Abstract
This work explores the use of ultrasound (US) as a means of intensifying the impregnation of apple cubes with vitamin B12 (cyanocobalamin). The effect of different US power densities (90 and 200 WL−1) and treatment times (5, 10, and 15 min) was evaluated, on vitamin load, vitamin stability, and physicochemical and microstructural properties of the fruit matrix. The US enhanced the impregnation producing high cyanocobalamin content products (0.12–0.19 mg vitamin/g db.). Vitamin losses in the sonication medium due to US application were not significant. Impregnated samples exhibited higher moisture and lower soluble solids with respect to the untreated fruit. Changes in chromatic coordinates were well correlated to vitamin uptake. Only at the highest treatment intensities (200 WL−1, 10, and 15 min) was a marked softening observed, which agreed with the microstructural changes observed in fruit tissues. Results permit US-assisted impregnation to be considered a promising technology in the preparation of vitamin B12 fortified apple cubes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin B12 (cobalamins) comprises a set of water-soluble micronutrients naturally present in meat and other animal origin foods. They act as a coenzyme in the methyl metabolism, being essential for human growth and development in all lifespan stages (Banjari & Hjartåker, 2018). Various factors, such as cultural, religious, or economic, among others, may restrict the daily consumption of animal-source foods, and this goes some way toward explaining the increasing deficiency of vitamin B12, recently noticed in many groups of the population (Allen, 2018). In addition, there are some physiological conditions, occurring mostly in the elderly, related to difficulties in absorbing this vitamin from food due to the lack of gastric acid or the intrinsic factor required for vitamin B12 absorption. In all cases, the prevalence of vitamin B12 deficiency implies adverse effects, which have been well documented and updated in recently published works (Allen, 2018; Bajaj & Singhal, 2020).
The fortification of foods with vitamin B12 has been proposed as a way of meeting these nutritional requirements (Bajaj & Singhal, 2020; Saffarionpour & Diosady, 2021) and could be a feasible alleviation strategy against malnutrition and food insecurity (Qiu et al., 2019). The most common vitamin B12 fortified foods include cereals, dairy, milk substitutes, sweets, fat spreads, non-alcoholic and instant beverages, and desserts. In most cases, the vitamin is directly added to non-structured products or produced in situ by fermentation (Bajaj & Singhal, 2020).
Plant tissues, including fruits and vegetables, constitute interesting alternatives as food matrices for fortification with vitamin B12, conforming to a demand for healthy and nutritious products (Joshi et al., 2020; Mieszczakowska-Frąc et al., 2016). Additionally, fortified fruits may result in attractive raw materials for the development of snacks or topping for dairy, among other possibilities, where the preservation of the plant structure is desirable (Tavera-Quiroz et al., 2014). However, the incorporation of nutrients in cell structured foods represents a challenge (Rojas et al., 2019). In this context, several innovative technologies, such as vacuum (Castagnini et al., 2015), ohmic heating (Moreno et al., 2016), high-pressure (George et al., 2016), and ultrasound (Miano & Augusto, 2018), have been evaluated as approaches (in individual or combined form) to change the composition of cellular foods with nutrients or bioactive compounds (Hamedi et al., 2018).
Ultrasound (US) has been proposed as a promising technology for the intensification of mass transfer unit operations in the food industry (Villamiel et al., 2017; Wiktor et al., 2016). It is based on the formation of longitudinal ultrasonic waves in a liquid medium, where pressure fluctuations generate gas/vapor-filled bubbles which form, grow, and collapse, in a phenomenon known as cavitation (de Medeiros et al., 2019). The implosion of these microbubbles results in various mechanical and thermal effects, which favor the mass transference (Villamiel et al., 2017).
It has been reported that when plant tissues are immersed in the transmission medium, the US promotes a series of compressions and expansions in the material (also called the “sponge effect”), which favors the entry of the solvent through pores and capillaries (Rodríguez et al., 2015). The solvent penetration increases the internal surface area easing the mass transfer processes (Hamedi et al., 2018; Li et al., 2020). Thus, it is expected that US promotes the bidirectional migration of compounds between the food structure and the external medium, when it is applied in osmotic dehydration, impregnation, or extraction processes (de Medeiros et al., 2019; Feng et al., 2019).
In this context, we hypothesized that ultrasound can improve the impregnation process of sensitive nutrients such as vitamin B12 in cell structured foods (fruit tissue). Several studies have described the use of US as pre-treatment, post-treatment, or applied simultaneously with vacuum (Feng et al., 2019; Mashkour et al., 2018; Yılmaz & Bilek, 2018). However, to the best of our knowledge, the available literature on the effect of only ultrasound on the impregnation of nutrients in plant tissues is very scarce. Furthermore, the effectiveness of US-assisted impregnation of fruit tissue with vitamin B12 has not been reported. Therefore, the objective of this work was to evaluate the US-assisted impregnation of vitamin B12 in apple cubes, by monitoring the impact on the physicochemical and microstructural properties of fruit tissue, and the vitamin stability in the immersion media. In particular, the effect of different US power densities and treatment times was evaluated.
Materials and Methods
Materials
Apples (Malus domestica var. Granny Smith) were purchased from a local shop in Mallorca (Spain) and stored at 4 °C, for 2–3 days until use. Apples with total soluble solid content of 12 ± 1°Brix (25 °C) and moisture of 85.1 ± 1.1% wb. were selected. Cyanocobalamin was chosen as a bioactive form of vitamin B12, since it can be relatively easily produced in large quantities (Chen et al., 2010). A cyanocobalamin food supplement (1000 µg per tablet) was commercially acquired (Solgar®, The Nature’s Bounty Co. Ltd., Spain). Cyanocobalamin used as a standard was purchased from Sigma Chemical Co. (St. Louis, MO, USA). HPLC-grade acetonitrile was purchased from Scharlau (Barcelona, Spain). All other chemicals used were analytical grade. All aqueous solutions were prepared with double distilled water obtained from the Milli-Q system (Millipore, Bedford, MA, USA).
Impregnation Treatments
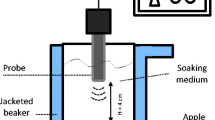
The apples were washed, peeled, cored, and cut into cubes of 10.0 ± 0.1 mm edge with a stainless steel grid cutter just before the impregnation treatments. About 10 ± 1 g of apple cubes (~ 10 units) was immersed in 50 ml of freshly prepared food supplement solution containing 150 μg mL−1 of cyanocobalamin. Apple cubes and impregnation solution were placed within a jacketed vessel (inner diameter: 52.5 mm). The temperature of the impregnation medium during sonication processes was maintained at 21 ± 3 °C by circulating refrigerant liquid through the jacket. The ultrasound application was performed by using a UP400S ultrasonic generator with 400 W power and 24 kHz ultrasonic frequency (Hielscher Ultrasonics GmbH, Germany), equipped with a titanium probe of 40 mm. The probe was immersed 5 mm below the impregnation solution surface. Pulse duration was set at 0.5 s. Two different amplitudes 20 and 100% were used to achieve two different power densities: 90 ± 2 and 200 ± 3 WL−1, respectively, as determined previously following the calorimetric method described by Dalmau et al. (2020). The US treatments were performed for 5, 10, and 15 min.
A no-US assisted impregnation process was carried out for comparative purposes at the same fruit/impregnation solution ratio, vitamin concentration, geometry conditions, and treatment times but with agitation. The apple cubes were kept gently moving (50 rpm) in a stirrer (RZR 2021, Heidolph, Germany) equipped with a four-blade propeller (50 mm diameter) placed at 2 cm from the liquid interface, and the temperature was also maintained by circulating liquid refrigerant at 21 ± 2 °C through the jacket. Considering the photosensitivity of vitamin B12, all experiments were performed in vessels covered with aluminum foils to avoid exposure to light.
Physicochemical Properties
Treated samples were removed from the impregnation media, drained, rinsed three times with doubly distilled water, and superficially dried with paper towels. Physicochemical analyses (including weight, moisture, and soluble solids) were determined immediately after cutting fresh samples and after each impregnation treatment to prevent time-dependent changes. The moisture content was gravimetrically determined according to the AOAC Official Method 934.06 (2000) and expressed as % wet basis (wb.). The moisture fraction (w) was calculated as moisture/100 and expressed as unit fraction. Then, the solid fraction (s) was calculated considering that w + s = 1. A mass balance, based on the change of mass (M), water fraction (w), and solid fraction (s) of samples before and after each treatment, was performed. The water (∆W%) and solid (∆S%) variations were expressed as a percentage according to the following equations:
where subscripts 0 and 1 indicate before and after treatment, respectively.
Total soluble solids (°Brix) were determined by refractometer. For this purpose, the apple cubes were crushed in a mortar and the liquid fraction was filtered and analyzed in a hand refractometer (ZUZI, Spain) at 25 °C. Similarly, the pH values were measured in sample homogenates with a pH meter (Crison pH25, Spain). Sample homogenates were made at 25,000 rpm with an Ultraturrax T25 (IKA, Staufen, Germany).
Vitamin Extraction and Quantification
Approximately 5 g of the impregnated fruit was homogenized in the presence of 5 mL of phosphate buffer pH 5.8 using an Ultraturrax T25 (IKA, Staufen, Germany) at 15,000 rpm for 15 s. The mixture was then centrifuged (4000 rpm, 10 min). The supernatant was collected and the volume was made up to 10 mL with phosphate buffer, before filtering the solution through a 0.45 µm nylon filter Magna (GVS, Roma, Italy).
Vitamin content in fruit extracts and impregnation solutions (before and after US treatment) were quantified by HPLC–DAD as described by Qiu et al. (2019), with slight modifications. Chromatographic separations were achieved using an HPLC analytical system (Waters, Milford, MA, USA) consisting of a Waters 600E pump, a Waters 2966 photodiode array detector, and a Waters 717 plus autosampler controlled by software (Empower). For each analysis, 10 μL of the extract was injected in a 150 × 4.6 mm C18 Gemmini® column (5 μm, 110 Å) at 30 °C. An isocratic mobile phase (87% of 20 mM phosphate buffer pH 3, and 13% of LC-grade acetonitrile) was set at a flow rate of 1 mL min−1. A cyanocobalamin peak was identified at RT = 6.4 min and 360.6 nm. The cyanocobalamin content was determined using a calibration curve y = 1·108x (R2 = 0.99) with QL = 0.00052 mg/mL, and the results were expressed as mg vitamin B12 g−1 of sample db., or, eventually, mg vitamin B12 mL−1 impregnation solution.
Color and Textural Analysis
The surface color of fresh and impregnated samples was determined immediately after cutting the cubes to avoid enzymatic browning, and then immediately after the impregnation treatments. For this purpose, a CM-5 spectrophotometer (Konica Minolta, Japan) was used and chromatic properties were evaluated in terms of CIELab* color coordinates. Readings were performed using a D65 illuminant reference system, with a 10 opening angle and considering the excluded specular component. The intensity of the color change after impregnation was calculated in terms of total color difference as described in Umaña et al. (2020) according to:
where the subscript 0 refers to average L*, a*, and b* parameters of fresh sample. Ten readings were taken from the faces of different apple cubes for each trial.
Textural analysis of fresh and treated samples was immediately performed after cutting cubes and immediately after the impregnation treatments. For this purpose, a material testing machine Z100 (Zwick, Germany) was used. Compression of 60% strain was carried out using a 75 mm diameter cylindrical P/2 probe with a testing speed of 6 mm/s and trigger force of 3 N. The maximum force (N) and elasticity modulus (N mm−2) values were obtained by testXpert (Zwick, Germany) software. Textural measurements were performed on 5 apple cubes for each trial.
Microstructural Analysis by Light Microscopy
Microstructure observation was performed on fresh and impregnated samples according to the methodology described by Eim et al. (2012). Immediately after cutting the fresh samples and after the impregnation treatments, samples were immersed in a formaldehyde solution (10% v/v) for at least 24 h, dehydrated with ethanol, and finally embedded in paraffin at 60 °C, for 3 h. Treated samples were sectioned into 4–5 µm slices with a microtome Finesse 325 (Thermo Shandon, Cheshire, UK). Slices were stained with acid periodic acid-Schiff to visualize cell structures. A light microscope (Olympus BX60FS, Japan) equipped with a digital image capture system Moticam 3.0 MP (Xiamen, China) was used to obtain images at 50 × magnification. The microstructure was analyzed from both the periphery and the center of the inner face of cut-in-half sample cubes. Shown images guarantee representative fields.
Statistical Analysis
Impregnation treatments were evaluated at least in duplicate, and for each one, two trials were carried out. The first trial was intended for analysis of vitamin content, moisture, color, and microstructure, while the second allowed sampling for texture, Brix, and pH measurements. Both analytical and instrumental measurements were performed at least in triplicate. Data were evaluated by employing one-way ANOVA at a significance level of p < 0.05. To find the significant difference between the mean values, the post hoc Tukey’s test was used. The statistical analysis and artwork were performed through GraphPad Version 4 (GraphPad, Software Inc., San Diego, CA, USA).
Result and discussion
Moisture, Water, and Solid Changes
Table 1 shows the moisture (% wb.) and soluble solids (°Brix) of raw and treated samples, and the water (∆W%) and solid variations (∆S%) due to the impregnation treatments.
The moisture of apple cubes after the US-assisted impregnation treatment increased by 3.1–4.3%, in comparison with the untreated samples, regardless of both the US power density and the treatment time (Table 1). In experiments with agitation (no-US), the samples showed an intermediate moisture increase (1.1–2.8%). Similar increases in moisture were reported for apple (3.29–4.06%) (Mieszczakowska-Frąc et al., 2016), mango (2.74%) (de Medeiros et al., 2019), and banana (5.17%) (Azoubel et al., 2010) after immersion in water or in very diluted media.
During impregnation processes, simultaneous changes in water and dry matter content can occur, consequently modifying the total weight of samples and hindering the comparison in terms of moisture variation, especially when it is expressed on wet basis (% wb.). To clarify the effect of the US on variation of water and solid fractions of the fruit during impregnation treatments, a mass balance (Eqs. 1 and 2) was proposed (Table 1).
In most cases, the ∆W% was close to zero and no significant differences was found among the treatments, indicating that there was no major changes in the water content of the samples studied. However, at 200 WL−1 for 15 min, positive values of ∆W% indicated that water gain had occurred. According to Wiktor et al. (2016), water changes in immersed and sonicated samples require a consideration of both the sample and medium properties, as well as the treatment conditions.
In the present study, the impregnation solution may be considered hypotonic with regard to fruit cells. Hence, water transfer may occur toward fruit tissue by simple diffusion. However, water uptake was not observed for most treatments, except for those samples subjected to the highest intensity treatment (200 WL−1, 15 min). Water uptake might be explained considering a hydrodynamic type mechanism, as well as the occurrence of microstructural changes which would have prevailed only in this operating condition.
The hydrodynamic mechanism has been described in the vacuum-assisted osmotic dehydration processes (Feng et al., 2019). It comprises the outflow of the intercellular air by vacuum application, and the filling of empty intercellular spaces with the osmotic solution when pressure is restored. A similar effect could be considered in the present study, where the alternating compression and decompression of material caused by ultrasound waves (sponge effect) would favor the outflow of intercellular air, with the consequent penetration of the impregnation solution (mainly water) into plant tissue.
On the other hand, it is well known that above a certain intensity, mechanical effects induced by ultrasonic waves modify the capillary structures of plant tissues, as well as the intense cavitation altering cell permeability (Mashkour et al., 2018). Both effects help explain why penetration of the liquid phase into plant tissues (involving water uptake) occurred only at the highest treatment intensity.
Water gain was also reported in other high moisture vegetable matrixes such as mangoes (de Medeiros et al., 2019), apples (Wiktor et al., 2016), and melons (da Silva et al., 2016) exposed to US during immersion of samples in water or hypotonic media.
With regard to solid variations, negative values of ∆S% were observed for all impregnation treatments, indicating that the samples lost solids (Table 1). In all cases, the solid concentration gradient existing between the samples and the diluted impregnation medium drove the mass transfer (da Silva et al., 2016; Feng et al., 2019; Mieszczakowska-Frąc et al., 2016). Although data variability did not allow a finding of distinctive effects on ∆S% among the studied conditions, marked differences were best noticed when treated samples were compared with fresh apples in terms of soluble solids (°Brix).
US promoted the leakage of soluble solids and this effect was more evident at the highest ultrasound power density and at the longest treatment time. This could be explained by considering that shear forces generated by cavitation led to the gradual formation of microchannels, the erosion of surfaces, and probably the fragmentation of the tissues, which increased the rate of extraction of cell compounds as the treatment intensity rose (Goula et al., 2016; Lelas, 2007; Yao, 2016). Similar losses of water-soluble solid were also reported for apples (Wiktor et al., 2016) and mangos (de Medeiros, 2019) by sonication in water or diluted media.
Leakage of food components has been considered an undesirable side effect when US is applied to impregnation or osmotic dehydration processes (Mashkour et al., 2018; Mieszczakowska-Frąc et al., 2016), since most natural compounds of nutritional or functional interest, like pigments, aromas, or antioxidants, may be extracted (Wiktor et al., 2016; Yılmaz & Bilek, 2018).
US-Assisted Impregnation of Apple Cubes with Vitamin B12
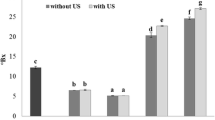
The effect of US power density and treatment time on vitamin impregnation in apple cubes was evaluated. Figure 1 shows the content of vitamin B12 in samples after US and no-US treatments.
Concentration of vitamin B12 in apple cubes after impregnation treatments by agitation (no-US) and ultrasound assisted at 90 and 200 WL−1 for 5, 10, and 15 min. Mean and standard deviation of at least three replicates (n = 3) are shown. Different letters indicate statistical differences (p < 0.05) according to the Tukey’s test
The samples showed varying amounts of vitamin B12. The US-assisted impregnated samples revealed higher contents of vitamin in comparison with the no-US treated samples, indicating that the US improved the diffusion of the vitamin from the impregnation solution toward the fruit tissue.
Among the US-assisted treatments, the studied US power densities produced significant differences (p < 0.05) in the vitamin load (Fig. 1). At the lowest US power density (90 WL−1), time did not exert significant effects, and the samples exhibited an average vitamin content of 0.12 ± 0.01 mg/g db. At the highest power US (200 WL−1), a sharp increase in vitamin load with treatment time was observed. The highest US power density increased the vitamin load in the apples treated for 10 and 15 min, by 33% and 69%, respectively, in comparison with those impregnated at 5 min. This last processing condition led to vitamin infusions comparable with those obtained at 90 WL−1.
As observed, US positively affected the vitamin transfer toward the fruit tissue, the amount of infused vitamin in the apple cubes being proportional to the treatment intensity. The improvement of vitamin diffusion by US may be explained by considering the different mechanical effects caused by ultrasonic waves (Yılmaz & Bilek, 2018).
Gradient vitamin concentration between the external media and samples (devoid of vitamin B12) constitutes the main driving force for mass transfer. Thus, the penetration of the immersion medium into the apple cubes largely explains the vitamin diffusion, as a result of an increased exposed area. In this sense, the hydrodynamic mechanism induced by the sponge effect (Wiktor et al., 2016), besides water gain, also caused the vitamin uptake. In addition, cavitation phenomena could also contribute to the mass transfer, its effect being more noticeable at the highest US intensities (Yao, 2016). In fact, at a fixed US frequency, the higher the US power density, the higher the number of cavitation bubbles (Mashkour et al., 2018). When the bubbles collapse, microscopic channels in the fruit tissue are created, which modify the cell permeability and ease the solvent penetration (Mashkour et al., 2018). Thus, the diffusion of vitamin into the sample is not constrained by molecular size. As US intensity increases, cell rupture may also occur (Lelas, 2007) which further extends the mass transfer area. In those conditions, mass transfer increases with treatment time, as was observed for the longest immersion periods.
In the present study, the highest vitamin infusion was verified at 200 WL−1 and 15 min (Fig. 1), and this was related to the net influx of water into the fruit matrix (Table 1). From this, it could be assumed that the vitamin entered the plant tissue along with water, in a process favored by mechanical effects of acoustic energy. de Medeiros et al. (2019) also reported an improvement in the uptake of phenolic compounds in mango, when US-assisted impregnation at atmospheric pressure in a grape residue extract was compared with simple dipping. Similarly, Yılmaz and Bilek (2018) achieved higher contents of antioxidant compounds in apple discs by increasing the US power density in combined ultrasound/vacuum impregnation treatments using black carrot concentrate as immersion media.
In the present study, US-assisted impregnation allowed apple cubes to be fortified with vitamin B12 which represents an improvement in the nutritional quality of this type of product. The vitamin B12 content of US-treated samples varied between 1350 and 2160 µg per 100 g of product (wb.), a result highly superior to those reported for foods considering sources of cobalamin such as tuna fish (9.43 µg per 100 g wb.), beef (1.47 µg per 100 g wb.), or dairy (0.45 µg per 100 g wb.) (Banjari & Hjartåker, 2018). Thus, at the condition studied, in which vitamin B12 infusion was the highest (200 WL−1, 15 min), 50 g of obtained product could provide an amount of cyanocobalamin equivalent to that present in a tablet of commercial dietary supplement (~ 1000 µg). Therefore, its consumption could possibly assist in preventing a deficiency of vitamin B12, since it comfortably covers the recommended dietary intake of cobalamin (0.9 to 2.4 µg per day). Despite the fact that there is no toxicological risk related to high doses of vitamin B12 (Allen, 2018), the amount to incorporate in a food formulation must finally be decided on the basis of its bioavailability, the frequency of consumption, the targeted population, and the expected product quality (Joshi et al., 2020).
Impact of US Treatments on Net Change and Stability of Vitamin B12 in the Impregnation Media
Figure 2a shows the net variation of vitamin B12 concentration in the impregnation medium after each treatment, with reference to the initial solution concentration.
In all cases, a reduction in vitamin B12 concentration was observed (negative percentages). Except for the treatment carried out with US at 90 WL−1 for 5 min, the application of US, regardless of both the power and the impregnation time, caused similar changes to those found for no-US assisted impregnation over 15 min. This last could indicate that treatment time exerts a significant effect on vitamin concentration change.
The change in vitamin B12 concentration after treatment may be interpreted as being caused by the combined effect of the net transport of vitamin to the inner fruit tissue, as well as the loss of vitamin by chemical degradation. To evaluate the vitamin stability during the impregnation process, a quantitative analysis was performed. For this purpose, the loss of vitamin B12 was calculated for each treatment considering the initial, residual, and infused vitamin amounts (directly measured). Figure 2b depicts the residual, infused, and lost vitamin fractions expressed as percentages of the initial concentration for each treatment.
At the lowest US power density (90 WL−1), the percentage of vitamin loss increased with treatment time (p < 0.05), and the observed values were similar to those found for the no-US treatments. This result could indicate that vitamin degradation was mainly due to impregnation time regardless of US application.
At the highest US power density (200 WL−1), the vitamin B12 loss did not show a clear correlation with treatment time (Fig. 2b). In fact, at 15 min, the percentage of lost vitamin (2.9 ± 0.4%) was markedly lower than that produced at 90 WL−1 (6.1 ± 0.4%) and with no-US (6.2 ± 0.1%), evaluated at the same time. The improved vitamin stability at the highest power US and longest treatment time could be explained by considering the greatest infusion of vitamin into the fruit tissue achieved under this particular processing condition (Fig. 1).
Vitamin B12 is typically unstable and is easily degraded by different mechanisms involving pH, light, oxygen, and by interaction with food components. Particularly, cyanocobalamin in solution is mainly pH-sensitive, being more stable at mildly acidic conditions (Bajaj & Singhal, 2020; Qiu et al., 2019). In this study, the impregnation solution containing vitamin B12 was prepared by dissolving a commercial dietary supplement at 1% w/v in water. The vitamin and the filler ingredients used in tablet formulation (e.g., stearic acid, magnesium stearate, mannitol, maltodextrin, gum arabic, modified cellulose, and flavoring) were at a very low concentration, resulting in a solution pH of 7.1 ± 0.1. In addition, the pH of apples (3.31 ± 0.06) remained unmodified throughout the impregnation process (p > 0.05). In this sense, the acidic cell medium could contribute to stabilizing the vitamin, and the cyanocobalamin introduced into the fruit tissue could have a relatively higher protection in comparison with those exposed to the pH of impregnation media. Thus, the higher the amount of impregnated vitamin, the lower the loss of vitamin.
Color and Textural Properties
Chromatic properties of samples were instrumentally evaluated in the CIELab* color space (Fig. 3).
Chromatic coordinates L*, a*, and b* and color change (ΔE) for fresh apple and apple cubes impregnated with vitamin B12 by agitation (no-US) and power ultrasound (at 90 and 200 WL−1) during 5, 10, and 15 min. Mean and standard deviation of five replicates (n = 5) are shown. Different letters indicate statistical differences (p < 0.05) according to the Tukey’s test
Fresh apples (FA) showed L* (60 ± 2), a* (− 2 ± 1), and b* (16 ± 2) values in agreement with the typical greenish pale color of green apple pulp (var. Granny Smith) (Aguirre-García et al., 2020; Tavera-Quiroz et al., 2014). In all cases, the color of samples varied with the processing conditions (p < 0.05), and the differences in color coordinates were more noticeable when US was applied.
A gradual reduction in luminosity (L*) was observed by increasing treatment intensity. Darkening may be explained by considering the uptake of the colorful impregnation solution of cyanocobalamin. Similar results have been reported in apples impregnated in highly colored blueberry juice (Castagnini et al., 2015) or mango infused with grape residue extract (de Medeiros et al., 2019).
A noticeable increase in redness (a*) was observed, and this was more evident at long impregnation times and higher US power densities. This change agreed with the visual perception of the pink color of the impregnated samples, and it was attributed to the deep red color of cyanocobalamin present in the impregnation medium (Allen, 2018). Production of colored fresh fruits has been described as a novel approach to develop healthy and attractive snacks in food technology (Yılmaz & Bilek, 2018). The fortification with vitamin B12 could achieve an additional purpose by imparting desirable nutritional and sensory properties.
In most cases, a decrease in yellowness (b*) with respect to the fresh sample was observed, and this could be partially explained by the diffusion of fruit pigments into the solution. As was reported by Feng et al. (2019), the immersion of fruits can cause the loss of some food components in osmotic or impregnation solutions. This may be caused by the hydrophilic nature of pigments and the cell rupture by the US effect. However, no clear correlation between b* values and the different treatment intensities was found, and the observed variability could be attributed to the prevailing effect of natural variations in fruit color.
Finally, the total color difference was calculated for each studied impregnation condition, taking as references the average color coordinates of the untreated samples. As expected, a gradual increase in ∆E with the intensity of treatment was observed, and this was directly proportional (Pearson’s r = 0.92) to the vitamin content after impregnation.
The assessment of textural properties provides useful insights into macrostructural changes due to US application during the impregnation process. Figure 4 shows the elastic modulus (N mm−2) and the maximum force (N) evaluated for paired sets of fresh and treated samples for each operating condition.
Textural parameters, (a) elastic modulus and (b) maximum force, obtained for apple cubes after (
 ) and before (
) and before (
 ) impregnation treatment with vitamin B12, by agitation (no-US) and assisted with power ultrasound (90 and 200 WL−1) during 5, 10, and 15 min. Mean and standard deviation of five replicates (n = 5) are shown. Asterisks represent significance level: ***p < 0.001, **0.001 < p < 0.01
) impregnation treatment with vitamin B12, by agitation (no-US) and assisted with power ultrasound (90 and 200 WL−1) during 5, 10, and 15 min. Mean and standard deviation of five replicates (n = 5) are shown. Asterisks represent significance level: ***p < 0.001, **0.001 < p < 0.01
Despite natural variability found in the texture of fresh apples, only a significant decrease (p < 0.05) in the elastic modulus and the maximum force was observed for samples impregnated at the treatments performed at 200 WL−1 for 10 and 15 min. Lower elastic modulus and lower maximum force indicate lower rigidity and lower resistance in the material, which could be interpreted as a softening of the apple cubes. The change in both parameters might be explained by considering the loss of the cell structure integrity as a consequence of the ultrasonic vibration (Lelas, 2007). In fact, cell rupture, and microchannel formation induced by US, has been previously related to firmness reduction in plant tissues (de Medeiros et al., 2019). At the highest intensity treatment, the modification in texture parameters agreed with the impregnation effectiveness (Fig. 1) and hydration (Table 1) induced by US application.
Microstructural Changes
Figure 5 shows representative optical microscopic images of fresh and impregnated samples at 90 WL−1 for 15 min and at 200 WL−1 for 10 and 15 min.
For most of the conditions studied (images provided as supplementary file), and even for the treatment performed at 90 WL−1, for 15 min, apple cubes showed a cell structure similar to those of the untreated samples. Only after 10 min of impregnation treatment with the highest power density ultrasound (200 WL−1) did the samples exhibit noticeable changes in the intracellular structure (Fig. 5).
The microscopic pictures of samples treated at 90 WL−1 for 15 min revealed that the impregnation achieved in this processing condition (Fig. 1) occurred without tissue damage. However, at 200 WL−1 for 10 and 15 min, acoustic energy negatively affected the cell walls, the medial lamella, and the plasmalemma integrity, causing a clear disruption of cellular structures. These changes were more appreciable at the apple cubes’ periphery than in the center (Fig. 5).
More specifically, the tissue of samples treated at 200 WL−1 for 15 min was severely distorted showing numerous breakdowns and voids. The cellular damage observed in these samples provides enough evidence to explain the impact of this treatment on soluble solid loss, water uptake (Table 1), and changes in textural properties (Fig. 4). In fact, the ruptured cell walls and membranes increased the transfer area, easing the leakage of cell constituents (Lelas, 2007). The higher porosity and presence of voids could improve the absorption capacity of the tissue resulting in high water uptake (Feng et al., 2019) and, consequently, of the vitamin. Finally, as expected, the structural changes affected mechanical attributes (Villamiel et al., 2017).
Although the impregnation assisted by power ultrasound at 200 WL−1 promoted the highest vitamin B12 infusion, the extent of cell damage, loss of compounds naturally present in apples, and the softening of texture may negatively affect the quality properties of the final product. Additionally, in damaged tissues, the leakage of cell content during storage could provoke the leakage of the impregnated vitamin, and finally, the fortification would be lost.
According to results, there exist a range of intermediate intensities of treatment, for which it is possible to incorporate useful amounts of vitamin B12 in apple cubes preserving the tissue integrity. As reported, impregnation strategies only succeed if substances stay in the tissue long enough for the product to be commercialized, purchased, and consumed, and this condition is achieved in tissues with cells that have not been irreversibly damaged.
A similar effect of US on plant tissue was previously reported (Feng et al., 2019; Rodríguez et al., 2015; Yılmaz & Bilek, 2018). In most cases, authors found it desirable to achieve the highest impregnation with the least cell disruption and the lowest solid leakage (Mashkour et al., 2018; Neri et al., 2016).
Conclusions
This work considers the use of US as a means of intensifying the impregnation of apple cubes with vitamin B12. US enhanced the bidirectional mass transfer between fruit tissue and diluted immersion media containing cyanocobalamin. Increasing US power density and treatment time improved the vitamin impregnation. US-impregnated samples showed cyanocobalamin contents which varied between 1350 and 2160 µg vitamin B12 per 100 g of product (wb.). Vitamin uptake was related to sample color changes. For most of the evaluated processing conditions, apple cubes preserved the fresh-like texture. However, at the highest treatment intensity, a marked softening of fruit tissue was noticed, which was related to a deep disruption of cell structure. The introduction of useful amounts of vitamin B12 with the preservation of the quality attributes of the fruit is possible with the proper selection of US treatments. Since obtained samples are highly perishable, future research is required to evaluate the effect of US-assisted impregnation on the subsequent drying process, and the resultant quality and stability food attributes, to complete the product development.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Aguirre-García, M., Hernández-Carranza, P., Cortés-Zavaleta, O., Ruiz-Espinosa, H., Ochoa-Velasco, C., & Ruiz-López, I. (2020). Mass transfer analysis of bioactive compounds in apple wedges impregnated with beetroot juice: A 3D modelling approach. Journal of Food Engineering, 110003. https://doi.org/10.1016/j.jfoodeng.2020.110003
Allen, L. H. (2018). Efficacy and safety of vitamin B12 fortification. Food Fortification in a Globalized World (pp. 255–261): Elsevier. https://doi.org/10.1016/B978-0-12-802861-2.00026-2
AOAC. (2000). Official method Cd 934.06 moisture in dried fruits.
Azoubel, P. M., Baima, M. d. A. M., da Rocha Amorim, M., Oliveira, S. S. B. (2010). Effect of ultrasound on banana cv Pacovan drying kinetics. Journal of Food Engineering, 97(2), 194–198. https://doi.org/10.1016/j.jfoodeng.2009.10.009.
Bajaj, S. R., & Singhal, R. S. (2020). Degradation kinetics of vitamin B12 in model systems of different pH and extrapolation to carrot and lime juices. Journal of Food Engineering, 272, 109800. https://doi.org/10.1016/j.jfoodeng.2019.109800
Banjari, I., & Hjartåker, A. (2018). Dietary sources of iron and vitamin B12: Is this the missing link in colorectal carcinogenesis? Medical Hypotheses, 116, 105–110. https://doi.org/10.1016/j.mehy.2018.05.003
Castagnini, J., Betoret, N., Betoret, E., & Fito, P. (2015). Vacuum impregnation and air drying temperature effect on individual anthocyanins and antiradical capacity of blueberry juice included into an apple matrix. LWT-Food Science and Technology, 64(2), 1289–1296. https://doi.org/10.1016/j.lwt.2015.06.044
Chen, P., Wolf, W. R., Castanheira, I., & Sanches-Silva, A. (2010). A LC/UV/Vis method for determination of cyanocobalamin (VB 12) in multivitamin dietary supplements with on-line sample clean-up. Analytical Methods, 2(8), 1171–1175. https://doi.org/10.1016/j.lwt.2015.06.044/10.1039/C0AY00177E
da Silva, G. D., Barros, Z. M. P., de Medeiros, R. A. B., de Carvalho, C. B. O., Brandão, S. C. R., & Azoubel, P. M. (2016). Pretreatments for melon drying implementing ultrasound and vacuum. LWT, 74, 114–119. https://doi.org/10.1016/j.lwt.2016.07.039
Dalmau, E., Rosselló, C., Eim, V., Ratti, C., & Simal, S. (2020). Ultrasound-assisted aqueous extraction of biocompounds from orange byproduct: Experimental kinetics and modeling. Antioxidants, 9(4), 352. https://doi.org/10.3390/antiox9040352
de Medeiros, R. A. B., da Silva Júnior, E. V., da Silva, J. H. F., Neto, O. d. C. F., Brandão, S. C. R., Barros, Z. M. P., da Rocha, O. R., Azoubel, P. M. (2019). Effect of different grape residues polyphenols impregnation techniques in mango. Journal of Food Engineering, 262, 1–8. https://doi.org/10.1016/j.jfoodeng.2019.05.011
Eim, V. S., García-Pérez, J. V., Rosselló, C., Femenia, A., & Simal, S. (2012). Influence of the addition of dietary fiber on the drying curves and microstructure of a dry fermented sausage (Sobrassada). Drying Technology, 30(2), 146–153. https://doi.org/10.1080/07373937.2011.628428
Feng, Y., Yu, X., Yagoub, A. E. A., Xu, B., Wu, B., Zhang, L., & Zhou, C. (2019). Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic slices dehydration. Ultrasonics Sonochemistry, 50, 363–372. https://doi.org/10.1016/j.ultsonch.2018.09.038
George, J. M., Selvan, T. S., & Rastogi, N. K. (2016). High-pressure-assisted infusion of bioactive compounds in apple slices. Innovative Food Science & Emerging Technologies, 33, 100–107. https://doi.org/10.1016/j.ifset.2015.11.010
Goula, A. M., Thymiatis, K., & Kaderides, K. (2016). Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food and Bioproducts Processing, 100, 132–144. https://doi.org/10.1016/j.fbp.2016.06.016
Hamedi, F., Mohebbi, M., Shahidi, F., & Azarpazhooh, E. (2018). Ultrasound-assisted osmotic treatment of model food impregnated with pomegranate peel phenolic compounds: Mass transfer, texture, and phenolic evaluations. Food and Bioprocess Technology., 11(5), 1061–1074.
Joshi, A., Prajapati, U., Sethi, S., Arora, B., & Sharma, R. R. (2020). Fortification in fresh and fresh-cut horticultural products Fresh-Cut Fruits and Vegetables (pp. 183–204): Elsevier. https://doi.org/10.1016/B978-0-12-816184-5.00009-4
Lelas, V. (2007). Ultrasonic effect on pH, electric conductivity, and tissue surface of button mushrooms, brussels sprouts and cauliflower. Czech Journal of Food Science, 25(2), 90–100. https://doi.org/10.17221/757-CJFS
Li, Y., Wang, X., Wu, Z., Wan, N., & Yang, M. (2020). Dehydration of hawthorn fruit juices using ultrasound-assisted vacuum drying. Ultrasonics sonochemistry, 68, 105219.
Mashkour, M., Maghsoudlou, Y., Kashaninejad, M., & Aalami, M. (2018). Effect of ultrasound pretreatment on iron fortification of potato using vacuum impregnation. Journal of Food Processing and Preservation, 42(5), e13590. https://doi.org/10.1111/jfpp.13590
Miano, A. C., & Augusto, P. E. D. (2018). The ultrasound assisted hydration as an opportunity to incorporate nutrients into grains. Food Research International, 106, 928–935. https://doi.org/10.1016/j.foodres.2018.02.006
Mieszczakowska-Frąc, M., Dyki, B., & Konopacka, D. (2016). Effects of ultrasound on polyphenol retention in apples after the application of predrying treatments in liquid medium. Food and Bioprocess Technology., 9(3), 543–552.
Moreno, J., Espinoza, C., Simpson, R., Petzold, G., Nuñez, H., & Gianelli, M. (2016). Application of ohmic heating/vacuum impregnation treatments and air drying to develop an apple snack enriched in folic acid. Innovative Food Science & Emerging Technologies, 33, 381–386. https://doi.org/10.1016/j.ifset.2015.12.014
Neri, L., Di Biase, L., Sacchetti, G., Di Mattia, C., Santarelli, V., Mastrocola, D., & Pittia, P. (2016). Use of vacuum impregnation for the production of high quality fresh-like apple products. Journal of Food Engineering, 179, 98–108. https://doi.org/10.1016/j.jfoodeng.2016.02.002
Qiu, X., Zhang, H., Yin, Y., Brandes, H., Marsala, T., Stenerson, K., Cramer, H., & You, H. (2019). Determination of active vitamin B12 (cobalamin) in dietary supplements and ingredients by reversed-phase liquid chromatography: Single-laboratory validation. Food Chemistry, 298, 125010. https://doi.org/10.1016/j.foodchem.2019.125010
Rodríguez, Ó., Llabrés, P. J., Simal, S., Femenia, A., & Rosselló, C. (2015). Intensification of predrying treatments by means of ultrasonic assistance: Effects on water mobility, PPO activity, microstructure, and drying kinetics of apple. Food and Bioprocess Technology., 8(3), 503–515.
Rojas, M. L., Alvim, I. D., & Augusto, P. E. D. (2019). Incorporation of microencapsulated hydrophilic and lipophilic nutrients into foods by using ultrasound as a pre-treatment for drying: A prospective study. Ultrasonics Sonochemistry, 54, 153–161. https://doi.org/10.1016/j.ultsonch.2019.02.004
Saffarionpour S. & Diosady L. L. (2021). Multiple emulsions for enhanced delivery of vitamins and iron micronutrients and their application for food fortification. Food and Bioprocess Technology, 1–39.
Tavera-Quiroz, M. J., Urriza, M., Pinotti, A., & Bertola, N. (2014). Development and characterization of a baked snack from rings of green apples. Food and Bioprocess Technology., 7(8), 2218–2227.
Umaña, M., Turchiuli, C., Rosselló, C., & Simal, S. (2020). Addition of a mushroom by-product in oil-in-water emulsions for the microencapsulation of sunflower oil by spray drying. Food Chemistry, 128429. https://doi.org/10.1016/j.foodchem.2020.128429
Villamiel, M., García-Pérez, J. V., Montilla, A., Carcel, J. A., & Benedito, J. (2017). Ultrasound in Food Processing: Recent Advances: John Wiley & Sons. https://doi.org/10.1002/9781118964156
Wiktor, A., Sledz, M., Nowacka, M., Rybak, K., & Witrowa-Rajchert, D. (2016). The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Applied Acoustics, 103, 136–142. https://doi.org/10.1016/j.apacoust.2015.05.001
Yao, Y. (2016). Enhancement of mass transfer by ultrasound: Application to adsorbent regeneration and food drying/dehydration. Ultrasonics Sonochemistry, 31, 512–531. https://doi.org/10.1016/j.ultsonch.2016.01.039
Yılmaz, F. M., & Bilek, S. E. (2018). Ultrasound-assisted vacuum impregnation on the fortification of fresh-cut apple with calcium and black carrot phenolics. Ultrasonics Sonochemistry, 48, 509–516. https://doi.org/10.1016/j.ultsonch.2018.07.007
Acknowledgements
The authors acknowledge the Agencia Estatal de Investigación MCIN/AEI/10.13039/501100011033 through project (PID2019-106148RR-C43) and Universidad Nacional del Chaco Austral (UNCAUS – PI N° 79/18). They are grateful to the Fundación Carolina and the Ministry of Education, Culture, Science and Technology of Argentina, for the postdoc scholarship awarded to F. E. Vasile, making this work possible. We would also like to thank Joan Cifre, Josep Pablo Cànaves, and Fernando Hierro from Serveis Cientificotècnic of the Universitat de les Illes Balears for their assistance with textural analysis, HPLC, and microstructure analysis, respectively.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the Agencia Estatal de Investigación (PID2019-106148RR-C43/AEI/10.13039/501100011033), the Universidad Nacional del Chaco Austral, Argentina (UNCAUS – PI N° 79/18), and Fundación Carolina and the Ministry of Education, Culture, Science and Technology of Argentina.
Author information
Authors and Affiliations
Contributions
Franco Emanuel Vasile: conceptualization, methodology, validation, formal analysis, writing–review and editing, project administration. Susana Simal: conceptualization, resources, supervision, project administration, funding acquisition. Carmen Rosselló: conceptualization, resources, supervision, project administration, funding acquisition. Valeria Eim: conceptualization, resources, supervision, writing–review and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasile, F.E., Simal, S., Rosselló, C. et al. Power Ultrasound-Assisted Impregnation of Apple Cubes with Vitamin B12. Food Bioprocess Technol 15, 219–229 (2022). https://doi.org/10.1007/s11947-021-02752-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02752-6





 ), infused (
), infused (
 ), and lost (
), and lost (
 ) fractions of vitamin B12 expressed as percentages of initial concentration for each treatment
) fractions of vitamin B12 expressed as percentages of initial concentration for each treatment

