Abstract
The effect of sonication time and kind of liquid medium on polyphenol retention and microstructure changes during predrying treatment of apple tissue was investigated. The apple cubes from ‘Idared’ cultivar were submerged in water or sucrose solution and sonicated indirectly in beakers placed in a water bath fitted with ultrasound transducers (25 kHz, 0.1 W/cm3) at 40 °C. The treatment was conducted with and without ultrasound applied for 45 and 90 min. The content of individual polyphenols was monitored by a reverse-phase high-performance liquid chromatography (RP-HPLC) method. The dominant phenolic compounds in apple were procyanidins, accounting for 56 % of total polyphenols. While a significant effect of sonication on mass transfer intensification was observed when the samples were dehydrated in sucrose solution, almost no negative effects of ultrasound application were perceived on polyphenols concentration, except for dimers of catechin. When using ultrasound in water solution, an increase in polyphenol compound losses was noted. Furthermore, the ultrasound energy caused an apple tissue structure modification which additionally affected polyphenol retention during predrying treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The regular consumption of fruit and vegetables as part of a balanced diet has been associated with health benefits in different epidemiological studies. Many of them clearly identify the connection between the intake of fruit and vegetables and a reduced risk of cardiovascular disease and cancer, resulting from a high content of polyphenols (Knekt et al. 2002; WHO 2003; Manach et al. 2004; Hackman et al. 2008; Link et al. 2010; Hyson 2011). Among the species available in the temperate climatic zone, apples are one of the most often consumed, which makes them a vital source of bioactive compounds, especially polyphenols, offering well-documented health benefits (Hyson 2011; Francini and Sebastiani 2013).
The five predominant classes of polyphenols, secondary plant metabolites, recognized in apples are phenolic acids (e.g., chlorogenic acid), flavan-3-ols (e.g., (+)-catechin, (−)-epicatechin, and procyanidins), dihydrochalcones (unique to apple, e.g., phloridzine and phloretin-3-xyloglucoside), flavonols (e.g., quercetin and its glucosides), and also anthocyanins (e.g., cyanidin-3-galactoside) (Podsędek et al. 2000; Khanizadeh et al. 2007; Wojdyło et al. 2008). Particular classes of bioactive compounds can be found in unequal measures in various parts of the apple fruit. Phenolic acids are mainly present in the flesh and seeds, flavanols both in the skin and in fruit flesh, dihydrochalcons are most abundant in the seeds, while flavonols and anthocyanins are generally located in the skin (Łata et al. 2009; Awad et al. 2000; Khanizadeh et al. 2007; Fromm et al. 2012; Francini and Sebastiani 2013). According to literature, apple peel possesses a higher content of phenolic compounds than the flesh (Khanizadeh et al. 2007; Drogoudi et al. 2008), which to some extent restricts consumer access to some polyphenol groups, which are discarded along with the apple peel. Another reason explaining why vital polyphenol intake could be restricted is the fact that consumers fail to take full advantage of the benefits on offer when fresh fruit consumption is replaced by processed products such as juices or dried fruit, where processing operations can severely affect bioactive compounds. It is therefore advisable to use less detrimental processing methods, thus allowing the maximum possible level of polyphenols to be retained in the processed product. In the case of dried apple products, the main reason of polyphenol deterioration is related to prolonged material exposure to air flow and elevated temperature which substantially speed up oxidation as well as further condensation processes (Krokida et al. 1998; Devic et al. 2010b). These undesirable polyphenol alterations, visible as browning reactions, diminish external product quality and are also considered an indicator of bioactive components losses (Akyıldız and Öcal 2006). To improve dried product quality, certain types of pretreatments of fruit prior to drying are practiced, such as osmotic dehydration or, more recently, application of ultrasound (US). Both osmotic dehydration and ultrasound sonication of plant tissue before drying are considered effective forms of biomaterial pretreatment (Lenart 1996; Nowacka et al. 2012), which can greatly reduce drying time, especially when applied together (Cárcel et al. 2007; Fernandes and Rodrigues 2008). During tissue sonication, ultrasound affects the matrix structure by formation of microscopic channels that favor both mass transfer intensification during osmotic treatment, and higher water diffusivity during subsequent air-drying (Nowacka et al. 2014). From a technological point of view, the ultrasound seemed to be very promising, but unfortunately, in some cases, especially when a liquid medium during sonication was applied, substantial losses of bioactive compounds were reported (Stojanovic and Silva 2007; Opalić et al. 2009; Pingret et al. 2013). Although the available literature presents many experiments with ultrasound application before and during fruit and vegetable dehydration process (Siucińska and Konopacka 2014), the majority of them focus on monitoring the dynamics of mass transfer or physical properties, such as sugar gain, water loss, or changes in color or microstructure of plant tissue (Cárcel et al. 2007; Deng and Zhao 2008; Garcia-Perez et al. 2012; Nowacka et al. 2012; Schössler et al. 2012). With regard to phenolic compound retention during ultrasound-assisted fruit tissue treatment, the reports on negative effect of sonication on phenolic compound content and antioxidant activity (Stojanovic and Silva 2007; Opalić et al. 2009), more often than not, indicate a positive influence (Keenan et al. 2012). However, in both cases, the data is based on the total phenolic content measurements. To our knowledge, no study has been carried out to date, on the impact of high-power ultrasounds on the alteration of individual phenolic compounds during apple tissue treatment, except the studies dedicated to the ultrasound-assisted extraction of bioactive compounds in analytical methods (Vilkhu et al. 2008; Abid et al. 2014).
The aim of this study was to explain how the ultrasound-assisted drying pretreatment might influence the losses of particular polyphenol groups naturally contained in apple tissue. The effect of sonication time and kind of liquid medium on polyphenol retention during predrying treatment of apple cubes was investigated.
Materials and Methods
Chemicals
Food grade, commercial sucrose was used for the osmotic solution. Acetonitrile, glacial acetic acid (HPLC solvents were gradient grade), methanol, hydrochloric acid, formic acid, and sodium acetate were purchased from Mallinckrodt Baker Ltd. (Deventer, Netherlands). Phloroglucinol and L-ascorbic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA). All phenolic standards—(−)-epicatechin, (+)-catechin, 5-caffeoylquinic acid (chlorogenic acid), p-coumaric acid, and phloretin—were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Predrying Treatment
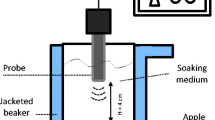
The common apple variety ‘Idared’ was selected as a raw material. Apples, picked at harvest maturity, were purchased from the Experimental Orchard of the Research Institute of Horticulture in Dąbrowice, Poland. The apples were peeled and cored by hand and then cut into 1-cm cubes with a shredder (Hällde RG-100, Kista, Sweden), after which they were submerged in liquid medium (distilled water or 60 °Bx sucrose solution) at a fruit-to-liquid medium ratio of 1:4. The treatment was conducted for 45 and 90 min with or without ultrasound application. The samples were sonicated at 40 °C in beakers placed in a water bath (InterSonic, Olsztyn, Poland) fitted with ultrasound transducers (25 kHz, 0.1 W/cm3, 5-μm wave amplitude) and a shaking plate (30 rpm). After treatment, the apple cubes were removed from bakers, strained and rinsed for a few seconds in tap water, and then blotted with absorbent paper to remove any excess solution. The samples were weighed and checked for their soluble solids and dry matter contents. To characterize mass transfer intensity, the weight reduction (WR), water loss (WL), and solid gain (SG) were calculated according to following formulas (Fernandes et al. 2008b):
where w is the sample mass (g), X is the sample moisture on wet basis (g water/g), Xs is the sample soluble content (g solid/g), and the subscripts i = initial and f = final state of sample.
For each treatment, two technological repetitions were carried out. The obtained material was immediately frozen at −20 °C to preserve it for further analyses. The apple cubes, destined for microstructure and procyanidin measurements, were additionally freeze-dried (−50 °C, pressure 20 Pa, FreeZone 6, Labconco, USA). As a reference material, freshly cut apple cubes, frozen without any treatment, were taken. The experiment was carried out in two technological replications. For each sample, the all chemical analyses were performed in two analytical repetitions.
Soluble solids contents (°Bx) were measured with a digital refractometer (Mettler-Toledo, type RE50).
Dry matter content (%) was determined using the gravimetric method by drying to constant weight at 70 °C under vacuum (3 · 103 Pa) according to PN-90/A-75101/03.
Extraction of Phenolic Compounds
The frozen apple cubes were disintegrated in dry ice using a grinder (Blixer 4, Robot Coupe, Vincennes Cedex, France) into a homogeneous powder. Extractions of phenolic compounds from apple powder (10 g) were carried out by homogenization for 2 min with a 70 % aqueous methanol solution (30 mL) containing 1 % of ascorbic acid (Ultra Turrax T25 Basic IKA-Werke, Staufen, Germany). The sludge was then transferred to a volumetric flask and filled to the 50-mL mark with 70 % methanol. The mixture was filtered through Whatman no. 3 filter paper. Before HPLC injection, all samples were diluted 1:3 in acetate buffer (mobile phase A).
Extraction of Procyanidins—Phloroglucinolysis
Quantification of procyanidins was performed according to the analytical method by Kennedy and Jones (2001), using phloroglucinol as a trapping reagent. The freeze-dried apple cubes were disintegrated in liquid nitrogen using a grinder (IKA A11 Basic, IKA-Werke, Staufen, Germany) in order to obtain a representative powdered sample. Direct phloroglucinolysis: The amount of 800 μL methanolic solution containing phloroglucinol (75 g/L) with ascorbic acid (15 g/L), and 400 μL of 0.3 N hydrochloric acid in anhydrous methanol were added to freeze-dried powder (∼100 mg, precisely weighed into a 2.2-mL Eppendorf vial) and mixed. Then, the sample was incubated for 30 min in a thermostatic water bath at 50 °C with vortexing after 15 and 25 min. The reaction was stopped by mixing 700 μL of the reaction medium with an equal amount of a 0.2 M buffered sodium acetate solution, and then centrifuged immediately at 3000×g for 7 min (MPW-55, Warsaw, Poland), filtered (PTFE, 0.45 μm), and transferred into a 1.5-mL HPLC vial sintered with a butylteflon cap. Free monomer catechins analysis: The freeze-dried powder (about 100 mg) was extracted with 1200 μL of 1 % formic acid in 60 % methanol in an ultrasonic water bath (Model 6CT, Warsaw, Poland) for 20 min. The media were centrifuged (3000×g; 7 min) and filtered with a 0.45-μm PTFE filter before HPLC analysis. The degree of procyanidin polymerization (DPn) was calculated as follows: sum of phloroglucinol adducts and terminal catechins was divided by the terminal catechins (catechins in phloroglucinolysis minus free catechins). Results were calculated according to (+)-catechin standard.
Analysis of Phenolic Compounds by HPLC
The polyphenolic compounds were determined by a modified version of the HPLC method of Tsao and Yang (2003) using a Synergi 4 μm Fusion-RP 80A column (250 nm × 4.6 mm) with a guard column (Phenomenex®, Torrance, USA). An Agilent 1100 series HPLC (Hewlett-Packard, Palo Alto, USA) system equipped with a DAD detector was used. The mobile phase consisted of 10.2 % (v/v) acetic acid in 2 mM sodium acetate (solvent A) and acetonitrile (solvent B). The flow rate was kept constant at 0.5 mL/min at 25 °C. The analysis was conducted with a gradient program: 0–20 min, 3 % B linear; 20–40 min, 17 % B linear; 40–65 min, 40 % B linear; 65–68 min, 90 % B linear; 68–72 min, 90 % B isocratic; and 72–73 min, 0 % B linear, followed by washing and reconditioning of the column. Phenolic compounds were detected and quantified at 280 nm (flavan-3-ols, dihydrochalcones) and 320 nm (hydroxycinnamic acids) according to external standards. Results were expressed in mg/kg of dry matter (DM).
Microstructure of Apple Sample
For SEM preparation, slices were cut from a middle part of the freeze-dried apple cubes, parallel to the object’s side. Due to extremely high hygroscopicity of freeze-dried apple tissue, before being coated with gold (Hayat 1976), the tissue was additionally desiccated with critical point drying CO2. This operation allows for both removing residual water molecules from matrix, which increased the image sharpness, and preventing samples from getting damp while maintaining. The changes in microstructure of apple cubes were examined using the scanning electron microscope JEOL JSM-6390LV in Mossakowski Medical Research Centre Polish Academy of Sciences in Warsaw.
Statistical Analysis
Results were subjected to three-way analysis of variance (ANOVA) using Statistica V. 8.0 software (Statsoft Inc., Tulsa, OK, USA). As the main source of variance, the liquid solution, US application, and time of treatment were considered. The significance of the differences between sampling dates was estimated with Tukey’s HSD test at p = 0.05.
Results and Discussion
Mass Transfer Characteristics
The effects of applied predrying treatments on mass transfer characteristics are presented in Table 1. Irrespective of treatment time, the operation of soaking the apple cubes in distilled water, brought about marginal and not significant weight reductions in samples (1.87–4.23 %). The observed changes were the result of the action of two opposite mass fluxes. The substantial losses of soluble solids (48.0–62.1 %) were accompanied by a slight increase in water content (3.29–4.06 %). Our results correspond with data reported by other teams investigating the mass transfer efficiency during fruit tissue predrying operation. For example, when subjecting pineapple slices to 30 min in distilled water, Fernandes et al. (2009) reported a loss of 23.2 % of fruit sugars, in which, although less than we obtained, the difference can be attributed to factors such as much longer trials and the more open and spongy structure of apple tissue. The same research team described the relation between treatment time and sugar transfer, where a prolonged time of immersion of papaya pieces in distilled water led to a higher solid decline (Fernandes et al. 2008c). In the case of apple cubes soaked in distilled water, the treatment time extension from 45 to 90 min increased the solid losses from 48.0 to over 60 %. Surprisingly, no statistically significant sonication effects on soluble solids leakage were noticed (Fig. 1), which contrasts with the result obtained by Fernandes et al. (2008c), where the ultrasound effect in enhancing the removal of soluble solids from papaya fruit to the distilled water was clearly established.
In our experiment, the expected positive effect of ultrasound application on mass transfer intensification turned out to be noticeable, only when the sucrose solution was used as the liquid medium (Fig. 1). Along with comparable levels of water loss and weight reduction for samples treated or non-treated with US, irrespective of soaking time, those sonicated revealed higher values of solid gain. In this case, our data remained in consensus with results obtained by other researchers and confirmed that high-intensity ultrasounds can affect mass transfer processes during the treatment of apple samples in a hypertonic sucrose solution (Cárcel et al. 2007).
Initial Composition of Phenolic Compounds in Raw Apple Cubes
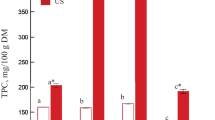
Three classes of polyphenols were detected in the investigated apple cubes: flavan-3-ols, hydroxycinnamic acids, and dihydrochalcons. Among the flavan-3-ols, the following compounds were quantified: monomers [(+)-catechin and (−)-epicatechin], dimers of catechins, and procyanidins, with contents of 57, 137, 728, and 2005 mg/kg dry weight (DW), respectively (Table 2). The degree of procyanidin polymerization (DPn) in the fresh apple was about 11.3. As is easily noticeable, the procyanidins occurred as the predominant phenolic compound, accounting for 56 % of the total polyphenols detected. The obtained results are similar to that reported by other researchers, which also indicated the procyanidins as the dominant phenolic group usually presented in different cultivars of apples (Guyot et al. 2002; Vrhovsek et al. 2004; Wojdyło et al. 2008). The second most important group of polyphenols in the investigated raw material was the hydroxycinnamic acids, which accounted for about 36 % of total detected polyphenols. The predominant compound in this group was chlorogenic acid (1253 mg/kg DW) followed by p-coumaroylquinic acid (49 mg/kg DW). According to Podsędek et al. (2000) and Markowski et al. (2009), the high content of chlorogenic acid is a characteristic displayed specifically by the ‘Idared’ cultivar. The last dihydrochalcone group, represented by phloridzin (82 mg/kg DW) and phloretin xyloglucoside (24.5 mg/kg DW), constituted only about 3 %. Finally, the calculated sum of phenolic compounds in raw apple cubes ‘Idared’ cv. was measured at 3608 mg/kg DW (Fig. 2).
Polyphenol Alterations During Ultrasound-Assisted Predrying Treatments
The amount of total polyphenols retained after predrying treatment strongly depended on the kind of medium used and processing time (Fig. 2). Unfortunately, the more efficient dehydration process using sucrose solution (Table 1) led to significantly higher polyphenol losses (ranging between 35 and 54 %) than when the apple tissue was soaked in distilled water (11–45 %). When lengthening predrying treatment time, substantially higher losses were observed.
Also, the US assistance had an effect on the polyphenol transfer from the apple tissue into the liquid solution, the degree of which depended on both the kind of solute and the application period. Only when apple samples were immersed in distilled water for 90 min did the sonication effect become noticeable, expressed by unfavorably (27 %) higher losses of polyphenols than those obtained for control treatment. Although, as was mentioned above, osmotic dehydration in sucrose syrup caused significantly higher polyphenol losses, additional US application did not cause any extra declines.
In Fig. 3, the losses of individual groups of polyphenolic compounds versus processing time are presented. Generally, it can be observed that irrespective of the phenolic group, the speed of losses strongly depends on the kind of medium. When the process was carried out in sucrose solution, the rate of polyphenol losses in the first 45 min was clearly higher than during the second part of the treatment (the only exception being dihydrochalcons). When, in turn, the apple cubes were immersed in distilled water, the dynamics of losses run in an opposite manner. In the first 45 min, the polyphenol losses were much slower than those observed for sucrose solution; however, along with time prolongation, the leakage clearly intensified, especially when the ultrasound assistance was applied.
a–e Illustrate the losses (%) of individual groups of phenolic compounds making comparisons as follows: black line, predrying treatment of water; grey line, sucrose solution; dotted line, without US; plain line, with US application. f the total polyphenol losses using the same format. Bars depict a standard deviation
Procyanidin Losses
In Fig. 3a, the details relating to the dynamics of procyanidin losses are given. Typically as for other investigated groups, when samples were soaked for 45 min, neither in distilled water nor in sucrose solution, no significant effect of sonication on losses of these compounds was observed. The indicated losses were around 13 % for samples soaked in water and 34 % when sucrose solution was used, which equated to amounts of 1750–1759 and 1237–1314 mg/kg DW, respectively (Table 2). With US treatment prolonged to 90 min, changes of tissue structure were observed, which along with the presence of distilled water led to substantial leakage of the procyanidins. This effect did not appear when regular osmotic dehydration in sucrose syrup was used. Final retention of procyanidins in samples pretreated in water for 90 min differed between 81 and 64 % (which related to 1626 mg/kg DW for not sonicated samples and 1273 mg/kg DW for those treated with US). In the case of treatment in sucrose solution, procyanidin retention was on average 47.6 % which related to 944–970 mg/kg DW (Table 2). Substantial losses of procyanidins during osmotic dehydration of apple cubes had been already reported by Devic et al. (2010c); however, after comparable soaking time in the 60 °Bx sucrose solution carried out at 45 and 60 °C, the related losses did not exceed 20 %. One possible reason for the obtained divergence could be due to the fact that their experiment was conducted on cider apple cultivars, characterized by a very dense structure, which could restrict osmotic agent penetration and retard the release of procyanidins. According to Devic et al. (2010c), the procyanidin retention should be also linked to their degree of polymerization (DPn), as the highly polymerized molecules of greater capacity to bind to cell wall polysaccharides need more energy to be diffused into osmotic solution. In the case of our experiment, the DPn of investigated ‘Idared’ cultivar tissue did not change substantially; however, the tendency toward DPn lowering when ultrasounds were applied was clearly outlined (Table 2). According to the literature, the probable mechanisms linking ultrasound and degradation of polymers (Shim et al. 2002) or pectins (Zhang et al. 2013) are connected with a cavitation effect causing mechanical or chemical degradation of the polymerized molecules (Grönroos et al. 2004).
The Other Flavan-3-ol Alterations
Irrespective of the type of predrying treatments considered, of the all monitored polyphenolic compounds, the dimers of catechins occurred to be the most susceptible to degradation. The quantified losses ranged between 22 and 67 % of their initial concentration (Fig. 3b). Our results remain in consensus with data provided by Devic et al. (2010a), which reported flavan-3-ols as being especially prone to losses during osmotic dehydration of apple tissue. Although US application initiated higher losses of dimers of catechins in every combination tested, statistical significance was proved only for the prolonged 90-min treatments (Table 2). It must be noted, however, that the losses depended on the type of liquid medium, being lower when sucrose solution was used (about 38 % in water and 28 % in sucrose solution when compared to treatment without US).
With regard to monomeric catechins, the 45-min predrying treatment without US caused a decrease of their contents, ranging from 10 % (for water medium; 175 mg/kg DW) to 34 % (sucrose solution; 129 mg/kg DW) (Fig. 3c and Table 2). Using the US during the pretreatment in water (45 min) resulted in a reverse phenomenon, causing the monomeric catechin content to increase by about 11 % (215 mg/kg DW). This increase might be explained by fact that degradation of dimers and procyanidins (which was observed in our study) resulted in the release of monomers such as (+)-catechin and (−)-epicatechin. On the other hand, the prolonged immersion in water for 90 min with US brought about a rapid reduction in the (63 %) monomeric catechin content (71 mg/kg DW), which may diffuse into the water liquid. In the case of using sucrose solution, we did not observe any significant differences of monomer content between treatment with US and without US.
Retention of Hydroxycinnamic Acids and Dihydrochalcons
Losses of hydroxycinnamic acids (Fig. 3d) showed the same pattern as that observed for dimeric catechins (Fig. 3b). Although the hydroxycinnamic acid resistance toward applied process conditions was regularly higher than that indicated for dimeric catechins (on average about 10 points lower percentage losses), they both displayed a tendency to be more sensitive to ultrasound application. As opposed to other phenolic compounds, the significant effect of US treatment was also noticeable when sucrose solution as a liquid medium was used. The decrease of hydroxycinnamic acids during 90-min treatment in water ranged between 30 and 54 % (respectively without and with US), while in the case of regular osmotic dehydration, the decrease amounted to 48 and 55 %, respectively.
Dihydrochalcons, the final phenolic compound to come under consideration, displayed relatively minor losses during predrying treatment (below 40 %). As with the other phenolic compounds, however, losses increased significantly (63 %) when immersed in water for 90 min with US assistance (Fig. 3e).
Microstructure of Apple Parenchyma Tissue
In Fig. 4, the microstructure changes caused by the applied treatments are illustrated. Treatment in water caused the intercellular spaces to fill up and become more rounded (Fig. 4b, c) than in the untreated material (Fig. 4a). Furthermore, the samples treated in water have thinner and more delicate cell walls, with a more open looking structure (Fig. 4b, c) than the samples dehydrated in sucrose syrup (Fig. 4d, e), which are characterized by a more compact structure. Many sources of literature confirm the occurrence of changes in the structure of plant tissue as a result of water diffusion between plant tissue and the surrounding medium (Deng and Zhao 2008; Fernandes et al. 2008a; Nowacka et al. 2014). After subjecting apple tissue to 90 min of gentle shaking in distilled water, formation of a compact layer of cells on the surface of the apple cubes was observed (Fig. 4b). The ultrasound application action clearly inhibited formation of compact surface, which resulted in creating an “open structure” with hardly any tissue–medium barrier (Fig. 4c). The opposite phenomenon was observed in the case of apple cube dehydration in sucrose solution, where US encouraged the formation of more compact and thicker subsurface of the tissue (Fig. 4e) than in the sample treated without US assistance (Fig. 4d). Perhaps this phenomenon would be caused by a deeper penetration of sucrose medium into apple tissue when ultrasound-assisted treatment was applied. Deng and Zhao (2008) noticed more prominent changes in the structure of apple tissue after ultrasound-assisted osmotic dehydration than when apples were subjected to osmotic dehydration with agitation or osmotic dehydration with a pulsed vacuum. Moreover, Fernandes et al. (2009) observed that ultrasound-assisted osmotic dehydration of pineapple induced greater changes of cell structure and created bigger cell interspaces than when a distilled water medium was used. In our study, we observed the deeper penetration of sucrose medium into apple tissue when ultrasound-assisted treatment was applied.
Scanning electron micrograph showing a cross section of freeze-dried apple cubes: untreated (a), after 90-min predrying treatment in water (b, c) and sucrose (d, e). Panel (b)/(d) relates to samples where soaking was supported by gentle shaking, while (c)/(e) were additionally treated with ultrasound. White arrows show the area of compact layer of cells (magnification ×30)
It is probable that these microstructure changes triggered by ultrasound application caused a significant loss of bioactive compounds when samples were immersed in distilled water for 90 min (Fig. 2; water 90). The lack of a barrier in the form of a compact layer of cells on the surface contributed to larger leakage of bioactive compounds from the apple cubes to the surrounding medium.
Conclusion
Our results confirm that the ultrasound-assisted dehydration of apple cubes in sucrose solution brings about positive effects of water loss and solids gain intensification. Analysis proved that the ultrasound interaction with apple tissue structure strongly depended on the kind of liquid medium used for wave propagation. When using distilled water, the US led to “opening” tissue structure, while in sucrose solution, the US led to a more intensive impregnation of the outer layers. This observation seems crucial when describing the changes in retention of individual phenolic compounds caused by the ultrasound application. Parallel to structure modification, the ultrasound energy brought about several expected chemical interventions, the most evident of which (in our study) was the lowering of procyanidin polymerization, degradation of the dimers of catechins, and probably simultaneously weakening of binding between procyanidins and cell walls. Further courses of possible alteration pathways depended on the structure changes that in turn were connected with the liquid medium used. In the case of distilled water, the open structure provoked by the US allowed a significant, almost uncontrolled increase of polyphenol leakage, while when considering sucrose solution, the superficial impregnated layer was able to efficiently prevent the leakage of highly polymerized molecules. In the case of smaller molecules like hydroxycinnamic acids, the prevention was not so evident.
Summarizing the above, the US assistance could be recommended as an effective factor in the intensification of predrying treatments, provided sucrose syrup is used as the liquid medium, which would ensure only marginal losses of total phenolic compounds.
References
Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Hu, B., Lei, S., et al. (2014). Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrasonics Sonochemistry, 21(1), 93–97.
Akyıldız, A., & Öcal, N. D. (2006). Effects of dehydration temperatures on colour and polyphenoloxidase activity of Amasya and Golden Delicious apple cultivars. Journal of the Science of Food and Agriculture, 86(14), 2363–2368.
Awad, M. A., de Jager, A., & van Westing, L. M. (2000). Flavonoid and chlorogenic acid levels in apple fruit: characterisation of variation. Scientia Horticulturae, 83(3–4), 249–263.
Cárcel, J. A., Benedito, J., Roselló, C., & Mulet, A. (2007). Influence of ultrasound intensity on mass transfer in apple immersed in a sucrose solution. Journal of Food Engineering, 78(2), 472–479.
Deng, Y., & Zhao, Y. (2008). Effects of pulsed-vacuum and ultrasound on the osmodehydration kinetics and microstructure of apple (Fuji). Journal of Food Engineering, 85(1), 84–93.
Devic, E., Guyot, S., Daudin, J. D., & Bonazzi, C. (2010a). Effect of temperature and cultivar on polyphenol retention and mass transfer during osmotic dehydration of apples. Journal of Agricultural and Food Chemistry, 58(1), 606–614.
Devic, E., Guyot, S., Daudin, J. D., & Bonazzi, C. (2010b). Kinetics of polyphenol losses during soaking and drying of cider apples. Food and Bioprocess Technology, 3(6), 867–877.
Devic, E., Guyot S., Daudin, J. D., & Bonazzi, C. (2010c). Retention of polyphenols and ascorbic acid in apples: impact of osmotic dehydration and convective drying. 17th International Drying Symposium (IDS), 1470–1477.
Drogoudi, P. D., Michailidis, Z., & Pantelidis, G. (2008). Peel and flesh antioxidant content and harvest quality characteristics of seven apple cultivars. Scientia Horticulturae, 115(2), 149–153.
Fernandes, F. A. N., Gallão, M. I., & Rodrigues, S. (2008a). Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: melon dehydration. LWT - Food Science and Technology, 41(4), 604–610.
Fernandes, F. A. N., Linhares, F. E., Jr., & Rodrigues, S. (2008b). Ultrasound as pre-treatment for drying of pineapple. Ultrasonics Sonochemistry, 15(6), 1049–1054.
Fernandes, F. A. N., Oliveira, F. I. P., & Rodrigues, S. (2008c). Use of ultrasound for dehydration of papayas. Food and Bioprocess Technology, 1(4), 339–345.
Fernandes, F. A. N., & Rodrigues, S. (2008). Application of ultrasound and ultrasound-assisted osmotic dehydration in drying of fruits. Drying Technology, 26(12), 1509–1516.
Fernandes, F. A. N., Gallão, M. I., & Rodrigues, S. (2009). Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. Journal of Food Engineering, 90(2), 186–190.
Francini, A., & Sebastiani, L. (2013). Phenolic compounds in apple (Malus x domestica Borkh.): compounds characterization and stability during postharvest after processing. Antioxidants, 2(3), 181–193.
Fromm, M., Bayha, S., Carle, R., & Kammerer, D. R. (2012). Characterization and quantitation of low and high molecular weight phenolic compounds in apple seeds. Journal of Agricultural and Food Chemistry, 60(5), 1232–1242.
Garcia-Perez, J. V., Ortuno, C., Puig, A., Carcel, J. A., & Perez-Munnuera, I. (2012). Enhancement of water transport and microstructural changes induced by high-intensity ultrasound application on orange peel drying. Food and Bioprocess Technology, 5(6), 2256–2265.
Grönroos, A., Pirkonen, P., & Ruppert, O. (2004). Ultrasonic depolymerization of aqueous carboxymethylcellulose. Ultrasonics Sonochemistry, 11(1), 9–12.
Guyot, S., Bourvellec, C. L., Marnet, N., & Drilleau, J. F. (2002). Procyanidins are the most abundant polyphenols in dessert apples at maturity. LWT - Food Science and Technology, 35(3), 289–291.
Hackman, R. M., Polagruto, J. A., Zhu, Q. Y., Sun, B., Fujii, H., & Keen, C. L. (2008). Flavanols: digestion, absorption and bioactivity. Phytochemistry Reviews, 7(1), 195–208.
Hayat, M. A. (1976). Summary of specimen preparation for SEM. In M. A. Hayat (Ed.), Principles and techniques of scanning electron microscopy (pp. 10–11). New York: Van Nostrand Reinhold Co.
Hyson, D. A. (2011). A comprehensive review of apples and apple components and their relationship to human health. Advances in Nutrition, 2(5), 408–420.
Keenan, D. F., Tiwari, B. K., Patras, A., Gormley, R., Butler, F., & Brunton, N. P. (2012). Effect of sonication on the bioactive, quality and rheological characteristics of fruit smoothies. International Journal of Food Science and Technology, 47(4), 827–836.
Kennedy, J. A., & Jones, G. P. (2001). Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. Journal of Agricultural and Food Chemistry, 49(4), 1740–1746.
Khanizadeh, S., Tsao, R., Rekika, D., Yang, R., & DeEll, J. (2007). Phenolic composition and antioxidant activity of selected apple genotypes. Journal of Food, Agriculture and Environment, 5(1), 61–66.
Knekt, P., Kumpulainen, J., Järvinen, R., Rissanen, H., Haliövaara, M., Reunanen, A., et al. (2002). Flavanoid intake and risk of chronic diseases. American Journal of Clinical Nutrition, 76(3), 560–568.
Krokida, M. K., Tsami, E., & Maroulis, Z. B. (1998). Kinetics on color changes during drying of some fruits and vegetables. Drying Technology, 16(3–5), 667–685.
Lenart, A. (1996). Osmo-convective drying of fruit and vegetables: technology and application. Drying Technology, 14(2), 391–413.
Link, A., Balaguer, F., & Goel, A. (2010). Cancer chemoprevention by dietary polyphenols: promising role of epigenetics. Biochemical Pharmacology, 80(12), 1771–1792.
Łata, B., Trampczynska, A., & Paczesna, J. (2009). Cultivar variation in apple peel and whole fruit phenolic composition. Scientia Horticulturae, 121(2), 176–181.
Manach, C., Scalbert, A., Morand, C., Rémésy, C., & Jiménez, L. (2004). Polyphenols: food sources and bioavailability. American Journal of Clinical Nutrition, 79(5), 727–747.
Markowski, J., Mieszczakowska, M., & Płocharski, W. (2009). Effect of apple cultivar and enzyme treatment on phenolic compounds content during clear apple juice production. International Journal of Food Science and Technology, 44(5), 1002–1010.
Nowacka, M., Wiktor, A., Śledź, M., Jurek, N., & Witrowa-Rajchert, D. (2012). Drying of ultrasound pretreated apple and its selected physical properties. Journal of Food Engineering, 113(3), 427–433.
Nowacka, M., Tylewicz, U., Laghi, L., Rosa, M. D., & Witrowa-Rajchert, D. (2014). Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chemistry, 144, 18–25.
Opalić, M., Domitran, Z., Komes, D., Belščak, A., Horžic, D., & Karlović, D. (2009). The effect of ultrasound pre-treatment and air-drying on the quality of dried apples. Czech Journal of Food Science, 27(4), 297–300.
Pingret, D., Fabiano-Tixier, A. S., & Chemat, F. (2013). Degradation during application of ultrasound in food processing: a review. Food Control, 31(2), 593–606.
Podsędek, A., Wilska-Jeszka, J., Anders, B., & Markowski, J. (2000). Compositional characterization of some apple varieties. European Food Research and Technology, 210(4), 268–272.
Schössler, K., Jäger, H., & Knorr, D. (2012). Effect of continuous and intermittent ultrasound on drying time and effective diffusivity during convective drying of apple and red pepper. Journal of Food Engineering, 108(1), 103–110.
Shim, S. E., Ghose, S., & Isayev, A. I. (2002). Formation of bubbles during ultrasonic treatment of cured poly(dimethyl siloxane). Polymer, 43(20), 5535–5543.
Siucińska, K., & Konopacka, D. (2014). Application of ultrasound to modify and improve dried fruit and vegetable tissue: a review. Drying Technology, 32(11), 1360–1368.
Stojanovic, J., & Silva, J. L. (2007). Influence of osmotic concentration, continuous high frequency ultrasound and dehydration on antioxidants, colour and chemical properties of rabbiteye blueberries. Food Chemistry, 101(3), 898–906.
Tsao, R., & Yang, R. (2003). Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromotagraphy. Journal of Chromatography A, 1018(1), 29–40.
Vilkhu, K., Mawson, R., Simons, L., & Bates, D. (2008). Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innovative Food Science & Emerging Technologies, 9(2), 161–169.
Vrhovsek, U., Rigo, A., Tonon, D., & Mattivi, F. (2004). Quantitation of polyphenols in different apple varieties. Journal of Agricultural and Food Chemistry, 52(21), 6532–6538.
WHO (World Health Organization), (2003). Diet, nutrition and the prevention of chronic diseases. WHO Technical Report Series 916. (http://www.fao.org/DOCREP/005/AC911E/ac911e00.htm. Accessed 20 November 2014)
Wojdyło, A., Oszmiański, J., & Laskowski, P. (2008). Polyphenolic compounds and antioxidant activity of new and old apple varieties. Journal of Agricultural and Food Chemistry, 56(15), 6520–6530.
Zhang, L., Ye, X., Ding, T., Sun, X., Xu, Y., & Liu, D. (2013). Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrasonics Sonochemistry, 20(1), 222–231.
Acknowledgments
This research had been conducted within the “BIOSUSZ” PBS Project financed by the National Centre for Research and Development (PBS1/A8/13/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mieszczakowska-Frąc, M., Dyki, B. & Konopacka, D. Effects of Ultrasound on Polyphenol Retention in Apples After the Application of Predrying Treatments in Liquid Medium. Food Bioprocess Technol 9, 543–552 (2016). https://doi.org/10.1007/s11947-015-1648-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1648-z