Abstract
In this study, bionanocomposite coating solutions were created using polyvinyl alcohol (PVA) and chitosan (Cs), with different concentrations of cinnamon essential oil in nanoemulsion (n-CEO; 0%, 5%, 10%, and 20%) and TiO2 nanoparticles (TiO2-NPs). The bionanocomposite was characterized using Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy with EDX, and mechanical and barrier property assessment. Additionally, antimicrobial and antioxidant properties and total phenols were evaluated. Generally, mechanical and barrier properties were enhanced with increasing n-CEO concentrations with a favorable distribution in film matrix. Moreover, total phenols, antioxidant, and antimicrobial activities were also enhanced a broader inhibition pattern against A. flavus, gram-positive, and gram-negative bacteria. The influence of n-CEO and TiO2-NPs blended into bionanocomposite on preservation of fresh chicken breast fillets during 21 days of refrigeration was evaluated. Added n-CEO concentration, especially 20%, and TiO2-NPs enhanced antimicrobial properties and extended preservation time up to 14 days compared to uncoated samples. Furthermore, weight loss was decreased during storage of coated samples. Thus, PVA/Cs/TiO2–NPs with n-CEO bionanocomposites may be useful as a coating for chicken breast fillets to control microbial growth and reduce weight loss during cold storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active packaging can improve durability, safety, quality, and sensory properties of foodstuffs. Packaging materials are typically dependent on petroleum-based materials. Nowadays, the global environment faces serious challenges. For example, materials may accumulate in the environment and require several hundred years to degrade. Furthermore, many materials are expensive and require substantial energy to produce. Thus, researchers worldwide currently focus on solutions to issues of environmental protection and lowering costs and energy requirements. One promising solution is to exploit biodegradable polymers from natural sources that readily decompose into less hazardous products (Youssef et al., 2019; Qasim et al., 2021). Chitosan (Cs) is an example of a biodegradable polysaccharide composed of glucosamine and N-acetyl glucosamine units connected with β-1-4-glycosidic linkages. Cs is degraded by enzymes in humans into nontoxic products (Crini & Badot, 2008; El-Mohamedy et al., 2019). Furthermore, Cs is a possible polymeric material for food packaging applications, especially in edible coatings; Cs efficiently forms films and is frequently blended with other polymers or cross-linked to improve functional properties. Inter- or intramolecular cross-linking is achieved through reactive amino and hydroxyl functional groups (Wang et al., 2018; Herrera et al., 2018).
Polyvinyl alcohol (PVA) is a multiscale polymer with many industrial applications. This polymer may be the only synthesized polymer with a backbone mainly composed of carbon atoms that is entirely biodegradable. PVA exhibits ideal mechanical properties despite its synthetic nature (Matsumura et al., 1999). It shows a planar zigzag structure that permits efficient packing of polymer chains in neat blended form. PVC displays elevated polarity and high water solubility (Pal et al., 2007).
Some nanomaterials of metal oxide are effective antimicrobial agents. They inhibit bacterial growth by interacting with cellular membranes, generating reactive oxygen species, disrupting protein structure, and inhibiting DNA replication and enzymatic reactions. TiO2-NPs are an interesting nanomaterial widely used in active packaging (Azizi-Lalabadi et al., 2020; Youssef et al., 2022).
Essential oils (EOs) are natural compounds and promising candidates for food applications. These oils are potent antifungal, antiviral, antioxidant, and antibacterial agents (Paudel et al., 2019; Ferreira et al., 2010; Giatrakou et al., 2010). It can readily dissolve cytoplasmic membranes of bacterial cells in the hydrophobic domain. Cinnamon is a spice from the inner bark of tree species in the genus Cinnamomum. Cinnamon essential oil (CEO) contains two important compounds, cinnamaldehyde and eugenol, that are effective inhibitors of a broad spectrum of microbes, making it ideal for a variety of products. CEO is an alternative natural and safe antimicrobial to standard antimicrobial agents (Ghosh et al., 2014). However, the application of crude cinnamon oil is limited due to lipophilicity and associated insolubility in water, along with a high minimum inhibitory concentration (MIC) (Donsì et al., 2012). One strategy for dealing with hydrophobic compounds is use of a nanoemulsion delivery system (Shah et al., 2012). Nanoemulsions are colloidal solutions of two immiscible phases, either oil-in-water or water-in-oil, with an emulsifier, such as tween 20 or tween 80, and droplet sizes between 20 and 300 nm (Yildirim et al., 2017; Anton & Vandamme, 2009). Fabrication of nanoemulsions is achieved with high- and low-energy methods (Acosta, 2009). Radi et al. (2022) illustrated that the CEO loaded in the nanostructured lipid carriers has a good effect against P. Citrinum and P. Expansum. Tiwari et al. (2022) used chitosan nanoemulsion encapsulating cinnamomum glaucescens essential oil (CGEO) as a green preservative against fungi.
The preserving of chicken breast fillets can be carried out by various methods such as UV radiation (Park & Ha, 2015), extracts of garlic (Diao et al., 2020) or basil (Tsiraki & Savvaidis, 2013), and polymer nanocomposites as Cs/ZnO-NPs (Amjadi et al., 2019) or PVA/Ag-NPs (Azlin-Hasim et al., 2016) to protect them from bacteria and fungi and increase the shelf life time.
Herein, the cinnamon essential oil was successfully prepared in nanoemulsion form (n-CEO). In addition, Cs/PVA/TiO2/n-CEO bionanocomposite was prepared as a coating film for preserving fresh chicken breast fillets to improve their resistant to microbial and fungi to increasing their shelf life time. Furthermore, the morphology and thermal and barrier properties of the bionanocomposites films were investigated. Also, the microbiological changes and the weight loss of chicken meat coated were illustrated.
Materials and Methods
Materials
Chitosan (Cs, high molecular weight, Mwt ≈ 310,000 to 375,000, deacetylation ≈ 90 %), polyvinyl alcohol (PVA; Mwt = 30,000), and TiO2-NPs (99.5% and particle size > 40 nm) were purchased from Sigma-Aldrich. Acetic acid (AcOH) and glycerol were obtained from Elnasr Pharmaceutical Chemicals Co. Extra-pure citric acid (CA) and Tween 20 were obtained from SDFCL, India. CEO was from the Oil Recovery unit, National Research Centre. All chemicals were used in analysis grade without any purification required. Fresh chicken breast fillets were obtained from a local market in Giza Governorate, Egypt. Fillet samples were washed with freshwater, dried, and stored under refrigeration.
Methods
Preparation of Cinnamon Essential Oil Nanoemulsion (n-CEO)
Oil-in-water nanoemulsion was prepared using CEO (8 g), Tween 20 (nonionic surfactant), and distilled H2O (92 g). Initially, the coarse emulsion was formed by adding water to the organic phase containing oil and surfactant in a ratio of 1:4 using a magnetic stirrer at 400 rpm for 5 min and then heating at 70 °C for 5 h with stirring. The mixture was then placed in a Daihan Scientific Homogenizer at 25,000 rpm for 5 min.
Preparation of Bionanocomposite (Cs/PVA/TiO2/n-CEO)
Bionanocomposites were prepared first by mixing Cs with AcOH solution (2% V) with stirring at 50 °C to form a homogeneous viscous solution (2% wt). In a separate beaker, PVA (3 g) was dissolved in 100 ml distilled H2O at 70 °C. The combination of Cs/PVA was obtained by mixing polymer solutions (40%:60% wt/wt). Separately, CA (30% wt/wt of polymer blend) was sonicated with TiO2-NPS (1% wt) to disperse nanomaterials. Next, the CA/TiO2-NPs mixture was added to the polymer blend with continuous mechanical stirring. The mixture was cured at 140 °C for 10 min to form a cross-linked blend. The solution was left to cool to room temperature and mixed with glycerol (25% wt/wt) and cinnamon oil nanoemulsion in ratios of 5%, 10%, and 20% v/v with the polymer blend. The obtained suspension was poured into transparent rounded glass Petri dishes and left at room temperature to allow evaporation of solvent and film formation (Table 1).
Coating Chicken Fillets
Chicken breast fillets were distributed into six groups. The first group was control without coating, fillets in the second and third groups were coated with Cs/PVA (B1) and Cs/PVA/TiO2-NPs (B2), respectively, and fillets in the fourth, fifth, and sixth groups were coated with Cs/PVA/TiO2-NPs bionanocomposite films that contain 5%, 10%, and 20% n-CEO, respectively. All chicken fillets were stored at 4 °C ± 1 °C for 21 days. Microbiological quality was monitored to assess general quality.
Characterizations
Fourier Transform Infrared (FTIR)
The transmission FTIR spectrum was measured for the thin film of bionanocomposite in the range 500–4000 cm−1 using a FTIR-8400S (Shimadzu Europe Co., Kyoto, Japan).
X-ray Diffractometer
XRD patterns of bionanocomposite films were obtained using X-ray diffractometer (Diano Co., USA) with a CoKα radiation source at 45 kV and a Philips X-ray diffractometer (PW1930 generator, PW 1820 goniometer) with a CuKα radiation source (ƛ = 0.15418 nm). The lattice d-spacing was calculated from the diffraction patterns via Bragg’s equation (n λ=2d sin θ).
Transmission Electron Microscope (TEM)
The morphology and size of n-CEO droplets were demonstrated using TEM (JEM-1230, Japan; 120 kV, magnification of 600,000, and a resolution is 0.2 nm). A drop of n-CEO was placed on a carbon-coated copper grid and then left to dry in the air before insert into a TEM device.
Dynamic Light Scattering (DLS)
Mean droplet size and size distribution of n-CEO were determined by DLS with a Zeta sizer (Nano ZS model ZEN 3600, Malvern, UK) at a fixed scattered angle of 173° and a temperature of 25 °C.
Scanning Electron Microscopy with a Dispersive Energy Spectrometer (SEM-EDX)
The morphology of films was viewed with SEM-EDX (JSM 6360LV, JEOL/Noran, USA). Before examination, samples were sputter-coated with a fine layer of gold at a low deposition rate, refrigerated, and placed at the maximum distance from the target to prevent damage. Images were obtained using an accelerating voltage of 10–15 kV.
The Thermal Gravimetric Analysis (TGA)
TGA was carried out by using a PerkinElmer TGA7 apparatus (PerkinElmer, Waltham, MA, USA) under nitrogen gas with a flow of 30 ml/min, a heating rate of 10 °C/min, and a temperature range of 25–600 °C.
Barrier Properties
Water vapor transmission rate (WVTR, GBPI Co., China) was measured using the cup method with GBPI W303-B Water Vapor Permeability Analyzer. WVTR was calculated as the amount of water vapor that permeates the unit area per unit time under specific humidity (4–10%) and temperature (38 °C) conditions. Assessments followed standards JIS Z0208, 53122-1, TAPPI T464, ASTM D1653, ISO 2528, and ASTM E96. Oxygen transmission rate (OTR) was measured with an N530-B Gas Permeability Analyzer (GBPI Co., China), following ASTM D1434-82 (2003) and ISO2556-2001.
Determination of Antioxidant Activity
Antioxidant activity was assessed by the 2, 2-diphenyl-1-picrylhydrazil (DPPH) radical scavenging as described by Moustafa et al. (2021)
Determination of the Total Phenolic Content
Total phenolic content in CEO and Cs/PVA/TiO2/n-CEO bionanocomposite films was evaluated colorimetrically with a Folin-Ciocalteu reagent as definite by Lafka et al. (2007). Total phenolic content was calculated using a gallic acid standard curve and reported as mg gallic acid equivalent (GAE/g).
Pathogenic Strains
Pathogenic bacterial strains (Bacillus cereus B-3711; Staphylococcus aureus ATCC 6538; Listeria monocytogenes 598; Escherichia coli ATCC 8739; Pseudomonas aeruginosa ATCC 15442; Salmonella typhmirum14028s; Candida albicans ATCC 10231) were obtained from the Dairy Department at National Research Centre, Egypt. Tested strains were incubated in nutrient broth at 35 °C/24h and diluted with saline solution (0.9% NaCl, w/v) to ~1×105 CFU/ml before assessing antimicrobial properties of coating solutions.
Antimicrobial Activity of Coating Emulsion Cs/PVA/TiO2 with Different Concentrations of n-CEO
Antimicrobial activity was established with nutrient agar and a well-diffusion method, according to Youssef et al. (2020). One-tenth milliliter of suspensions of test strains were spread on nutrient agar medium and allowed to sit for 2 h to saturate the medium with pathogens. Wells were cut in the agar layer with a 0.5-cm cork borer. Each well was filled with 100 µl of a different coating solution. Diameters of clear inhibition zones surrounding wells were measured after incubation for 24 h at 37 °C. n-CEO was also examined for antimicrobial activity in the same manner. The antimicrobial activity test was carried out in triplicate and mean values with standard error (SE).
Microbiological Evaluation of Uncoated and Coated Chicken Meat During Cold Storage
Samples for microbial analysis were assessed after dipping chicken fillets in coating solutions and storing them in the cold for 21 days. At each sampling interval, 10 g of fillet was mixed well in sterile saline solution for 5 min to prepare an initial dilution (10−1). Subsequent dilutions (10−1 to 10−8) were prepared in test tubes. Suitable serial dilutions were plated on plate count agar medium (Sigma) with incubation at 35 °C for 48 h and colonies counted to assess total aerobic bacterial CFU. Total anaerobic bacterial CFU was assessed similarly using plate count agar medium but incubated anaerobically at 35 °C for 48 h (Wehr et al., 2004). Violet red bile glucose agar medium (Sigma) was used assessing coliforms with incubation at 37 °C for 24 h (Omar & Wilson, 2002). Staphylococcus aureus was evaluated by spreading 0.1 ml of a suitable dilution on Baird–Parker agar medium (Sigma) supplemented with egg yolk and potassium tellurite solution with incubation at 35 °C for 48 h (Omar & Wilson, 2002). Listeria counts were estimated by spreading 0.1 ml of a suitable dilution using Listeria on Oxford agar medium with Listeria supplement (Oxford) with incubation at 37 °C for 48 h (Heisick et al., 1995)). Mold and yeast counts were assessed by spreading 0.1 ml of a suitable dilution on rose bengal chloramphenicol agar (Sigma) plates with aerobic incubation at 25 °C for 4 days (Bengal, 1995). Finally, psychrotrophic bacteria were detected by plate count agar medium (Sigma) with incubation at 5 °C for 7 days. Microbial colonies were counted and expressed as log CFU/g (Omar & Wilson, 2002). All microbiological evaluation was done using independent three replicates from each uncoated and coated chicken meat samples, and the results of the microbial counts were determined as the average of three replicate values with standard deviation (SD).
Weight Loss of Chicken Fillets
All chicken fillet samples were separately weighted initially and during storage. Weight loss (WL) of fillets was calculated as:
where Wf is the first weight and Wt is the weight at time t.
Results and Discussion
Characterizations
The particle size distribution and the morphology of cinnamon essential oil nanoemulsion (n-CEO) are illustrated in Fig. 1. TEM images of n-CEO showed spherical particles with irregular sizes from 100 to 300 nm (Fig. 1a). In addition, DLS illustrated that the average particle size of n-CEO was 152 ± 79 nm (Fig. 1b).
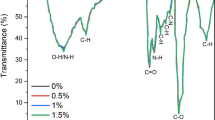
The FT-IR pattern of Cs exhibited a broad absorption band at 3200–3600 cm−1 related to −OH contribution and −NH stretching vibration (Fig. 2a). This peak shifted to 3100–3400 cm−1 for biocomposites due to interaction between amino groups of Cs and hydroxyl groups of PVA. Furthermore, the C=O group of the amide of Cs was stretched at 1650 cm−1, which shifted to 1730–1750 cm−1 in biocomposites. This shift may be due to intra-/inter-hydrogen bonds formed between amino and carbonyl groups of Cs chains. The effect of hydrogen bonds for biocomposites decreased because amino groups of Cs were involved in the formation of Cs/PVA complexes.
Furthermore, bands at 1152 and 1075 cm−1 are related to C–O–C symmetric stretching and C–O stretching, respectively (Mahmoud et al., 2018). TiO2-NPs showed an absorption peak in the fingerprint region below a wavelength of 1000 nm at about 650 cm−1 for Cs/PVA/TiO2 and Cs/PVA/TiO2-20% n-CEO bionanocomposites.
The XRD pattern of TiO2-NPs fit well with reference anatase TiO2 (JCPDS No. 21-1272), with the main peak at 2ϴ ≈ 25.21° corresponding to 101 and other peaks at 2ϴ ≈ 37.28°, 47.99°, 53.95°, 62.65°, and 75.38° corresponding to 004, 200, 105, 204, and 215 planes, respectively. Cs showed a diffraction peak at 2θ ≈ 20.9° ascribed to reflection plane 220); PVA shows typical peaks at 2θ = 19.6° and 40.8° of 101 and 102 planes of PVA. Additionally, TiO2 shows a small hump at 25.21° in bionanocomposite spectra (Fig. 2b) (Chen et al., 2018; Aziz et al., 2017).
The surface morphology of the prepared bionanocomposite film as well as their corresponding EDX obtained from Cs-PVA (B1), Cs/PVA/TiO2 (B2), Cs/PVA/TiO2-5%n-CEO (C1), and Cs/PVA/TiO2-20%n-CEO (C3) are illustrated in Fig. 3. The SEM images of samples B1 and B2 showed high compatibility between Cs and PVA; in addition, sample C1 showed good distribution of the n-CEO in the matrix, while sample C3 showed aggregation of n-CEO as its content become 20%. The elemental analysis of sample B1 illustrated that it consists mainly of carbon, oxygen, and nitrogen atoms of about 39%, 47%, and 12%, respectively. This can be detected by the presence of the peaks in the EDX analyses less than 1 keV which is concerned with the K-shell electrons of carbon, oxygen, and nitrogen atoms. The addition of the TiO2-NPs to bionanocomposites (B2, C1, and C3) leads to the appearance of a new beak at 4.5 keV which is concerned with the titanium atom.
The chemical component and content of inorganic materials are the most significant factors that affect the thermal behavior of nanocomposites (Doh et al., 2005). The thermal degradation analyses were carried out to study the thermal properties of the prepared samples (Fig. 4). TGA curves showed a four-step WL profile. The first step exhibited mass loss at temperatures below 120 °C that may be due to loss of absorbed water; all samples lost 6–9% of their weight. The second step was a WL of about 20% at temperatures up to 240 °C attributed to the deacetylation of Cs and dehydration of PVA through removal of fragments such as CH2–OH and OH groups, respectively. The third step occurred at a peak in the range of 344 °C due to decomposition of glycerol. The final step was degradation of the main backbone of polymer chains at temperatures of 420 to 500 °C. The inclusion of CEO in Cs-PVA-TiO2-20% n-CEO caused a shift in the DTG curve to 403 °C.
Oxygen and water vapor transmission rates (OTR and WVTR) are critical aspects for preparation of packaging materials. Nanomaterials create an impediment in polymer films for diffusion of gases and water molecules and enhance barrier properties of nanocomposites. Table 2 demonstrated the WVTR for the fabricated bionanocomposite films. Composite films display low WVTR compared to B1 and B2. Increasing n-CEO loading in bionanocomposites thus produces a significant drop in water transmissibility. Permeation of water vapors reflects the number of transmission phases in which water first liquefies and condenses on nanocomposite film surfaces, followed by liquid water diffusion across the film, and then evaporation from the opposite side of the film. The presence of n-CEO enhances hydrophobicity of C2 and C3 bionanocomposites (Dirim et al., 2004; Polat et al., 2018; Son et al., 2021).
An increase in OTR was seen after introducing n-CEO into the B2 matrix (Table 2). The OTR for B2 was 29.38 cc/M2-day, which increased to 39.98, 40.04, and 42.79 (cc/M2-day) as the proportion of n-CEO increased to 5%, 10%, and 20%, respectively. Bionanocomposites C1, C2, and C3 thus become suitable packaging materials for food products.
Antioxidant Properties and Total Phenol
Bionanocomposite films based on Cs/PVA (B1) and Cs/PVA/TiO2 (B2) exhibited the lowest antioxidant activity and total phenolic content (Table 3). The addition of 5–20% n-CEO increased DPPH scavenging by C1, C2, and C3 progressively to 53.36%, 63.59%, and 67.06%, respectively. Likewise, total phenolic increased to 75.00, 91.46, and 123.25 mg/g. The impact of n-CEO reflects its volatile compound content. DPPH scavenging and phenolic content of n-CEO were 78.89% and 172.75 mg/g oil, respectively. These values are likely associated with bioactive compounds, such as benzaldehyde, cinnamic acid, catechin, caffeic acid, and cinnamyl aldehydes (El-Sayed & Youssef, 2019; Andrade et al., 2016). Thus, cinnamon shows significant potential for novel applications in food packaging with health benefits for consumers.
Antimicrobial Properties of Coatings
Overall, the antimicrobial activity of composites was variable among tested strains (Table 4). Composites with n-CEO showed broader inhibition against A. flavus and gram-positive and gram-negative strains. Diameters of inhibition zones ranged between 14 and 25 mm. Antimicrobial effect was enhanced with n-CEO integrated with CH/PVA/TiO2 due to TiO2-NPs (Youssef et al., 2015; Apjok et al., 2019). The blank bionanocomposite coating showed inhibition zones from 8 to 14 mm. Inhibition was increasingly enhanced as n-CEO content increased. With 5% n-CEO, the zone ranged from 13 to 18 mm depending on strain, and A. flavus, S. aureus, and P. aeruginosa proved more sensitive. Inhibition significantly increased with bionanocomposites containing 10% n-CEO, ranging between 15 and 23 mm, and increased further at 20% n-CEO, 19–29 mm. A. flavus, gram-positive bacteria, and P. aeruginosa were more sensitive to bionanocomposite coatings with n-CEO.
The CH/PVA bionanocomposite coating did not inhibit growth of tested stains. Chitosan in these coating solutions displays weak antimicrobial activity and was added in smaller proportions (Goy et al., 2009; Yilmaz Atay, 2019). Our funding are consistent with El Atki et al. (2019) and Unlu et al. (2010), who observed that antimicrobial activity in cinnamon may be associated with trans-cinnamaldehyde. Raeisi et al. (2015) showed that gram-negative bacteria were more resistant than gram-positive organisms to EO due to differences in membrane structure. Direct contact between hydrophobic components and the phospholipid layer of the cell membrane occurs in gram-positive bacteria because of the lack of an outer phospholipid membrane. This structure allows greater ion permeation.
The Effect of Coating Types on the WL of Chicken
Generally, a continuous WL was seen for all tested samples during storage, especially after 14 days of cold storage; however, greater WL was noted for uncoated chicken (control) on day 21 (4.08% ± 1.24%), mainly attributed to moisture loss (Fig. 5). Mir et al. (2017) reported that the water-holding capacity of chicken breast declines quickly as proteins in muscle fiber collapse. Less WL was observed for fillets coated with bionanocomposites combined with n-CEO. This decrease in WL was most prominent for coatings with 20% n-CEO on day 21 of storage. Loss varied as 1.37% ± 0.23%, 1.51% ± 0.25%, 1.84% ± 0.23%, 2.03% ± 0.24%, and 2.67% ± 0.29%, respectively, for C3, C2, C1, B2, and B1, respectively (Fig. 5). Less WL in fillets coated with composites with a high concentration of n-CEO content can be explained by their more effective barrier to water permeation. Similar findings were observed by El-Sayed et al. (2020) who combined bionanocomposite films with Roselle extract and found that WVTR decreased.
Microbiological Changes of Chicken Meat Coated with Different Coating During Cold Storage
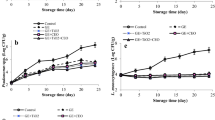
The initial bacterial coliform count for fillets was 1.30 log CFU/g. Numbers of bacterial coliforms increased with storage time in uncoated samples and samples coated with B1 and B2. However, coliforms were not detected on samples coated with C1, C2, and C3 on day 7 of storage. Also, a significant difference was observed between uncoated fillets (7.12 log CFU/g) and fillets coated with C1, C2, and C3 (3.20, 2.88, and 1.98 log CFU/g, respectively) after 21 days of storage. Coliform counts were 6.80 and 4.49 log CFU/g, respectively, for samples coated with B1 and B2, indicating that added TiO2 enhanced antimicrobial activity (Youssef et al., 2018). Incorporating n-CEO extended the shelf life of chicken fillets over 21 days of cold storage, and this effect reflects its antimicrobial activity, as confirmed in the present findings and other researchers (Klein et al., 2013; Nabavi et al., 2015; Aminzare et al., 2018).
Furthermore, total bacterial counts from fillets coated with C1, C2, and C3 showed reduced microbial counts compared with samples coated with B1 during storage (Fig. 6b). Total bacterial count on the first day was 3.97 log CFU/g and gradually increased during storage in controls and samples coated with B1. In contrast, samples coated with C1, C2, and C3 decreased to 3.00, 2.95, and 2.89 log CFU/g, respectively, at day 7. Counts then increased to 7.88, 5.70, 5.22, 4.75, and 4.20 for chicken fillets coated with B1, C1, C2, and C3, respectively, after 21 days of storage. Uncoated samples showed 8.30 log CFU/g. Also, bacterial counts for C2 and C3 stayed within acceptable levels of 6 log CFU/g (Foods ICoMSf, 1996). Thus, coating fillets with C3 significantly increased shelf life of fillets by more than 14 days for storage at 4 °C.
Anaerobic bacterial counts during storage showed initial levels of 2.50 log CFU/g. These counts rose significantly with increased storage time (Fig. 6c). n-CEO-containing coating materials exhibited significant antimicrobial activity, and counts from samples coated with C1, C2, and C3 were 3.80, 3.59, and 3.30 log CFU/g, respectively, in comparison with uncoated samples, 7.00 log CFU/g, at day 21 of storage. Additionally, TiO2 in coating materials enhanced antimicrobial effects as evidenced by anaerobic bacteria in samples coated with B2, 4.96 log CFU/g, at day 21 of storage in comparison with 6.25 log CFU/g for B1.
The same trend was observed for S. aureus. Counts were initially 1.50 log CFU/g and gradually increased during storage in uncoated samples and samples coated with B1 and B2. Counts reached 5.84, 5.00, and 4.60 log CFU/g, respectively, after 21 days of storage (Fig. 6d). Also, S. aureus were not observed in samples coated with C1, C2, and C3 at day 7 and during the remaining storage time.
Listeria was not detected initially or after 21 days of storage for samples coated with C1, C2, and C3. Some colonies were found in uncoated samples and samples coated with B1 and B2 at day 14. At day 21 of storage, counts increased to 2.34, 2.04, and 1.90 log CFU/g, respectively (Fig. 6e).
Coating and storage times had a significant effect on psychrotrophic counts in chicken fillets (Fig. 6f). Initial psychrotrophic count was 3.05 log CFU/g and increased in uncoated samples and samples coated with B1 and B2 only. The lowest numbers of psychrotrophic bacteria during storage were observed in samples coated with C1, C2, and C3—4.60, 3.85, and 30.16 log CFU/g, respectively—at day 21 of storage. Uncoated fillets reported 7.20 log CFU/g. Coating with n-CEO augments preservation of fillets due to a reduction in oxygen exchange. Pseudomonas species are obligate aerobes unable to survive without oxygen (Alghooneh et al., 2015; Behbahani et al., 2017).
Finally, in the first week, all samples were free from mold and yeast. Low counts, 3.15 log CFU/g, were found in uncoated samples and increased to 5.70 log CFU/g on day 21. Also, 3.00 and 2.25 log CFU/g were seen in samples coated with B1 and B2 at 14 days, respectively, which increased to 5.00 and 3.80 log CFU/g, respectively, at the end of storage. Samples coated with C1, C2, and C3 were mold and yeast free during storage time.
Conclusions
A novel bionanocomposite coating based on polyvinyl alcohol, chitosan, TiO2 nanoparticles, and cinnamon essential oil in nanoemulsion form (n-CEO) were fabricated. A nanoemulsion of CEO was prepared with an average particle size of 152 nm. The bionanocomposite showed high content of antioxidants and phenolic compounds. Also, it was an effective antimicrobial against A. flavus and gram-positive and gram-negative bacteria. The presence of n-CEO in the bionanocomposite reduced oxygen and water permeability, suggesting utility for coating foodstuffs. WL of fillets decreased when coated with the bionanocomposite in comparison with uncoated fillets and fillets coated with Cs/PVA or Cs/PVA/TiO2-NPs.
Data Availability
Not applicable.
References
Acosta, E. (2009). Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid and Interface Science, 14(1), 3–15.
Alghooneh, A., Behbahani, B. A., Noorbakhsh, H., & Yazdi, F. T. (2015). Application of intelligent modeling to predict the population dynamics of Pseudomonas aeruginosa in Frankfurter sausage containing Satureja bachtiarica extracts. Microbial Pathogenesis, 85, 58–65.
Aminzare, M., Tajik, H., Aliakbarlu, J., Hashemi, M., & Raeisi, M. (2018). Effect of cinnamon essential oil and grape seed extract as functional-natural additives in the production of cooked sausage-impact on microbiological, physicochemical, lipid oxidation and sensory aspects, and fate of inoculated Clostridium perfringens. Journal of Food Safety, 38(4), e12459
Amjadi, S., Emaminia, S., Nazari, M., Davudian, S. H., Roufegarinejad, L., & Hamishehkar, H. (2019). Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food and Bioprocess Technology, 12(7), 1205–1219.
Andrade, W. A., Firon, A., Schmidt, T., Hornung, V., Fitzgerald, K. A., Kurt-Jones, E. A., Trieu-Cuot, P., Golenbock, D. T., & Kaminski, P.-A. (2016). Group B Streptococcus degrades cyclic-di-AMP to modulate STING-dependent type I interferon production. Cell Host and Microbe, 20(1), 49–59.
Anton, N., & Vandamme, T. F. (2009). The universality of low-energy nano-emulsification. International Journal of Pharmaceutics, 377(1–2), 142–147.
Apjok, R., Mihaly Cozmuta, A., Peter, A., Mihaly Cozmuta, L., Nicula, C., Baia, M., & Vulpoi, A. (2019). Active packaging based on cellulose-chitosan-Ag/TiO2 nanocomposite for storage of clarified butter. Cellulose, 26(3), 1923–1946.
Aziz, S. B., Abdulwahid, R. T., Rasheed, M. A., Abdullah, O. G., & Ahmed, H. M. (2017). Polymer blending as a novel approach for tuning the SPR peaks of silver nanoparticles. Polymers, 9(10), 486.
Azizi-Lalabadi, M., Ehsani, A., Ghanbarzadeh, B., & Divband, B. (2020). Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. International Journal of Food Microbiology, 312, 108375
Azlin-Hasim, S., Cruz-Romero, M. C., Morris, M. A., Padmanabhan, S. C., Cummins, E., & Kerry, J. P. (2016). The potential application of antimicrobial silver polyvinyl chloride nanocomposite films to extend the shelf-life of chicken breast fillets. Food and Bioprocess Technology, 9(10), 1661–1673.
Behbahani, B. A., Shahidi, F., Yazdi, F. T., Mortazavi, S. A., & Mohebbi, M. (2017). Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. International Journal of Biological Macromolecules, 94, 515–526.
Bengal R (1995) Rose Bengal chloramphenicol (RBC) agar. In: Corry JEL, Curtis GDW & Baird RM (eds) Progress in Industrial Microbiology, 34, 431–433. Elsevier.
Chen, Y.-N., Jiao, C., Zhao, Y., Zhang, J., & Wang, H. (2018). Self-assembled polyvinyl alcohol–tannic acid hydrogels with diverse microstructures and good mechanical properties. ACS Omega, 3(9), 11788–11795.
Crini, G., & Badot, P.-M. (2008). Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Progress in Polymer Science, 33(4), 399–447.
Diao, X., Huan, Y., & Chitrakar, B. (2020). Extending the shelf life of ready-to-eat spiced chicken meat: Garlic aqueous extracts-carboxymethyl chitosan ultrasonicated coating solution. Food and Bioprocess Technology, 13(5), 786–796.
Dirim, S. N., Özden, H. Ö., Bayındırlı, A., & Esin, A. (2004). Modification of water vapour transfer rate of low density polyethylene films for food packaging. Journal of Food Engineering, 63(1), 9–13.
Doh, G.-H., Lee, S.-Y., Kang, I.-A., & Kong, Y.-T. (2005). Thermal behavior of liquefied wood polymer composites (LWPC). Composite Structures, 68(1), 103–108.
Donsì, F., Annunziata, M., Vincensi, M., & Ferrari, G. (2012). Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. Journal of Biotechnology, 159(4), 342–350.
El-Mohamedy, R., Abd El-Aziz, M. E., & Kamel, S. (2019). Antifungal activity of chitosan nanoparticles against some plant pathogenic fungi in vitro. Agricultural Engineering International: CIGR Journal, 21(4), 201–209.
El-Sayed, S. M., El-Sayed, H. S., Ibrahim, O. A., & Youssef, A. M. (2020). Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohydrate Polymers, 239, 116234
El-Sayed, S. M., & Youssef, A. M. (2019). Potential application of herbs and spices and their effects in functional dairy products. Heliyon, 5(6), e01989
El Atki, Y., Aouam, I., El Kamari, F., Taroq, A., Nayme, K., Timinouni, M., Lyoussi, B., & Abdellaoui, A. (2019). Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. Journal of Advanced Pharmaceutical Technology & Research, 10(2), 63.
Ferreira, J., Alves, D., Neves, O., Silva, J., Gibbs, P., & Teixeira, P. (2010). Effects of the components of two antimicrobial emulsions on food-borne pathogens. Food Control, 21(3), 227–230.
Foods ICoMSf. (1996). Microorganisms in foods 5: Characteristics of microbial pathogens, vol 5. Springer Science & Business Media.
Ghosh, V., Mukherjee, A., & Chandrasekaran, N. (2014). Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids and Surfaces B: Biointerfaces, 114, 392–397.
Giatrakou, V., Ntzimani, A., & Savvaidis, I. (2010). Effect of chitosan and thyme oil on a ready to cook chicken product. Food Microbiology, 27(1), 132–136.
Goy, R. C., Britto, Dd., & Assis, O. B. (2009). A review of the antimicrobial activity of chitosan. Polímeros, 19(3), 241–247.
Heisick, J. E., Rosas-Marty, L. I., & Tatini, S. R. (1995). Enumeration of viable Listeria species and Listeria monocytogenes in foods. Journal of Food Protection, 58(7), 733–736.
Herrera, A., De Ávilamontiel, G., & Polocorrales, L. (2018). Chitosan-based films with silver nanoparticles incorporated for food packaging applications. Indian Journal of Science and Technology, 11(9), 1–6.
Klein, G., Rüben, C., & Upmann, M. (2013). Antimicrobial activity of essential oil components against potential food spoilage microorganisms. Current Microbiology, 67(2), 200–208.
Lafka, T.-I., Sinanoglou, V., & Lazos, E. S. (2007). On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chemistry, 104(3), 1206–1214.
Mahmoud, S. H., Salama, D. M., & Abd El-Aziz, M. E. (2018). Effect of chitosan and chitosan nanoparticles on growth, productivity and chemical quality of green snap bean. Bioscience Research, 15(4), 4307–4321.
Matsumura, S., Tomizawa, N., Toki, A., Nishikawa, K., & Toshima, K. (1999). Novel poly (vinyl alcohol)-degrading enzyme and the degradation mechanism. Macromolecules, 32(23), 7753–7761.
Mir, N. A., Rafiq, A., Kumar, F., Singh, V., & Shukla, V. (2017). Determinants of broiler chicken meat quality and factors affecting them: A review. Journal of Food Science and Technology, 54(10), 2997–3009.
Moustafa, H., El-Sayed, S. M., & Youssef, A. M. (2021). Synergistic impact of cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable poly(butylene adipate-co-terephthalate)/clay platelets nanocomposites. Journal of Thermoplastic Composite Materials, 0892705721989771.
Nabavi, S. F., Di Lorenzo, A., Izadi, M., Sobarzo-Sánchez, E., Daglia, M., & Nabavi, S. M. (2015). Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients, 7(9), 7729–7748.
Omar, M. A., & Wilson, J. P. (2002). FDA adverse event reports on statin-associated rhabdomyolysis. Annals of Pharmacotherapy, 36(2), 288–295.
Pal, K., Banthia, A. K., & Majumdar, D. K. (2007). Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. Aaps Pharmscitech, 8(1), E142–E146.
Park, S. Y., & Ha, S.-D. (2015). Ultraviolet-C radiation on the fresh chicken breast: Inactivation of major foodborne viruses and changes in physicochemical and sensory qualities of product. Food and Bioprocess Technology, 8(4), 895–906.
Paudel, S. K., Bhargava, K., & Kotturi, H. (2019). Antimicrobial activity of cinnamon oil nanoemulsion against Listeria monocytogenes and Salmonella spp. on melons. LWT, 111, 682–687.
Polat, S., Fenercioğlu, H., & Güçlü, M. (2018). Effects of metal nanoparticles on the physical and migration properties of low density polyethylene films. Journal of Food Engineering, 229, 32–42.
Qasim, U., Osman, A. I., Al-Muhtaseb, A. A., Farrell, C., Al-Abri, M., Ali, M., Vo, D. V., Jamil, F., & Rooney, D. W. (2021). Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environmental Chemistry Letters, 19(1), 613–641.
Radi, M., Ahmadi, H., & Amiri, S. (2022). Effect of cinnamon essential oil-loaded nanostructured lipid carriers (NLC) against Penicillium citrinum and Penicillium expansum involved in tangerine decay. Food and Bioprocess Technology, 15(2), 306–318.
Raeisi, M., Tajik, H., Yarahmadi, A., & Sanginabadi, S. (2015). Antimicrobial effect of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Health Scope, 4(4).
Shah, B., Davidson, P. M., & Zhong, Q. (2012). Nanocapsular dispersion of thymol for enhanced dispersibility and increased antimicrobial effectiveness against Escherichia coli O157: H7 and Listeria monocytogenes in model food systems. Applied and Environmental Microbiology, 78(23), 8448–8453.
Son, S. R., An, J., Choi, J. W., & Lee, J. H. (2021). Fabrication of TiO2-Embedded Polyimide Layer with High Transmittance and Improved Reliability for Liquid Crystal Displays. Polymers, 13(3), 376.
Tiwari, S., Upadhyay, N., Singh, B. K., Singh, V. K., & Dubey, N. K. (2022). Facile fabrication of nanoformulated cinnamomum glaucescens essential oil as a novel green strategy to boost potency against food borne fungi, aflatoxin synthesis, and lipid oxidation. Food and Bioprocess Technology, 15(2), 319–337.
Tsiraki, M. I., & Savvaidis, I. N. (2013). Effect of packaging and basil essential oil on the quality characteristics of whey cheese “anthotyros.” Food and Bioprocess Technology, 6(1), 124–132.
Unlu, M., Ergene, E., Unlu, G. V., Zeytinoglu, H. S., & Vural, N. (2010). Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food and Chemical Toxicology, 48(11), 3274–3280.
Wang, H., Qian, J., & Ding, F. (2018). Emerging chitosan-based films for food packaging applications. Journal of Agricultural and Food Chemistry, 66(2), 395–413.
Wehr, H. M., Frank, J. F., & Association, A. P. H. (2004). Standard methods for the examination of dairy products. American Public Health Association Washington.
Yildirim, S. T., Oztop, M. H., & Soyer, Y. (2017). Cinnamon oil nanoemulsions by spontaneous emulsification: Formulation, characterization and antimicrobial activity. LWT, 84, 122–128.
Yilmaz Atay, H. (2019). Antibacterial activity of chitosan-based systems. In Functional Chitosan, 457-489. Springer, Singapore.
Youssef, A., Assem, F., Abd El-Aziz, M. E., Elaaser, M., Ibrahim, O., Mahmoud, M., & Abd El-Salam, M. (2019). Development of bionanocomposite materials and its use in coating of Ras cheese. Food Chemistry, 270, 467–475.
Youssef, A. M., El-Aziz, A., Mahmoud, E., & Morsi, S. M. (2022). Development and evaluation of antimicrobial LDPE/TiO2 nanocomposites for food packaging applications. Polymer Bulletin, 1–15.
Youssef, A. M., Assem, F. M., El-Sayed, H. S., El-Sayed, S. M., Elaaser, M., & Abd El-Salam, M. H. (2020). Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Advances, 10(62), 37857–37870.
Youssef, A. M., El-Sayed, S. M., El-Sayed, H. S., Salama, H. H., Assem, F. M., & Abd El-Salam, M. H. (2018). Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish). International Journal of Biological Macromolecules, 115, 1002–1011.
Youssef, A. M., El-Sayed, S. M., Salama, H. H., El-Sayed, H. S., & Dufresne, A. (2015). Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period. Carbohydrate Polymers, 132, 274–285.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Dr. Ahmed M. Youssef and Dr. Samah M. El-Sayed: Conceptualization. Dr. Ahmed M. Youssef, Dr. Mohamed Fouly, and Dr M.E. Abd El-Aziz: Methodology, formal analysis, and writing—original draft. Dr. Hoda S. El-Sayed and Dr. Samah M. El-Sayed: Methodology, formal analysis, and writing—original draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Youssef, A.M., El-Sayed, H.S., El-Sayed, S.M. et al. Novel Bionanocomposites Based on Cinnamon Nanoemulsion and TiO2-NPs for Preserving Fresh Chicken Breast Fillets. Food Bioprocess Technol 16, 356–367 (2023). https://doi.org/10.1007/s11947-022-02934-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02934-w