Abstract
Phycocyanin, a phycobiliprotein, is one of the few natural blue pigments available as food colourant, and it is largely used in food industry. We have devised an innovative two-step extraction process which allowed to obtain bright blue phycocyanin crude extracts with high purity grade P (within 2.5 and 3.5) directly from fresh biomass of Arthrospira platensis Gomont 1892 (commonly named Spirulina). We found out and for the first time exploited ammonium sulphate capability to minimize the release of water soluble phycobiliproteins in aqueous medium during ultrasound-assisted cell lysis/purification phase. The conventional sequence which is, extraction followed by purification, was reversed. The extraction phase was decoupled from biomass cell lysis. Cell lysis, accomplished by ultrasonication in ammonium sulphate solution, was merged with purification in a single step, before the pigment extraction/recovering phase. The process was entirely carried out in aqueous solutions. No downstream purification was required to obtain products suitable for the most common phycocyanin applications (i.e. foods, nutraceuticals). Production time, hours instead of days, was reduced to the advantage of the product quality. The process has the great advantages of (1) direct use of extracting solutions that cannot be used in the ordinary ultrasound-assisted extraction of phycocyanin (because of the extensive simultaneous extraction of contaminant molecules), (2) gain of high commercial value phycocyanin due to the elevated purity grade and (3) direct production of highly concentrated bright blue pigment crude extracts (up to about 5 mg mL−1) immediately in hand to the market.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthetic pigments are largely used in food as well as in other industrial sectors (Náthia-Neves & Meireles, 2018). Nevertheless, due to the great concern about the hazardous effects of synthetic colourants, safe natural pigments in foods are increasingly preferred by consumers (Nowruzi et al., 2022). However, not all colours are equally available in nature: on our “Blue Planet” actually blue pigments are particularly rare.
Phycocyanin (PC) is one of the few safe natural blue colourants available for food industries. To the best of our knowledge, among the natural blue pigments (anthocyanins and iridoids (Prado et al., 2020)), it is the most brilliant coloured and the one available in large quantity on the market.
PC is a water soluble, brilliant blue and highly fluorescent pigment-protein of the photosynthetic light-harvesting antenna complexes in, mainly, Cyanobacteria, Rhodophyta and Cryptomonads. PC is more than a safe natural colourant; it is a bioactive compound having antioxidant, anti-inflammatory, anticancer, antiviral, immunity-boosting and other therapeutic activities. PC has many biotechnological applications in food, cosmetic, pharmaceutical and medical sectors, as functional food, colourant, fluorescent tag, drug and photosensitizer in cancer photodynamic therapy (Ashaolu et al., 2021; de Morais et al., 2018; Kannaujiya et al., 2017; Sonani et al., 2016; Yu et al., 2017).
In Europe, PC aqueous extracts (eventually containing allophycocyanin (APC) another bluish phycobiliprotein) obtained from Arthrospira platensis have been approved by EFSA (EU Regulation No. 1333/2008 and No. 231/2012) as colouring foodstuff. The US Food and Drug Administration (FDA) classifies phycocyanin (21CFR73.1530) as a food natural colour additive and has confirmed GRAS status (i.e. generally recognized as safe) of PC. In fact, PC is one of the few approved food natural blue colorants in the USA, Europe and Asia (Meticulous Research, 2020). A. platensis is the microalga with the largest market in the food sector (Taragjini et al., 2022) and, together with A. maxima, is the main source of commercial PC. This organism can produce about 20% of PC (with PC/APC ratio usually around 3), respect to the biomass dry weight (d.w.) (de Morais et al., 2018; Kannaujiya et al., 2017).
The commercial value of PC is strongly dependent on its purity grade (P). The retail price of PC as a colorant in food (the largest market) sector is about 0.15 USD per mg and can reach as much as 180 USD per mg for therapeutic and diagnostic applications. The purity grade (P) of PC is generally evaluated by the ratio between the value of PC absorption peak, around 615–620 nm, and the absorbance value at 280 nm, which is related to the total amount of proteins in the product (Boussiba & Richmond, 1979). A product with P greater than 0.7 is usually considered as food grade, while greater than 1.5 as cosmetic grade, and greater than 3.9 as reactive grade, and finally greater than 4.0 as analytical grade (Lauceri et al., 2019).
However, despite the increasing demand of safe natural products (Nowruzi et al., 2022), the widespread use of phycocyanin, even if projected to increase according to market forecast (Meticulous Research, 2020), is still restrained by the costs of large-scale biomass production (Chaiklahan et al., 2022; Chini Zittelli et al., 2022) as well as of extraction and purification methods (Jaeschke et al., 2021; Meticulous Research, 2020). Both, extraction and purification are still too expensive, rather complex and time-consuming, and can affect the quality of the final product, since PC is photosensitive and not particularly stable even at ambient temperature. Generally, classical extraction and purification procedures involve numerous steps, which reduce product yield and increase the costs, hindering the exploitation at a large scale. Among them, repeated freeze–thaw process is recognized to be one of the most reliable, reproducible and robust extraction methods for many cyanobacteria species (including A. platensis), and the best way to achieve higher purity; but still it is time-consuming and more suitable to treat small quantity of biomass (Jaeschke et al., 2021; Kannaujiya et al., 2017). More, long-lasting PC extraction procedures at ambient temperature can lead to the development of a strong and unpleasant smell (Pan-utai & Iamtham, 2019), which can affect the organoleptic properties of the final product.
Finally, due to the increasing public awareness about human and environmental health, more health-safe and green, environmental friendly processes are required to food industries (Gomes-Diaz et al., 2022; Ochoa-Rivas et al., 2017).
Among the green methodologies applied in food sector and, particularly, in the extraction of bioactive compound from algae (Gomes-Diaz et al., 2022; Taragjini et al., 2022), ultrasound-assisted extraction is an alternative green method that ensures easer operation, reduced production time, minimum loss, low solvent consumption and high yield (Gomes-Diaz et al., 2022; Ochoa-Rivas et al., 2017). Ultrasound-assisted extraction has been applied to the extraction of bioactive compounds, such as polysaccharides (Gomes-Diaz et al., 2022) as well as protein fractions (Taragjini et al., 2022). Taragjini et al. (2022) recovered more than 85% of proteins from A. platensis biomass, stressing the good functionality maintained by proteins. However, they did not isolate PC, because they are interested to whole protein fraction and its valuable essential amino acid content more than PC application as a food colorant.
Indeed, ultrasound-assisted extraction represents an efficient and economic option for PC industrial production (Jaeschke et al., 2021; Kannaujiya et al., 2017) if no high degree of purity is required. However, conventional ultrasound-assisted extraction can cause a strong chlorophyll/carotenoid contamination, which affects the blue colour of the product (Fig. S1). As a consequence, the product is of low commercial value because of its low or moderate P and colour quality (Bachchhav et al., 2020; İlteret al., 2018). Therefore, low purity and/or chlorophyll/carotenoid contamination require application of some purification procedure after the extraction, in order to obtain a product with a middle-high commercial value (i.e. generally, with P > 2 and bright blue colour).
Here, we present a new extraction process, based on ultrasonication, which allows to obtain highly concentrated bright blue phycobiliprotein extracts directly by separating the extract from the biomass, said crude extract, reaching not only high pigment yield but also a high purity grade.
The original idea behind the process was to reverse the traditional approach usually applied to PC extraction and purification, by decoupling extraction from cell lysis and incorporating cell lysis and purification in one step. The process was completely carried out in aqueous medium to prevent fading of the pigment colour and protein structure destabilization that usually occurs when organic solvents are used in some steps, as it happens in processes aimed at the recovery of various co-products from the biomass (Wei & Ma, 2014).
Materials and Methods
Organism and Culture Conditions
Arthrospira platensis, strain M2M, from the culture collection of the Institute of Bioeconomy, CNR- IBE (Sesto Fiorentino, Italy) was used. The cells were grown in Zarrouk medium (Zarrouk, 1966) using glass flasks (1000 mL working volume) in a thermostatic chamber at 30 °C, operating in a batch mode. Cultures were exposed to continuous photon flux density of 130 ± 20 μmol m−2 s−1 supplied from one side.
Chemicals
Salt solutions of ammonium sulphate, ACS, Ph. Eur. Reagent (Merck KGaA), calcium chloride dihydrate, Ph.Eur. reagent (Carlo Erba Reagents) and sodium chloride, ACS, Ph.Eur. reagent (VWR Chemicals) were prepared with 18 MΩ Milli-Q water.
Innovative Two-Step Purification-Extraction Procedure
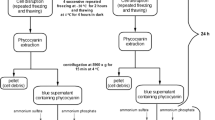
In this new approach, that is the core of our study presented herein, ultrasound cell lysis is not accomplished in the extracting solvent/solution, as it is typically done in the one-step conventional ultrasound-assisted extraction procedure (see SI−One-step conventional extraction procedure: evaluation […]): in our two-step procedure, fresh biomass cell lysis and phycobiliprotein extraction are carried out in distinct phases of the process: (1) the cell lysis/purification phase (in AS solution, that we found out minimizes the release of phycobiliproteins in solution) and (2) the extraction phase (after the removal of the AS solution). The biomass processing and the general purification-extraction process are schematised in Fig. 1.
Biomass Processing
Stock suspensions of A. platensis biomass were prepared as follows: aliquots of A. platensis culture were filtered using a nylon filter (mesh size 21 μm), to separate the biomass from the culture medium. The biomass was washed with ultrapure Milli-Q water, recovered with a small spatula, suspended in ultrapure Milli-Q water (biomass stock suspension) and used quickly after its preparation to avoid osmotic cell lysis. The d.w. of each biomass stock suspension was determined (see SI–Determination of biomass stock suspension dry weight) in order to assess the yield of the recovered PC versus the biomass processed.
Cell Lysis/Purification Phase
All the ultrasonication procedures were performed with a Hielscher Ultrasonic Processor UP200S (200 W, 24 kHz) using an S2 sonotrode.
The cell lysis/purification phase was optimized operating at R ≈ 250. Cell lysis was carried out by suspending the biomass in an ammonium sulphate (AS) solution (cleaning solution, Fig. 1). The effect of (a) AS concentration and (b) the number (one (b1) or two (b2)) of lysis/purification cycles were studied. Detailed description of the procedures is provided in the “Protocol of Cell lysis/purification phase procedures” section of SI.
The total ultrasonication time was 8 min both, in the one-cycle lysis/purification and in the two-cycle lysis/purification procedure.
Extraction Phase
The extraction phase was optimized operating at the same volume/biomass ratio as in the lysis/purification phase (R ≈ 250). The clean biomass pellets obtained by applying the cell lysis/purification procedures were suspended in 5 ml of extracting solution (CaCl2, NaCl or just Milli-Q water), shaken (300 rpm) for 3 h at ambient temperature (20–24 °C) by an orbital shaker and centrifuged (30 min, 12,000 × g, 15 °C). The supernatants (crude extracts) were recovered and phycobiliprotein content and PC purity grade determined by spectrophotometric analysis (Bennett & Bogorad, 1973; Boussiba & Richmond, 1979).
Spectrophotometric Determination of Phycobiliproteins
A. platensis produces PC and APC with a ratio usually around 3 (Kannaujiya et al., 2017). Both PC and APC were extracted applying the procedures used in this study, although often referred only to PC extraction. Being PC the most abundant phycobiliprotein in A. platensis, only the purity grade, P, of PC was considered (Eq. (1)); however, the yield of the total extracted (or recovered) phycobiliproteins (PC + APC) was also reported (Y%, Eq. (2)).
where APC is the absorbance maximum of PC and A280 is the absorbance at 280 nm (absorbance related to the whole protein content) (Boussiba & Richmond, 1979). CPBPs is the concentration (mg mL−1) of all the phycobiliproteins (PC + APC) present in the extract, VE and VB are the recovered extract volume and the biomass stock suspension volume (mL), respectively, and Bd.w. is the biomass stock suspension dry weight (mg mL−1).
PC and APC were determined using Eqs. (3) and (4) (Bennett & Bogorad, 1973). Absorbance values were corrected by subtracting the absorbance at 750 nm (Lawrenz et al., 2011).
A615 and A652 are the absorbance at 615 nm (PC absorption maximum) and 652 nm (APC absorption maximum), respectively.
Absorbance measurements were carried out with an UVmc2 spectrophotometer (SAFAS, Monaco, Munich). Suprasil quartz 1 cm light path cuvettes were used.
Results and discussion
The goal of this study was to produce PC of middle-high commercial value. To this aim, we planned to achieve the following objects: (1) to obtain a high yield and a highly concentrated PC crude extract with purity grade > 2.0 and bright blue colour, (2) to design a straightforward and time saving procedure that is able to maintain the chemical-physical and organoleptic properties of the product and (3) to design a simple process potentially suitable for large-scale production of phycocyanin.
Ultrasound-assisted extraction of phycocyanin from A. platensis biomass represents an efficient and a cost-effective option for industrial production of this pigment (Jaeschke et al., 2021; Kannaujiya et al., 2017). However, the product is usually characterized by a low purity and/or is contaminated by other pigments, such as chlorophyll and carotenoids, which affects its colour (Fig. S1) (Bachchhav et al., 2020; İlteret al., 2018). The application of conventional purification procedures after the extraction step tend to disadvantage the pigment concentration in the extract solution and that so, very often is necessary to increase its concentration, for example, by ultrafiltration (ultrafiltration can be also used to purify PC extracts, by eliminating contaminant molecules with molecular weights lower than PC), certainly with increase of production cost. Further, in the conventional ultrasonication procedures, biomass cell lysis and phycobiliprotein extraction occur simultaneously. On the contrary, the novelty proposed by us is that these two processes no longer occur at the same time. With our method, an efficient cell lysis was achieved by ultrasonication of the fresh biomass in AS solution (cleaning solution, cell lysis/purification phase, Fig. 1), by adopting mild conditions that reduced PC extraction, while the release of undesired biomolecules was still possible. PC was extracted by applying suitable conditions for the release of the pigment (extraction phase, Fig. 1) only after separating the biomass from the cleaning solution. A product with higher P was obtained (with respect to the control crude extract, obtained following the one-step control procedure (i.e. conventional ultrasound-assisted extraction)). This product was characterized by a bright blue colour and high PC concentration.
It is well known that the phycobiliprotein content of the biomass is not constant. It fluctuates, as phycobiliprotein production depends on various parameters, such as the culture age, pH, cell density, light intensity, etc. (Chini Zittelli et al., 2022). For this reason, each experiment was always matched with a control extraction, executed by applying the one-step control conventional procedure described in SI–One-step conventional ultrasound-assisted extraction: evaluation […], setting the experimental conditions in order to achieve an efficient cell lysis and maximum phycobiliprotein extraction, as discussed in the following section. The optimal conditions determined for the conventional procedure were also applied in the cell lysis/purification phase of here presented new two-step process.
One-Step Conventional Procedure for Control Extraction: Optimization of Cell Lysis and PC Extraction Conditions by Direct Ultrasonication in CaCl2 Extracting Solution
The conventional ultrasound-assisted extraction procedure was optimized (SI–One-step conventional ultrasound-assisted extraction: evaluation […]) using preferentially CaCl2 solutions to extract PC and APC as well. PC/APC ratio varied most often between 3 and 4. CaCl2 not only ensured a higher P in respect to other extracting solutions, such as NaCl or water, but also minimized chlorophyll and carotenoid contamination (ensuring a blue colour extract), while promoting phycobiliprotein extraction (İlter et al., 2018; Pott, 2019). On the contrary, PC extracted in water or NaCl solutions presented low P and was deeply green coloured (Fig. 2), hampering the determination of PBP content.
Absorbance spectra of PC crude extract solutions obtained after the lysis of A. platensis cells by ultrasonication in CaCl2 0.1 M (blue curve), NaCl 0.1 M (light green curve) and Milli-Q water (deep green curve). The same amount of biomass (20.52 mg d.w.) and the same volume of each extracting solution (5 mL) was used (R ≈ 250). The crude extracts were diluted for measurement. Insets: crude extracts. Only the extract in CaCl2 is blue
In order to understand the results obtained, biomass suspensions in CaCl2 0.1 M and NaCl 0.1 M were examined by optical microscopy before and after the ultrasonication treatment (Fig. S2). However, the images obtained under optical microscope did not reveal any relevant differences between the two suspensions, except a clear release of chlorophyll in NaCl solution after ultrasonication. The chlorophyll release mechanism needs a more in-depth study, as the cell wall of the two biomass suspensions looked very similar before and also after ultrasonication treatment. Finally, by applying ultrasound-assisted extraction in CaCl2 solution, characterized by simultaneous cell lysis and PC extraction, it was possible to obtain a blue extract; although, its purity barely exceeded grade 2.
Experiments were carried out to determine the conditions that allowed efficient cell lysis and PC extraction. Ultrasonication and extraction periods were evaluated, together with the effect of biomass density, operating at two different volume/biomass ratios: R ≈ 1200 and R ≈ 250 (Fig. 3a, b and c). The amount of total extracted phycobiliproteins (Fig. 3a) increased with the ultrasonication time within 6–8 min, and thereafter the yield attained differed by only about 1%. Similar yields (Fig. 3b, ultrasonication time = 8 min) and purity (Fig. 3c, ultrasonication time = 8 min) were obtained at R ≈ 1200 and R ≈ 250. The yield was only slightly lower when extraction followed immediately ultrasonication (Fig. 3b, extraction time = 0 h), whereas the purity was only slightly higher for extraction times equal or longer than 3 h (Fig. 3c). The most relevant difference among the various extracts was the content of chlorophyll and its decomposition derivatives (such as pheophytin), which provoked an absorbance in the range 400–450 nm and around 680 nm (Fig. 3d), affecting the blue colour brightness of the extract: the amount of those green pigments was maximum at extraction time 0 h and minimum at extraction time equal or longer than 3 h. Therefore, an ultrasonication time of 8 min and an extraction time of 3 h were adopted in all the two-step cell lysis/purification extraction procedures and in the control extraction in CaCl2 (one-step control standard procedure); R ≈ 250 was adopted. Nevertheless, it is worth noting that even at time = 0 h, the phycobiliprotein extraction was almost complete, while purity grade was not much different.
Evaluation of ultrasonication and extraction time: a yield % of total PC and APC extracted (YPBPs%) at various sonication times (extraction time = 4 h); b Y% (PC + APC) at various extraction time (sonication time = 8 min); c PC purity grade (PPC) at various extraction time (sonication time = 8 min); d absorbance spectra of extracts at extraction time = 0 h (green curve) and extraction time = 3 h (blue curve). Data are the average of 2 independent experiments
Innovative PC Ultrasound-Assisted Two-Step Extraction Process
AS solutions are commonly used in phycobiliprotein separation/purification processes by salting-out (Huo et al., 2022) or column (Chen et al., 2018) and membrane (Lauceri et al., 2019) chromatography (as eluents), downstream of the extraction process. In the process presented here, AS solutions were used upstream of the extraction procedure. Their important function (as a cleaning solutions) was to reduce/prevent the passage into the solution of phycobiliproteins, which remained trapped inside the cells, while the extraction of water-soluble contaminating biomolecules, less effectively trapped into the cells, occurred. In fact, the chemical compounds in the biomass cells are differently affected by the presence of AS: this salt may promote or impair non-covalent interactions between molecules or between molecules and cell organelles, in respect to chemical characteristics of a particular molecule. As an example, it is quite conceivable that the effect of AS on the interaction between proteins (having no or only few charges) is quite different from that between nucleic acids (which are polyanions). Indeed, these interactions inevitably affect the extraction of the various chemical compounds. In particular, nucleic acids were always released, even at the highest AS concentration applied (Fig. 4).
Absorbance spectra of the ammonium sulphate (AS) cleaning solution of two Spirulina biomass samples (one-cycle lysis/purification procedure): green curve, AS 0.4 M; red curve, AS 1.5 M. Both the solutions were diluted six times in order to execute the measurement. Inset: cleaning solutions at various AS concentrations obtained by applying the one-cycle lysis/purification (b1) procedure
The effect of AS was evaluated operating at R ≈ 250 (R = 244 ± 19), applying the one-cycle lysis/purification procedure (b1) and then extracting PC by keeping the “clean” biomass suspended in the extracting solution (CaCl2 0.1 M) for 3 h at ambient temperature. AS acted as a chemical barrier: it was able to reduce/impede the release of the phycobiliproteins into the media. However, this in-cell-trapping phenomenon still needs to be better studied. The effect of AS was concentration dependent (Fig. 4). It was quite small at a concentration of 0.4 M, but it increased greatly at higher AS concentrations (0.8–1.1 M) and finally almost reached a plateau at the highest concentrations applied (1.2–1.5 M). Selected absorbance spectra and the images of the cleaning solutions at various AS concentrations are shown in Fig. 4−inset, respectively. Most of the PC dissolved into the cleaning solution was actually lost at low AS concentrations. By increasing AS concentration, it was possible to minimize the PC loss that was almost negligible at AS 1.5 M (Fig. 4). This had a direct effect on the amount of the phycobiliproteins (PC + APC) recovered in the following extraction step (Fig. 5a). PC yield markedly increased with the increase of AS concentration (used in the cell lysis/purification phase). A yield lower than 2% (Y ≈ 1.5%) was obtained using AS 0.4 M; a quite tiny amount, considering that the yield obtained in the control experiment, performing cell lysing directly in the extracting solution, was about 28%. The yield exceeded 24% when AS 1.1 M was used and reached almost 27% when AS 1.5 M was used. Instead, PC purity was less affected (Fig. 5b). P initially increased simultaneously with the increase of AS concentration and then decreased slightly at the highest AS concentration. By applying the two-step purification/extraction procedure (b1) (in which cell lysis and extraction are not simultaneous), crude extracts with P > 2.5 were obtained (except for the extracts obtained after AS 0.4 M treatment (P ≈ 2.1)), while in the one-step control conventional (ultrasound-assisted) extraction procedure (cell lysis with concomitant extraction), P ≈ 1.7 was obtained.
Evaluation of the effect of ammonium sulphate concentration used in the cell lysis/purification phase on the total phycobiliprotein yield and on the phycocyanin purity obtained in the subsequent extraction phase. The one-cycle lysis/purification (b1) procedure was applied (R ≈ 250). a Yield % of total extracted phycobiliproteins (PC + APC) (YPBPs%); b PC purity grade (PPC). Average data of 4 independent experiments using two different biomass batches
By applying the two-cycle lysis/purification procedure (b2), it was possible to increase the purity of the recovered phycobiliproteins (Fig. 6b: control, P ≈ 1.6; one-cycle lysis/purification procedure, P ≈ 2.8; two-cycle lysis/purification procedure, P ≈ 3.3), while the yield was a little lower than that achieved by one-cycle lysis/purification procedure (b1) (Fig. 6a: control, Y ≈ 27%; one-cycle lysis/purification procedure, Y ≈ 22%; two-cycle lysis/purification procedure, Y ≈ 20%).
Furthermore, the purity and the yield of the extracted phycobiliproteins can be optimized tuning the concentration of CaCl2 in the extracting solution. In fact, an increase of the yield with CaCl2 concentration was observed (Fig. 7a), while purity first increased and then decreased, reaching the highest value in CaCl2 0.025–0.05 M extracting solutions (P ≈ 3.6, Fig. 7b). The yield and the purity of the control were 25% and 1.6, respectively. In this experiment set the one-cycle lysis/purification procedure (b1) was applied.
In Fig. 7, the yield and purity of PC extracts in Milli-Q water are also reported. A quite high yield was obtained when using water as an extracting solvent (Fig. 7a, [CaCl2] = 0 M), while purity, even if generally lower than in CaCl2 (P ≈ 2.6, Fig. 7b, [CaCl2] = 0 M), was however considerably higher than 2; the minimum value we are proposing to be able to achieve in this study.
In addition to the advantageous use of MilliQ water as an extracting solvent, the use of NaCl solutions was also assessed. The pigment yield was not much affected by NaCl concentration (Fig. S3a), as well as purity, which varied from 2.5 to 2.7 (Fig. S3b).
It is known that the selectivity of the process is highly dependent on the solvent/extracting solution used (Gomes-Diaz et al., 2022). However, to the best of our knowledge, only CaCl2 extracting solutions are usable in the conventional ultrasound-assisted PC extraction in order to obtain a blue extract (İlteret al., 2018).
Our results suggest that the separation of the cell lysis action from the phycobiliprotein extraction benefits the use of extracting solvent/solutions (e.g. water, NaCl solutions) that cannot be used in the ordinary ultrasound-assisted extraction, due to extensive simultaneous extraction of contaminant molecules (Fig. 2). This is an important achievement because, even if the presence of CaCl2 may not be a problem for some applications; for others, it could be a disadvantage, as this salt can affect stability of certain proteins (Huang et al., 2022). With our process, it is now possible to offer an extracting solution that complies with any PC application, in particular in the food sector.
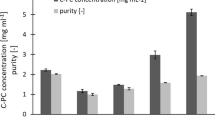
Finally, the process efficiency at low volume/biomass ratio (R = 45 ± 1) was tested. Both, the one-cycle (b1) and the two-cycle (b2) lysis/purification procedures were applied, while one-step control standard extractions by direct ultrasonication in CaCl2 0.1 M were executed at R ≈ 45 and at higher R (i.e. more diluted biomass suspension). Acceptable yields (Fig. 8a, 21.6% and 20.9% of biomass d.w. for procedure (b1) and (b2), respectively) and purity (Fig. 8b) were obtained. Similar yields were obtained at both R (45 and 660), indicating that phycobiliprotein extraction is almost complete at high cell density (R ≈ 45). Only a little yield reduction was observed after one-cycle lysis/purification procedure (b1) as well as after the two-cycle lysis/purification procedure (b2) respect to the control solutions. The pigment recover with respect to the control extract obtained by the one-step conventional ultrasound-assisted procedure (extraction Y = 23.2% of biomass d.w.) was about 93% and 89% for the one-cycle (b1) and the two-cycles (b2) procedure, respectively. In fact, some phycobiliproteins were lost in the cleaning solutions. But by applying the two-cycle lysis/purification procedure, a higher purity (P ≈ 3.2) was reached compared to one-cycle procedure (P ≈ 2.6), with values very similar to the those observed at R ≈ 250. These results are interesting, because the lowest ratio (R ≈ 45) ensures the production of an extract with a total phycobiliprotein concentration of about 4–5 mg mL−1. Such a concentration is sufficiently high to allow the direct commercialization of the pigment in solution as well as the direct drying of the product (for example, by spray-drying) avoiding extensive concentration treatments. No downstream purification is necessary to obtain products suitable for the most common PC applications (i.e. foods, nutraceuticals, cosmetics), which do not require, for instance, analytical purity grade, or separation of PC and APC, while it is needed to simplify/reduce the extraction and purification process steps to maintain high product yield and to contain production costs. The quality of the product is better preserved due to mild and quick operating conditions. In fact, the process is not excessively time-consuming. Moreover, the results achieved suggest that it is possible to further reduce the extraction time and, consequently, the whole production time (a few hours, including biomass harvesting, instead of days). Finally, the process is technically simple, and the only heavy initial investment and service cost, due to the use of centrifugation, should be effectively counterbalanced by the high commercial value of the pigment produced. All these characteristics suggest that the process may be suitable for the large-scale production of phycocyanin.
One-cycle lysis/purification (b1) procedure versus two-cycle lysis/purification (b2) procedure applied to suspensions having low volume/biomass ratio (R = 45 ± 1). a Yield % of total extracted phycobiliproteins (PC + APC) (YPBPs%); b PC purity grade (PPC). Data are the average of 4 independent experiments using two different biomass batches
Conclusions
Food grade bright blue PC crude extracts from A. platensis, with purity grade even higher than 3, were obtained by applying a novel, innovative ultrasound-assisted cell lysis process that exploits the capability of ammonium sulphate to minimize the release of phycobiliproteins in solution. No downstream purification step was required. Remarkably, high PC concentrations (4–5 mg mL−1) for a crude extract with P > 3 (unusually high for crude extracts with P > 2.5 as well) and high phycobiliprotein recovered yields (20–25%, which is most of the phycobiliproteins produced by the organism) were obtained by applying a procedure that ensures a short production time. The best conditions to maximize both PC purity grade and yield were 8 min of sonication (power 200 W) in AS 1.1 M (cell lysis/purification phase) and an extraction period of 3 h (extraction phase). The highest purity was achieved in CaCl2 extracting solution.
The proposed new procedure is promising in reducing the production costs, making the method economically worthwhile for a large-scale production of highly valuable bright blue phycocyanin.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information file.
References
Ashaolu, T. J., Samborska, K., Lee, C. C., Tomas, M., Capanoglu, E., Tarhan, Ö., Taze, B., & Jafari, S. M. (2021). Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. International Journal of Biological Macromolecules, 193, 2320–2331. https://doi.org/10.1016/j.ijbiomac.2021.11.064
Bachchhav, M. B., Kulkarni, M. V., & Ingale, A. G. (2020). Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Separation Science and Technology, 55(5), 932–944. https://doi.org/10.1080/01496395.2019.1580293
Bennett, A., & Bogorad, L. (1973). Complementary chromatic adaptation in a filamentous blue-green alga. The Journal of Cell Biology, 58(2), 419–435. https://doi.org/10.1083/jcb.58.2.419
Boussiba, S., & Richmond, A. E. (1979). Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Archives of Microbiology, 120(2), 155–159. https://doi.org/10.1007/BF00409102
Chaiklahan, R., Chirasuwan, N., Srinorasing, T., Attasat, S., Nopharatana, A., & Bunnag, B. (2022). Enhanced biomass and phycocyanin production of Arthrospira (Spirulina) platensis by a cultivation management strategy: Light intensity and cell concentration. Bioresource Technology, 343, 126077. https://doi.org/10.1016/j.biortech.2021.126077
Chen, K. H., Wang, S. S. S., Show, P. L., Lin, G. T., & Chang, Y. K. (2018). A rapid and efficient technique for direct extraction of C-phycocyanin from highly turbid Spirulina platensis algae using hydrophobic interaction chromatography in stirred fluidized bed. Biochemical Engineering Journal, 140, 47–56. https://doi.org/10.1016/j.bej.2018.09.005
Chini Zittelli, G., Mugnai, G., Milia, M., Cicchi, B., Benavides, A. S., Angioni, A., Addis, P., & Torzillo, G. (2022). Effects of blue, orange and white lights on growth, chlorophyll fluorescence, and phycocyanin production of Arthrospira platensis cultures. Algal Research, 61, 102583. https://doi.org/10.1016/j.algal.2021.102583
de Morais, M. G., da Fontoura Prates, D., Moreira, J. B., Duarte, J. H., & Costa, J. A. V. (2018). Phycocyanin from microalgae: Properties, extraction and purification, with some recent applications. Industrial Biotechnology, 14(1), 30–37. https://doi.org/10.1089/ind.2017.0009
Gomes-Diaz, J. S., Teixeira, J. A., & Rocha, C. M. (2022). Recent advances in the valorization of algae polysaccharides for food and nutraceutical applications: A review on the role of green processing technologies. Food and Bioprocess Technology, 1–29. https://doi.org/10.1007/s11947-022-02812-5
Huang, M., Wang, C., Cheng, M., Zhang, X., Jiang, H., & Wang, J. (2022). Effects of quantity and source of calcium on the behavior of goat milk after heating and acidification. LWT, 153, 112535. https://doi.org/10.1016/j.lwt.2021.112535
Huo, Y., Hou, X., Yu, Y., Wen, X., Ding, Y., Li, Y., & Wang, Z. (2022). Improving the thermal and oxidative stability of food-grade phycocyanin from Arthrospira platensis by addition of saccharides and sugar alcohols. Foods, 11(12), 1752. https://doi.org/10.3390/foods11121752
İlter, I., Akyıl, S., Demirel, Z., Koç, M., Conk-Dalay, M., & Kaymak-Ertekin, F. (2018). Optimization of phycocyanin extraction from Spirulina platensis using different techniques. Journal of Food Composition and Analysis, 70, 78–88. https://doi.org/10.1016/j.jfca.2018.04.007
Jaeschke, D. P., Teixeira, I. R., Marczak, L. D. F., & Mercali, G. D. (2021). Phycocyanin from Spirulina: A review of extraction methods and stability. Food Research International, 143, 110314. https://doi.org/10.1016/j.foodres.2021.110314
Kannaujiya, V. K., Sundaram, S., & Sinha, R. P. (2017). Phycobiliproteins: Recent developments and future applications. Springer Singapore. https://doi.org/10.1007/978-981-10-6460-9
Lauceri, R., Chini Zittelli, G., & Torzillo, G. (2019). A simple method for rapid purification of phycobiliproteins from Arthrospira platensis and Porphyridium cruentum biomass. Algal Research, 44, 101685. https://doi.org/10.1016/j.algal.2019.101685
Lawrenz, E., Fedewa, E. J., & Richardson, T. L. (2011). Extraction protocols for the quantification of phycobilins in aqueous phytoplankton extracts. Journal of Applied Phycology, 23(5), 865–871. https://doi.org/10.1007/s10811-010-9600-0
Meticulous Research. (2020). Phycocyanin market, global forecast to 2027. https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2027
Náthia-Neves, G., & Meireles, M. A. (2018). Genipap: A new perspective on natural colorants for the food industry. Food Public Health, 8(1), 21–33. https://doi.org/10.5923/j.fph.20180801.04
Nowruzi, B., Konur, O., & Anvar, S. A. A. (2022). The stability of the phycobiliproteins in the adverse environmental conditions relevant to the food storage. Food and Bioprocess Technology, 1-18. https://doi.org/10.1007/s11947-022-02855-8
Ochoa-Rivas, A., Nava-Valdez, Y., Serna-Saldívar, S. O., & Chuck-Hernández, C. (2017). Microwave and ultrasound to enhance protein extraction from peanut flour under alkaline conditions: Effects in yield and functional properties of protein isolates. Food and Bioprocess Technology, 10(3), 543–555. https://doi.org/10.1007/s11947-016-1838-3
Pan-utai, W., & Iamtham, S. (2019). Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochemistry, 82, 189–198. https://doi.org/10.1016/j.procbio.2019.04.014
Pott, R. W. (2019). The release of the blue biological pigment C-phycocyanin through calcium-aided cytolysis of live Spirulina sp. Coloration Technology, 135(1), 17–21. https://doi.org/10.1111/cote.12373
Prado, J. M., Veggi, P. C., Náthia-Neves, G., & Meireles, M. A. A. (2020). Extraction methods for obtaining natural blue colorants. Current Analytical Chemistry, 16(5), 504–532. https://doi.org/10.2174/1573411014666181115125740
Sonani, R. R., Rastogi, R. P., Patel, R., & Madamwar, D. (2016). Recent advances in production, purification and applications of phycobiliproteins. World Journal of Biological Chemistry, 7(1), 100. https://doi.org/10.4331/wjbc.v7.i1.100
Taragjini, E., Ciardi, M., Musari, E., Villaró, S., Morillas-España, A., Alarcón, F. J., & Lafarga, T. (2022). Pilot-scale production of A. platensis: Protein isolation following an ultrasound-assisted strategy and assessment of techno-functional properties. Food and Bioprocess Technology, 1–12. https://doi.org/10.1007/s11947-022-02789-1
Wei, D., & Ma, C. (2014). Method for extracting nutrients from spirulina in grading manner. Patent No. CN104086649.
Yu, P., Wu, Y., Wang, G., Jia, T., & Zhang, Y. (2017). Purification and bioactivities of phycocyanin. Critical Reviews in Food Science and Nutrition, 57(18), 3840–3849. https://doi.org/10.1080/10408398.2016.1167668
Zarrouk, C. (1966). Contribution a l’etude d’une cyanophycee. Influence de divers facteur physiques et chimiques sur la croissance et la photosynthese de Spirulina maxima, (Setch et Gardner) Geitler (p. 138). Universite’ de Paris. Ph.D. thesis.
Author information
Authors and Affiliations
Contributions
Rosaria Lauceri: Conceptualization, investigation, methodology, supervision, validation, visualization, writing—original draft preparation. Cristina Cavone: Data curation, visualization. Graziella Chini Zittelli: Resources (biomass production), writing—reviewing and editing. Lyudmila Kamburska: Visualization, writing—reviewing and editing. Simona Musazzi: Data curation; visualization; writing, original draft preparation; writing, reviewing and editing. Giuseppe Torzillo: Resources (biomass production), writing—reviewing and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that the extraction/purification process presented in this manuscript is the object of a patent application (WO2022144704A1) recently submitted by Rosaria Lauceri, Graziella Chini Zittelli, and Giuseppe Torzillo.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lauceri, R., Cavone, C., Chini Zittelli, G. et al. High Purity Grade Phycocyanin Recovery by Decupling Cell Lysis from the Pigment Extraction: an Innovative Approach. Food Bioprocess Technol 16, 111–121 (2023). https://doi.org/10.1007/s11947-022-02926-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02926-w