Abstract
The growing interest in minimal processing of foods due to its mild effects on product quality and taste has led to an increase in the research on novel techniques for food processing. One of these emerging techniques is atmospheric cold plasma (ACP) processing. This study investigated the effect of ACP processing on major milk proteins where plasma was generated using nitrogen–oxygen (N2–O2) and nitrogen (N2). Changes in whey protein denaturation and distribution, protein–protein interactions of aggregates, and antigenicity of milk proteins as a function of treatment time and feed gas composition were evaluated. Results show that, among whey proteins, ⍺-lactalbumin and β-lactoglobulin have the highest susceptibility to denaturation by N2–O2 and N2 plasma and that ACP changed the ratio of native β-lactoglobulin and native ⍺-lactalbumin present in milk serum. The aggregates formed by 1 min N2–O2 plasma treatment showed an increase in their disulfide bonding and a decrease in the hydrogen bonding. N2 and N2–O2 plasma decreased ⍺-lactalbumin antigenicity (as measured by ELISA) after 1 min and 4 min, respectively. β-Lactoglobulin antigenicity decreased with time only during N2–O2 plasma treatment, while casein antigenicity decreased with time, regardless of gas composition (N2–O2: 0.9 mol nitrogen and 0.1 mol oxygen per mol gas; N2: 1 mol nitrogen gas).
Industrial Relevance
Atmospheric cold plasma processing of foods is being extensively researched for its advantages over thermal processing techniques. This research shows the ability of plasma generated from different gases to partially denature and redistribute whey proteins between colloidal and serum phase of milk. Partially denatured whey proteins enhance the functional properties of milk such as gelling and foaming capacities which are of interest to the food industry. The ability of cold plasma to reduce the antigenicity of milk proteins is also investigated. This study shows the potential of atmospheric cold plasma to be used for processing of milk to create products with unique textures, properties, and reduced allergenicity.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Anema, S. G., & Li, Y. (2003). Effect of pH on the association of denatured whey proteins with casein micelles in heated reconstituted skim milk. Journal of Agricultural and Food Chemistry, 51(6), 1640–1646.
Augustin, M. A., Oliver, C. M., & Hemar, Y. (2011). Casein, caseinates, and milk protein concentrates. Dairy Ingredients for Food Processing, 1, 161–178.
Bravo, F. I., Felipe, X., López-Fandiño, R., & Molina, E. (2015). Skim milk protein distribution as a result of very high hydrostatic pressure. Food Research International, 72, 74–79. https://doi.org/10.1016/j.foodres.2015.03.014
Considine, T., Patel, H., Anema, S., Singh, H., & Creamer, L. (2007). Interactions of milk proteins during heat and high hydrostatic pressure treatments—A review. Innovative Food Science & Emerging Technologies, 8(1), 1–23.
Crowley, S. V., Dowling, A. P., Caldeo, V., Kelly, A. L., & O’Mahony, J. A. (2016). Impact of α-lactalbumin:β-lactoglobulin ratio on the heat stability of model infant milk formula protein systems. Food Chemistry, 194, 184–190. https://doi.org/10.1016/j.foodchem.2015.07.077
Dong, X., Wang, J., & Raghavan, V. (2021). Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Critical Reviews in Food Science and Nutrition, 61(2), 196–210. https://doi.org/10.1080/10408398.2020.1722942
Edwards, P. J. B., & Jameson, G. B. (2009). Structure and stability of whey proteins. In M. Boland & H. Singh (Eds.), Milk proteins: From expression to food (pp. 251–291). Elsevier.
Esteghlal, S., Gahruie, H. H., Niakousari, M., Barba, F. J., Bekhit, A. E. -D., Mallikarjunan, K., & Roohinejad, S. (2019). Bridging the knowledge gap for the impact of non-thermal processing on proteins and amino acids. Foods, 8(7), 262.
Fox, P. F. (2003). Milk proteins: General and historical aspects. In P. F. Fox & P. L. H. McSweeney (Eds.), Advanced dairy chemistry - 1 proteins (pp. 1–48). Springer.
Fox, P. F., & O’Mahony, J. (2013). Milk proteins: Introduction and historical aspects. In P. L. H. McSweeney & P. F. Fox (Eds.), Advanced dairy chemistry Vol 1A - Proteins: Basic aspects (4th ed., pp. 43–85). Springer.
Fuentes-Lemus, E., Jiang, S., Hägglund, P., & Davies, M. J. (2021). High concentrations of casein proteins exacerbate radical chain reactions and increase the extent of oxidative damage. Food Hydrocolloids, 121, 107060. https://doi.org/10.1016/j.foodhyd.2021.107060
Gaspard, S. J., Auty, M. A. E., Kelly, A. L., O’Mahony, J. A., & Brodkorb, A. (2017). Isolation and characterisation of κ-casein/whey protein particles from heated milk protein concentrate and role of κ-casein in whey protein aggregation. International Dairy Journal, 73, 98–108. https://doi.org/10.1016/j.idairyj.2017.05.012
Głąb, T. K., & Boratyński, J. (2017). Potential of casein as a carrier for biologically active agents. Topics in Current Chemistry (Cham), 375(4), 71–71. https://doi.org/10.1007/s41061-017-0158-z
Guyomarc’h, F., Famelart, M. -H., Henry, G., Gulzar, M., Leonil, J., Hamon, P., & Croguennec, T. (2015). Current ways to modify the structure of whey proteins for specific functionalities—A review. Dairy Science & Technology, 95(6), 795–814.
Jaskulski, M., Atuonwu, J. C., Tran, T. T. H., Stapley, A. G. F., & Tsotsas, E. (2017). Predictive CFD modeling of whey protein denaturation in skim milk spray drying powder production. Advanced Powder Technology, 28(12), 3140–3147. https://doi.org/10.1016/j.apt.2017.09.026
Joyce, A. M., Brodkorb, A., Kelly, A. L., & O’Mahony, J. A. (2017). Separation of the effects of denaturation and aggregation on whey-casein protein interactions during the manufacture of a model infant formula. Dairy Science & Technology, 96(6), 787–806. https://doi.org/10.1007/s13594-016-0303-4
Joyce, A. M., Kelly, A. L., & O’Mahony, J. A. (2018). Controlling denaturation and aggregation of whey proteins during thermal processing by modifying temperature and calcium concentration. International Journal of Dairy Technology, 71(2), 446–453.
Krska, R., Welzig, E., & Baumgartner, S. (2004). Detecting proteins with allergenic potential. In R. Yada (Ed.), Proteins in food processing (pp. 292–322). Woodhead Publishing Ltd.
Liu, K., & Hsieh, F. -H. (2008). Protein–protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. Journal of Agricultural and Food Chemistry, 56(8), 2681–2687.
Liu, Z. -W., Liu, L. -J., Zhou, Y. -X., Tan, Y. -C., Cheng, J. -H., Bekhit, A. E. -D., & Aadil, R. M. (2021). Dielectric-barrier discharge (DBD) plasma treatment reduces IgG binding capacity of β-lactoglobulin by inducing structural changes. Food Chemistry, 358, 129821. https://doi.org/10.1016/j.foodchem.2021.129821
Livney, Y. D. (2010). Milk proteins as vehicles for bioactives. Current Opinion in Colloid & Interface Science, 15(1), 73–83. https://doi.org/10.1016/j.cocis.2009.11.002
Manoharan, D., Stephen, J., & Radhakrishnan, M. (2021). Study on low-pressure plasma system for continuous decontamination of milk and its quality evaluation. Journal of Food Processing and Preservation, 45(2), 15138.
Marengo, M., Bonomi, F., Marti, A., Pagani, M. A., Elkhalifa, A. E. O., & Iametti, S. (2015). Molecular features of fermented and sprouted sorghum flours relate to their suitability as components of enriched gluten-free pasta. LWT - Food Science and Technology, 63(1), 511–518. https://doi.org/10.1016/j.lwt.2015.03.070
Mills, E. C., Sancho, A. I., Rigby, N. M., Jenkins, J. A., & Mackie, A. R. (2009). Impact of food processing on the structural and allergenic properties of food allergens. Molecular Nutrition & Food Research, 53(8), 963–969.
Moiseev, T., Misra, N., Patil, S., Cullen, P., Bourke, P., Keener, K., & Mosnier, J. (2014). Post-discharge gas composition of a large-gap DBD in humid air by UV–Vis absorption spectroscopy. Plasma Sources Science and Technology, 23(6), 065033.
Ng, S. W., Lu, P., Rulikowska, A., Boehm, D., O’Neill, G., & Bourke, P. (2021). The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chemistry, 342, 128283.
Nguyen, N. H. A., Anema, S. G., Guyomarc’h, F., Wong, M., & Havea, P. (2015). The effect of the addition of thiol reagents to heated milk on protein interactions and acid gelation properties. International Dairy Journal, 42, 42–50. https://doi.org/10.1016/j.idairyj.2014.10.005
Patel, H. A., Singh, H., Anema, S. G., & Creamer, L. K. (2006). Effects of heat and high hydrostatic pressure treatments on disulfide bonding interchanges among the proteins in skim milk. Journal of Agricultural and Food Chemistry, 54(9), 3409–3420.
Pereira, R. N., Teixeira, J. A., & Vicente, A. A. (2011). Exploring the denaturation of whey proteins upon application of moderate electric fields: A kinetic and thermodynamic study. Journal of Agricultural and Food Chemistry, 59(21), 11589–11597.
Pesic, M. B., Barac, M. B., Stanojevic, S. P., Ristic, N. M., Macej, O. D., & Vrvic, M. M. (2012). Heat induced casein–whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Ruminant Research, 108(1–3), 77–86.
Pizzano, R., Manzo, C., Adalgisa Nicolai, M., & Addeo, F. (2012). Occurrence of major whey proteins in the pH 4.6 insoluble protein fraction from UHT-treated milk. Journal of Agricultural and Food Chemistry, 60(32), 8044–8050. https://doi.org/10.1021/jf3024563
Qian, F., Sun, J., Cao, D., Tuo, Y., Jiang, S., & Mu, G. (2017). Experimental and modelling study of the denaturation of milk protein by heat treatment. Korean Journal for Food Science of Animal Resources, 37(1), 44.
Ryan, K. N., & Foegeding, E. A. (2015). Formation of soluble whey protein aggregates and their stability in beverages. Food Hydrocolloids, 43, 265–274. https://doi.org/10.1016/j.foodhyd.2014.05.025
Scholtz, V., Pazlarova, J., Souskova, H., Khun, J., & Julak, J. (2015). Nonthermal plasma—A tool for decontamination and disinfection. Biotechnology Advances, 33(6), 1108–1119.
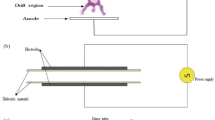
Sharma, S., & Singh, R. K. (2022). Effect of atmospheric pressure cold plasma treatment time and composition of feed gas on properties of skim milk. LWT-Food Science and Technology, 154, 112747. https://doi.org/10.1016/j.lwt.2021.112747
Sharma, S., Kumar, P., Betzel, C., & Singh, T. P. (2001). Structure and function of proteins involved in milk allergies. Journal of Chromatography B: Biomedical Sciences and Applications, 756(1), 183–187. https://doi.org/10.1016/S0378-4347(01)00107-4
Sutariya, S. G., Huppertz, T., & Patel, H. A. (2017). Influence of milk pre-heating conditions on casein–whey protein interactions and skim milk concentrate viscosity. International Dairy Journal, 69, 19–22.
Takai, E., Kitamura, T., Kuwabara, J., Ikawa, S., Yoshizawa, S., Shiraki, K., & Kitano, K. (2014). Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. Journal of Physics D: Applied Physics. https://doi.org/10.1088/0022-3727/47/28/285403
Vasbinder, A. J., & De Kruif, C. G. (2003). Casein–whey protein interactions in heated milk: The influence of pH. International Dairy Journal, 13(8), 669–677.
Xu, L., Hou, H., Farkas, B., Keener, K. M., Garner, A. L., & Tao, B. (2021). High voltage atmospheric cold plasma modification of bovine serum albumin. LWT - Food Science and Technology, 149, 111995. https://doi.org/10.1016/j.lwt.2021.111995
Yu, J. -J., Ji, H., Chen, Y., Zhang, Y. -F., Zheng, X. -C., Li, S. -H., & Chen, Y. (2020). Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. International Journal of Biological Macromolecules, 158, 1194–1203.
Zhang, R., Pang, X., Lu, J., Liu, L., Zhang, S., & Lv, J. (2018). Effect of high intensity ultrasound pretreatment on functional and structural properties of micellar casein concentrates. Ultrasonics Sonochemistry, 47, 10–16.
Acknowledgements
The authors are thankful to Dr. Catrin Tyl (Department of Chemistry, Biotechnology and Food Science, Norwegian University of Life Sciences) for her directions and technical expertise with the experiments and Mr. Carl Ruiz (Department of Food Science and Technology, University of Georgia) for his assistance with cold plasma processing. We also wish to extend gratitude to Mr. Clay Crippen (Szymanski lab, Complex Carbohydrate Research Center, University of Georgia) for assistance with electrophoresis experiments.
Author information
Authors and Affiliations
Contributions
Shruti Sharma: idea conception, study design, data collection and data analysis, and writing the original draft of the manuscript. Himanshu Prabhakar: data analysis and result compilation. Rakesh K. Singh: idea conception, direction in study design, supervision in data collection and analysis, and review and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, S., Prabhakar, H. & Singh, R.K. Atmospheric Cold Plasma-Induced Changes in Milk Proteins. Food Bioprocess Technol 15, 2737–2748 (2022). https://doi.org/10.1007/s11947-022-02915-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02915-z