
SPI is the purest form of soy protein, with a content of at least 90% protein. Due to its high protein content, SPI has a plethora of applications in the food industry (Astawan & Prayudani, 2020), the biomedical field (Tansaz & Boccaccini, 2016), the construction industry (Chen et al., 2022), among others. Due to its many applications, low-cost commercial SPI is widely available. On the other hand, the preparation of SPI in a laboratory is a time-consuming process. Therefore, the first assay in this work aimed at determining if LSPI could be replaced by CSPI in the production of SPI-sodium alginate microcapsules. To this end, the protein profiles in both LSPI and CSPI were compared by SDS-PAGE. LSPI evidenced a similar protein profile to those informed by other authors who obtained it by using similar techniques (Chen et al., 2016; Liu et al., 2007). Besides, as shown in Fig. 1, the patterns of both LSPI and CSPI were similar. In both cases, the main globular proteins in soybean—β-conglycinin (with subunits α, α’, and β) and glycinin (with subunits AS and BS)—were present. This would indicate that LSPI could be replaced by CSPI in the production of microcapsules with no consequences on the properties of the particles.
Optimization of the Microencapsulation of L. salivarius CRL2217
The particle size is an important physical characteristic that affects the texture and sensory attributes, such as mouthfeel (Jouki & Khazaei, 2022). It strongly influences the appearance, flowability, and dispersibility of microcapsule powders (Reineccius, 2004). Capsules with diameters higher than 100 µm may affect the organoleptic properties of feeds (Hansen et al., 2002). Besides, smaller particle sizes could be advantageous to achieve adequate dispersion and homogenization of the powder in the animal feeds (Ambrosio et al., 2020). Considering this, and based on a one factor at a time optimization, several parameters were analyzed to find the better conditions for each step involved in the microencapsulation of L. salivarius CRL2217 (source and nature of the oil, SPI and sodium alginate concentration, stirring equipment and time for emulsion formation, CaCl2 concentration, and absence or presence of stirring after the addition of the CaCl2 solution) to obtain the smaller possible particles. Besides mean diameter of microcapsules, the criteria to select the optimized conditions included the encapsulation efficiency, morphology of the obtained particles, and the complexity and cost of each step of the protocol.
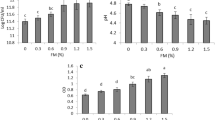
As shown in Fig. 2a, there were no significant differences between the diameters of microcapsules obtained using either olive or soybean oil (30.12 ± 10.57 µm and 29.25 ± 7.66 µm, respectively). Moreover the encapsulation efficiency in both cases was around 99.99% and the microcapsules presented spherical morphology. Soybean oil was selected as oil phase for the next assays due to its lower cost in comparison with olive oil. Then, the convenience of using raw soybean oil obtained at the laboratory instead of commercial soybean oil was evaluated (Fig. 2b). No significant differences between the diameters of microcapsules were found, with values near 29 µm in both cases. Besides, similar encapsulation efficiency (around 99.99%) and morphology—spherical particles—were found for both conditions. The next tests were performed using commercial soybean oil due to its low cost.
Diameter of microcapsules obtained through varying the source (a) and nature (b) of the oil, the concentration of SPI (c), alginate (d), and CaCl2 (e), and the stirring time (f) (gray bars). The EE(%) is represented as full circles. The results are shown as mean ± standard deviation. Different letters over bars in the same graphic indicate significant differences (p ≤ 0.05, test T)
Regarding SPI concentration (Fig. 2c), the microcapsules obtained using 100 mg/mL SPI showed significantly higher diameters (42.76 ± 19.93 µm, p ≤ 0.05) than those obtained employing 0, 30, or 60 mg/mL SPI (around 30 µm for the three concentrations). Besides, spherical particles were obtained in the four conditions assayed. Slightly lower encapsulation efficiency was observed in absence of SPI (99.69 vs. near 99.99% for the remaining conditions). Despite observing no differences among diameter, morphology, and encapsulation efficiency of particles obtained with either 30 or 60 mg/mL SPI, the latter concentration was selected for further assays to secure the protection of the encapsulated bacteria by the matrix (data not shown).
Figure 2d shows the results obtained when different concentrations of sodium alginate were tested. No significant differences were observed between the diameters of the particles, although they presented more uniformity in size when 0.2% alginate was employed. Regarding morphology, the particles were irregular in absence of the polysaccharide and spherical in presence of 0.2 and 1.0% sodium alginate. On the other hand, the encapsulation efficiency was approximately 99.99% in the three conditions. The following tests were performed using 0.2% sodium alginate because of the higher diameter uniformity of the microcapsules obtained in this condition.
No capsules were obtained when a magnetic stirrer was used, suggesting that the mixing capacity of this kind of equipment was not enough for the formation of the emulsion. Thus, an overhead stirrer was kept been used in the next tests. In another assay, stirring was maintained for 15, 30, or 60 min to form the emulsion after the addition of the aqueous phase containing bacteria and wall materials. As can be seen in Fig. 2e, and in concordance with the observed by Ma et al. (2020), the longer agitation led to microcapsules with significantly higher diameter, probably because the Tween 80 concentration used was not enough to avoid coalescing of the aqueous drops that collide in the emulsion after a long agitation. The particles were spherical for 30 and 60 min agitation and mostly oval for 15 min agitation. In all the conditions, the encapsulation efficiency was higher than 99.94%. Because of this, a 30-min agitation was selected as the best condition.
Concerning CaCl2 concentration, the capsules evidenced similar size for 0.01, 0.1, and 0.5 M CaCl2 (Fig. 2f). They were spherical, although many irregular particles were observed when 0.01 M CaCl2 was used. Moreover, the encapsulation efficiency was in the range of 99.90–99.99% for all the conditions. As consequence of more uniformity in the diameter of the microcapsules, a concentration of 0.1 M CaCl2 was established for the incoming assay.
The last condition evaluated was presence or absence of agitation after the addition of CaCl2. This step proved to be mandatory for the production of microcapsules, since there was no formation of microcapsules when it was omitted.
The final protocol for the encapsulation of L. salivarius CRL2217 with denatured SPI and sodium alginate described in the “Materials and Methods” section was defined considering the results obtained in these assays. With this optimized protocol, microcapsules with mean diameter of 30.16 ± 11.40 µm were obtained. Beads with similar size (52.57 µm) were obtained by Ma et al. (2020), who encapsulated a Lactobacillus plantarum strain using lactoprotein by the emulsion technique. However, the production of microcapsules with higher size was reported by other authors who used this technique. Ji et al. (2019) encapsulated a Bifidobacterium longum strain using sodium alginate and chitosan, obtaining round particles with mean diameters of 190 µm. Huang et al. (2021) produced 301.6-µm sodium alginate-whey protein isolate microcapsules loaded with a Limosilactobacillus reuteri strain. Sodium alginate-skim milk beads with a diameter of 325 µm containing a L. acidophilus strain were informed by My Dong et al. (2020), while Qi et al. (2019) obtained 300- to 500-µm sodium alginate particles filled with an Enterococcus faecium strain. As can be seen in Fig. 3a, the particles produced in the present work were almost spherical and uniform in size, with mean diameter of around 30 µm. The surface of the capsules presented a rough texture. Sensory evaluations of this kind of particles have reported rough and grainy texture (Ding & Shah, 2009). Nonetheless, the organoleptic properties of feeds are not affected by microcapsules with diameters smaller than 100 µm (Hansen et al., 2002). Figure 3b shows that bacteria were uniformly distributed throughout the volume of the capsules.
Survival to Gastric Digestion
The ability of probiotics to survive the gastric digestion is essential to exert their beneficial effect on the host. This is why the influence of simulated gastric digestion on the viability of free and microencapsulated L. salivarius CRL2217 was studied in this work. In the present study, the viability loss of encapsulated bacteria was significantly lower (p ≤ 0.05) than free bacteria after 1-h incubation in gastrointestinal conditions (0.18 ± 0.03 and 0.41 ± 0.06 log CFU/mL). When calcium alginate is the sole component of the wall, the microcapsules fail to provide a pronounced barrier effect at low pH because of the low density of the gel network. Thus, the porous structure of alginate microspheres permits the diffusion of acid into the particles (Shori, 2017). On the contrary, a combination of alginate and protein as wall materials results in less porous microcapsules due to crosslinking between opposite charged groups in both polymers, which confers higher protection against acidic environments (Dehkordi et al., 2020). Besides, as can be seen in Fig. 4, the physical integrity of the beads remained almost unaltered after the treatment. This agrees with other authors (Takka et al., 2010; Arora & Budhiraja, 2012), who reported that alginates do not swell at low pH, but a reversal of shrinkage takes place and the contents are not released. This was also observed by Zhang et al. (2016), who produced alginate-whey protein microcapsules loaded with lipophilic compounds and no damage on the particles after 2-h incubation in simulated gastric fluid at pH 2 was observed.
Survival and Release of Encapsulated Bacteria in Simulated Intestinal Juice
The release of L. salivarius CRL2217 from SPI-alginate microcapsules was investigated at pH 8.0 over 3 h. As determined using a Neubauer chamber, 6.22 ± 0.04 log microcapsules/mL were exposed to the simulated intestinal juice. Almost all the entrapped lactobacilli cells were released at the beginning of the treatment to give cell counts of 8.32 ± 0.03 log CFU/mL (Fig. 5). The counts of viable cells decreased slowly with time until reaching 6.16 ± 0.01 log CFU/mL after 3 h of incubation. On the contrary, a sharp decrease in counts of initially free viable cells was observed in the first hour of incubation, dropping from 8.90 ± 0.04 to 3.69 ± 0.07 log CFU/mL. The counts remained steady from that moment until the end of the test. Because of this, the viable counts of initially entrapped cells were significantly higher (p ≤ 0.05) than those of originally free bacteria from 1 h on. Similar results were informed by Wang et al. (2014), who encapsulated B. adolescentis in chickpea protein-alginate particles. They reported the release of most entrapped bacteria at the beginning of treatment with simulated intestinal juice and hypothesized that the higher viable cell count for cells released from the capsules relative to free cells may be attributed to protective effects of biopolymers free in solution (not in encapsulating form). In the present investigation, alginate and proteins from SPI in solution might exert a similar protective effect on L. salivarius CRL2217. In concordance with the viable counts observed, the treatment with simulated intestinal juice dissolved the microcapsules completely (Fig. 6a). On the other hand, no morphological differences could be noted by comparing released and undigested bacteria (Fig. 6b, d). Moreover, the released bacteria maintained their capability of binding wheat germ agglutinin throughput their surface (Fig. 6c), which indicates that they could effectively protect intestinal epithelial cells of poultry from the toxicity of this lectin.
Images of L. salivarius CRL2217 released from microcapsules after simulated intestinal digestion (a, TEM, 1000 × magnification; b, TEM, 10,000 × magnification; c, FITC-WGA bound-cells, fluorescence microscope, 1000 × magnification) and undigested L. salivarius CRL2217 (d, TEM, 10,000 × magnification)
Conclusion
In the present investigation, different parameters were optimized to microencapsulate L. salivarius CRL2217 with SPI and alginate. Thirty-micrometer spherical microcapsules were obtained using 60 mg/mL SPI and 0.2% sodium alginate. These particles effectively protected L. salivarius CRL2217 from the proteolytic activity and low pH present in the gastric environment. Besides, their content was released in contact with a simulated intestinal juice, as viable counts and binding of WGA after a simulated intestinal digestion indicated. This work paves the way for the design of a probiotic supplement for poultry including gastrointestinal digestion-susceptible bacteria.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Akter, Y., Hitchison, C., Liu, S., & O’Shea, C. J. (2017). Comparison of wheat and maize-based diets on growth performance and meat quality of broiler chickens. In 28th annual Australian poultry symposium, p. 233.
Ambrosio, C. M., Alvim, I. D., Contreras Castillo, C. J., & Da Gloria, E. M. (2020). Microencapsulation enhances the in vitro antibacterial activity of a citrus essential oil. Journal of Essential Oil Bearing Plants, 23(5), 985–997.
Arora, S., & Budhiraja, R. D. (2012). Chitosan-alginate microcapsules of amoxicillin for gastric stability and mucoadhesion. Journal of Advanced Pharmaceutical Technology & Research, 3(1), 68.
Astawan, M., & Prayudani, A. P. (2020). The overview of food technology to process soy protein isolate and its application toward food industry. World Nutrition Journal, 4(1–1), 12–17.
Attia, Y. A., Rahman, M. T., Hossain, M. J., Basiouni, S., Khafaga, A. F., Shehata, A. A., & Hafez, H. M. (2022). Poultry production and sustainability in developing countries under the COVID-19 crisis: Lessons learned. Animals, 12(5), 644.
Babot, J. D., Argañaraz-Martínez, E., Saavedra, L., Apella, M. C., & Perez Chaia, A. (2014). Selection of indigenous lactic acid bacteria to reinforce the intestinal microbiota of newly hatched chicken–Relevance of in vitro and ex vivo methods for strains characterization. Research in Veterinary Science, 97(1), 8–17.
Babot, J. D., Argañaraz-Martínez, E., Lorenzo-Pisarello, M. J., Apella, M. C., & Perez Chaia, A. (2016). Cytotoxic damage of soybean agglutinin on intestinal epithelial cells of broiler chicks: In vitro protection by Bifidobacterium infantis CRL1395. FEMS Microbiology Letters, 363(12).
Babot, J. D., Arganaraz Martinez, E., Lorenzo-Pisarello, M. J., Apella, M. C., & Perez Chaia, A. (2017). Lactic acid bacteria isolated from poultry protect the intestinal epithelial cells of chickens from in vitro wheat germ agglutinin-induced cytotoxicity. British Poultry Science, 58(1), 76–82.
Babot, J. D., Argañaraz-Martínez, E., Saavedra, L., Apella, M. C., & Perez Chaia, A. (2018). Compatibility and safety of five lectin-binding putative probiotic strains for the development of a multi-strain protective culture for poultry. Beneficial Microbes, 9(6), 927–935.
Babot, J. D., Argañaraz-Martínez, E., Quiroga, M., Grande, S. M., Apella, M. C., & Perez Chaia, A. (2021). Protection of the intestinal epithelium of poultry against deleterious effects of dietary lectins by a multi-strain bacterial supplement. Research in Veterinary Science, 135, 27–35.
Chen, X., Li, J., Essawy, H., Pizzi, A., Fredon, E., Gerardin, C., Du, G., & Zhou, X. (2022). Flame-retardant and thermally-insulating tannin and soybean protein isolate (SPI) based foams for potential applications in building materials. Construction and Building Materials, 315, 125711.
Chen, N., Zhao, M., Chassenieux, C., & Nicolai, T. (2016). Data on the characterization of native soy globulin by SDS-Page, light scattering and titration. Data in Brief, 9, 749–752.
De Man, J. C., Rogosa, D., & Sharpe, M. E. (1960). A medium for the cultivation of lactobacilli. Journal of Applied Bacteriology, 23(1), 130–135.
De Keyser, K., Kuterna, L., Kaczmarek, S., Rutkowski, A., & Vanderbeke, E. (2016). High dosing NSP enzymes for total protein and digestible amino acid reformulation in a wheat/corn/soybean meal diet in broilers. Journal of Applied Poultry Research, 25(2), 239–246.
Dehkordi, S. S., Alemzadeh, I., Vaziri, A. S., & Vossoughi, A. (2020). Optimization of alginate-whey protein isolate microcapsules for survivability and release behavior of probiotic bacteria. Applied Biochemistry and Biotechnology, 190(1), 182–196.
Ding, W. K., & Shah, N. P. (2009). An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. Journal of Food Science, 74(2), M53–M61.
El Kadri, H., Overton, T., Bakalis, S., & Gkatzionis, K. (2015). Understanding and controlling the release mechanism of Escherichia coli in double W 1/O/W 2 emulsion globules in the presence of NaCl in the W 2 phase. RSC Advances, 5(127), 105098–105110.
Gheorghita, R., Anchidin-Norocel, L., Filip, R., Dimian, M., & Covasa, M. (2021). Applications of biopolymers for drugs and probiotics delivery. Polymers, 13(16), 2729.
Gómez-Mascaraque, L. G., Tordera, F., Fabra, M. J., Martínez-Sanz, M., & Lopez-Rubio, A. (2019). Coaxial electrospraying of biopolymers as a strategy to improve protection of bioactive food ingredients. Innovative Food Science & Emerging Technologies, 51, 2–11.
Hansen, L. T., Allan-Wojtas, P. M., Jin, Y. L., & Paulson, A. T. (2002). Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiology, 19(1), 35–45.
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., Morelli, L., Canani, R. B., Flint, H. J., & Salminen, S. (2014). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11, 506–514.
Huang, X., Gänzle, M., Zhang, H., Zhao, M., Fang, Y., & Nishinari, K. (2021). Microencapsulation of probiotic lactobacilli with shellac as moisture barrier and to allow controlled release. Journal of the Science of Food and Agriculture, 101(2), 726–734.
Huq, T., Khan, A., Khan, R. A., Riedl, B., & Lacroix, M. (2013). Encapsulation of probiotic bacteria in biopolymeric system. Critical Reviews in Food Science and Nutrition, 53(9), 909–916.
Ji, R., Wu, J., Zhang, J., Wang, T., Zhang, X., Shao, L., Chen, D., & Wang, J. (2019). Extending viability of Bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Frontiers in Microbiology, 10, 1389.
Jouki, M., & Khazaei, N. (2022). Effects of active batter coatings enriched by quince seed gum and carvacrol microcapsules on oil uptake and quality loss of nugget during frying. Journal of Food Science and Technology, 59(3), 1104–1113.
Kadykalo, S., Roberts, T., Thompson, M., Wilson, J., Lang, M., & Espeisse, O. (2018). The value of anticoccidials for sustainable global poultry production. International Journal of Antimicrobial Agents, 51(3), 304–310.
Krasaekoopt, W., Bhandari, B., & Deeth, H. (2003). Evaluation of encapsulation techniques of probiotics for yoghurt. International Dairy Journal, 13(1), 3–13.
Leathem, A. J., & Brooks, S. A. (1997). Light microscopy, overview and basic methods, in: Rhodes, J.M. & Milton, J.D. (Eds) Lectin methods and protocols (Totowa, NJ, Humana Press).
Liu, H., Cui, S. W., Chen, M., Li, Y., Liang, R., Xu, F., & Zhong, F. (2019). Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Critical Reviews in Food Science and Nutrition, 59(17), 2863–2878.
Liu, H., Gong, J., Chabot, D., Miller, S. S., Cui, S. W., Ma, J., Zhong, F., & Wang, Q. (2016). Incorporation of polysaccharides into sodium caseinate-low melting point fat microparticles improves probiotic bacterial survival during simulated gastrointestinal digestion and storage. Food Hydrocolloids, 54, 328–337.
Liu, S., Zhou, R., Tian, S., & Gai, J. (2007). A study on subunit groups of soybean protein extracts under SDS-PAGE. Journal of the American Oil Chemists’ Society, 84(9), 793–801.
López, C. M., Bru, E., Vignolo, G. M., & Fadda, S. G. (2015). Identification of small peptides arising from hydrolysis of meat proteins in dry fermented sausages. Meat Science, 104, 20–29.
Ma, L., Shang, Y., Zhu, Y., Zhang, X., & E, J., Zhao, L., & Wang, J. (2020). Study on microencapsulation of Lactobacillus plantarum LIP-1 by emulsification method. Journal of Food Process Engineering, 43(8), e13437.
Mahmoud, M., Abdallah, N. A., El-Shafei, K., Tawfik, N. F., & El-Sayed, H. S. (2020). Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon, 6(3), e03541.
My Dong, L., Le Quyen, T. H., Duc Thang, T., Thuy, T. K., & D. (2020). The effects of extrusion and internal emulsion microencapsulation methods on the viability of Lactobacillus acidophilus. Journal of Human Environment and Health Promotion, 6(1), 1–5.
Qi, W., Liang, X., Yun, T., & Guo, W. (2019). Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. Journal of Food Science and Technology, 56(3), 1398–1404.
Reineccius, G. A. (2004). The spray drying of food flavors. Drying Technology, 22(6), 1289–1324.
Reque, P. M., & Brandelli, A. (2021). Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends in Food Science & Technology, 114, 1–10.
Sarao, L. K., & Arora, M. (2017). Probiotics, prebiotics, and microencapsulation: A review. Critical Reviews in Food Science and Nutrition, 57(2), 344–371.
Shori, A. B. (2017). Microencapsulation improved probiotics survival during gastric transit. HAYATI Journal of Biosciences, 24(1), 1–5.
Takka, S., & Gürel, A. (2010). Evaluation of chitosan/alginate beads using experimental design: Formulation and in vitro characterization. An Official Journal of the American Association of Pharmaceutical Scientists, 11(1), 460–466.
Tang, C. H., & Li, X. R. (2013). Microencapsulation properties of soy protein isolate and storage stability of the correspondingly spray-dried emulsions. Food Research International, 52(1), 419–428.
Tansaz, S., & Boccaccini, A. R. (2016). Biomedical applications of soy protein: A brief overview. Journal of Biomedical Materials Research Part A, 104(2), 553–569.
Teng, Z., Luo, Y., & Wang, Q. (2012). Nanoparticles synthesized from soy protein: Preparation, characterization, and application for nutraceutical encapsulation. Journal of Agricultural and Food Chemistry, 60(10), 2712–2720.
Verni, M., Rizzello, C. G., & Coda, R. (2019). Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Frontiers in Nutrition, 6, 42.
Wang, J., Korber, D. R., Low, N. H., & Nickerson, M. T. (2014). Entrapment, survival and release of Bifidobacterium adolescentis within chickpea protein-based microcapsules. Food Research International, 55, 20–27.
Zhang, J., Liang, L., Tian, Z., Chen, L., & Subirade, M. (2012). Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study. Food Chemistry, 133(2), 390–399.
Zhang, Y., Wang, Q. C., Yu, H., Zhu, J., de Lange, K., Yin, Y., Wang, Q., & Gong, J. (2016). Evaluation of alginate–whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. Journal of the Science of Food and Agriculture, 96(8), 2674–2681.
Acknowledgements
We sincerely thank Lic. Mabel Taljuk for her assistance in the search of bibliography.
Funding
This research was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) [grant number PICT 2016 Nº 0528], Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) [grants number PIP 678, P-UE 2017 N° 035] and Consejo de Investigaciones de la Universidad Nacional de Tucumán [grant number PIUNT D643/1].
Author information
Authors and Affiliations
Contributions
Jaime Daniel Babot: data collection and analysis, manuscript writing. Eloy Argañaraz-Martínez: data analysis, manuscript review. María Cristina Apella: manuscript review, funding acquisition. Adriana Perez Chaia: manuscript review, supervision, funding acquisition.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babot, J.D., Argañaraz-Martínez, E., Apella, M.C. et al. Microencapsulation of Probiotics with Soy Protein Isolate and Alginate for the Poultry Industry. Food Bioprocess Technol 16, 1478–1487 (2023). https://doi.org/10.1007/s11947-023-03007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03007-2