Abstract
Since rapeseed and sunflower meals are two of the most representative oilseed crops in the world, this study was focused on ethanol-wash solutes (EWS) obtained as wastes from the protein isolation process of rapeseed and sunflower meals. These meals have been previously valorised; however, the use of the EWS is unexplored. The present study is aimed at the characterisation of their phenolic profile, and antioxidant capacity for preventing lipid oxidation in rapeseed, sunflower, and soybean oil, which has been used as a reference oil. The sunflower EWS exhibited more total phenolic compounds (TPC) and antioxidant activity (119.39 ± 1.13 mg GA/g and 193.97 ± 9.77 mg TE/g, respectively) than the rapeseed one (103.44 ± 5.94 mg GA/g and 89.51 ± 3.17 mg TE/g). The phenolic identification showed hydroxybenzoic and protocatechuic acid in the rapeseed EWS, and pyrogallol and caffeic acid in the sunflower EWS, as the main representative phenols. Both EWS at 15% increased significantly (p < 0.05) the oxidative stability of the oils in the Rancimat equipment with values of antioxidant activity index (AAI) from 1.01 to 1.20, depending on the type of oil employed. In conclusion, the rapeseed and sunflower EWS showed great potential, and they could be used as a source of natural antioxidants within the food industry, replacing the synthetic ones, and promoting the circular economy since they are agro-food wastes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapeseed (Brassica napus) and sunflower (Helianthus annuus L.) are two of the most oilseed crops in the world, representing the third and fourth largest ones, respectively (Bouallegue et al., 2016; Kirpluks et al., 2017; Pan et al., 2011; Rauf et al., 2017). Both types of oils are considered healthy for cooking, particularly due to their beneficial balance of fatty acids (Bouallegue et al., 2016; Wroniak et al., 2016), but they are highly susceptible to oxidation (Hussain et al., 2018; Koski et al., 2003) due to their proportion of polyunsaturated linoleic and α-linolenic fatty acids.
Mechanical pressing and solvent extraction have been the two methods most proposed to produce oil from different seeds (Savoire et al., 2013). Furthermore, the meals recovered by the oil extraction process from rapeseed and sunflower seeds have a good nutritive value and they contain a high percentage of protein (almost 40%), fibre, and minerals (Ivanova et al., 2013, 2016; Pan et al., 2011; Wang et al., 2015). In addition, these co-products are rich in phenolic compounds, especially sinapine and sinapic acid in rapeseed meal (Hashmi & Satwadhar, 2010) and chlorogenic acid and caffeic acid in sunflower meal (Lomascolo et al., 2012). These meals are subjected to a further process with the aim of concentrating or isolating the proteins, which has been well explained previously in the literature (Kalaydzhiev et al., 2020; Rodrigues et al., 2017). Another important aim is to avoid possible non-desirable interactions with other compounds such as phenols (Karefyllakis et al., 2017). On the basis of these considerations, a previous washing of the meals with acetone, sodium phosphate, and ethanol is carried out in order to remove soluble non-protein components (Kalaydzhiev et al., 2020a, b; Li et al., 2017). Among all these solvents, the use of ethanol has been previously reported as a step to recover bioactive compounds from wastes and by-products meals, and it is an authorised solvent used within the food industry (Georgiev et al., 2021; Klinchongkon et al., 2019). The obtaining ethanol-wash solutes (EWS) contain phenolic compounds and flavonoids that could be useful and revalorised within the food industry (Gandova et al., 2021; Georgiev et al., 2021). This exploitation would be in agreement with previous research regarding the valorisation of several food wastes and by-products as a potential source of bioactive compounds (Sun-Waterhouse et al., 2013a, b; Wang 2020).
Lipid oxidation is one of the most relevant and studied reactions in food chemistry (Medina-Meza et al., 2014; Verardo et al., 2020) which affects the colour, texture, nutritive value, taste, and aroma, leading to rancidity and the consequent production of unpleasant odour and flavours (Amaral et al., 2018). Therefore, it is the main cause of deterioration in food such as oils, meat, emulsions, and nuts (Vieira et al., 2017), presenting a high quantity of polyunsaturated fatty acids, such as linoleic acid in the case of rapeseed and sunflower oils (Chernova et al., 2019), having low dissociation energy of their double bonds. For this reason, oxidative stability is one of the most important parameters assessed in the formulation of commercial oils (Tinello et al., 2018) since the undesirable compounds formed would impair the properties of not only the oil but also the fried food. In the free radical chain reaction of lipid oxidation, described by the stages of initiation, propagation, and termination, oxygen plays an important role, so the addition of antioxidants, as free radical scavengers before the propagation phase, is considered a strategy to control the lipid autoxidation and to enhance the lag time until the production of the volatile compounds, which are markers of rancidity (Tinello et al., 2020). For this reason, the food industry faces antioxidants as necessary additives (Choe & Min, 2009), such as the synthetic one butylated hydroxytoluene (BHT). Due to its potential health toxicity (Upadhyay & Mishra, 2015), employment is severally regulated in terms of use and dosage with the limit of 200 mg/kg in vegetable oils, established by the Codex Alimentarius. Nowadays, there is a growing search for natural alternatives to synthetic antioxidants (Ribeiro et al., 2019), a challenge based on the statement that polyphenols and some vitamins are the major natural antioxidants that can be either present or added in foods to increase their oxidative stability (Santos-Sánchez et al., 2017).
Up to our knowledge, there is little information about the study of the EWS from the rapeseed and sunflower oil industry, which are currently treated as wastes generated during the protein isolation process, without considering their potential as bioactive compounds sources. Therefore, the aims of this study were to preliminary characterise EWS from the rapeseed and sunflower oil industry, in terms of the content and type of phenols, the antioxidant activity, and to explore the valorisation of those wastes in preventing lipid oxidation in their respective oils (rapeseed and sunflower) as well as in soybean oil, which was used as a reference since it is another highly consumed oil in the world.
Literature Review
The oilseed extraction presents a considerable amount of by-products, meals being the most important. These meals are considered a source of proteins, mainly globulins and albumins (Moreno-González & Ottens, 2021). These proteins represent up to 50 and 48% in the case of rapeseed and sunflower meals, respectively (Lee et al., 2021), and it has been reported they exert some functional properties such as water-holding and gelling. In addition, meals have been recognised as a source of polyphenols, which are compounds with antioxidant and antimicrobial among other properties (Moreno-González & Ottens, 2021). However, the meal application is limited due to the antinutritional compounds they present (Jang et al., 2011; Wang et al., 2015). For this reason, a pre-treatment step to reduce the unwanted compounds is highly advised. Adsorption, among other techniques, has been widely applied for this purpose, for example, to recover phenolic compounds from sunflower meals (Moreno-González & Ottens, 2021; Pickardt et al., 2015); however, its application sometimes is not viable. Another option to separate these compounds is by the aqueous solution extraction, ethanol being recognised as an accurate solvent because of their capacity to extract phenols and other antinutrients such as glucosinolates (Adem et al., 2014) while increasing the protein level (Kalaydzhiev et al., 2020a, b). The residues generated after the meals pre-treatment step of proteins recovery has exhibited remaining bioactivity, at least in the case of rapeseed (Georgiev et al., 2021), demonstrating an initial potential to valorise these wastes and to turn them into useful commercial products.
Materials and Methods
Materials and Reagents
Rapeseed, sunflower, and soybean commercial oils were purchased in a local market (Padova, Italy). On the other hand, a local company in Bulgaria provided industrial rapeseed and sunflower meals. They were produced at an industrial scale after thermal treatment of rapeseed and sunflower seeds at 110–115 °C, followed by oil extraction with hexane at 60–65 °C for approximately 1 h. Ethanol, Folin-Ciocolateau’s phenols reagent, gallic acid, sodium carbonate, sodium hydroxide, Tween80, butylated hydroxytoluene (BHT), hydrochloric acid, acetic acid, iron chloride, 2,3,5-triphenyltetrazolium chloride (TPTZ), tetramethylchromane-2-carboxylic acid (Trolox), and HPLC standards (pyrogallol, hydroxybenzoic acid, protocatechuic acid, caffeic acid, syringic acid, and ferulic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of EWS from Rapeseed and Sunflower Meals
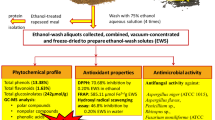
The rapeseed and sunflower EWS were obtained following the steps reported previously (Georgiev et al., 2021), and shown in Fig. 1. Briefly, the industrial rapeseed and sunflower meals were ground and sifted to collect 0.315-mm particles. After, 200 g of the meals were mixed with an aqueous ethanol solution (75%), to a final volume of 800 mL at room temperature in constant agitation for 30 min. Then, vacuum filtration was followed. This process was repeated three more times. The residue of the process (ethanol-treated rapeseed and sunflower meal) was then dried at room temperature and further used for alkaline extraction of proteins. The ethanol-wash aliquots obtained during this process were collected, mixed, vacuum-concentrated (RV 3 V Rotary Evaporator, IKA®-Werke GmbH & Co. KG, Staufen, Germany) at 50 °C to the 0.3-fold final reduction of the total volume and freeze-dried (Lyovac GT2, Leybold-Heraeus, Germany) to obtain both EWS.
Phenolic Extraction of the Rapeseed and Sunflower EWS
Figure 2 shows that both EWS (0.2 g) were solubilised in 10 mL of 70% v/v ethanol (Georgiev et al., 2021), mixed for 30 min using an orbital shaker at room temperature and then centrifuged for 5 min at 9500 gravitational force (g) at 4 °C (Hettich Zentrifugen, MOD: Universal 320R, Germany). Then, the supernatant (ethanolic extract) from both samples was filtered using a Whatman paper No 1 and the volume was measured. A second extraction at the same conditions was carried out with the exhausted EWS. Both supernatants obtained were mixed and stored at −18 °C until used.
Quantification of the Total Phenolic Compounds (TPC)
The TPC were quantified using the Folin-Ciocalteu method (Azuma et al., 1999). Briefly, 1 mL of diluted ethanolic extract was mixed with 5 mL of NaCO3 10%, containing NaOH 1 M, and 500 µL of Folin-Ciocalteau’s reagent previously diluted twice in distilled water. It was also prepared a blank solution with the dilution solvent. After waiting 30 min under darkness, the samples were filtered using 0.45-µm Millipore acetate cellulose filters (MA, USA). Hence, the absorbance value of the phenolic extract from the rapeseed and sunflower EWS was measured at 650 nm using a Varian Carry 50 Bio UV/Vis spectrophotometer. The results were expressed as mg of gallic acid equivalent per g of sample (mg GA/g), and the assay was performed in triplicate.
Phenolic Compound Identification
The phenolic profile of the rapeseed and sunflower EWS was determined by HPLC analysis, using a Thermo Finnigan SpectraSystem UV6000LP (Thermo Finnigan, San Jose, CA, USA) HPLC system with diode array detector, as reported previously (Thiyam et al., 2009). Before their injection in the column, the samples were filtered with a 0.22-µm cellulose acetate filter (Millipore, USA).
The phenols present in the sample were identified based on the retention time of the corresponding commercial standards (pyrogallol, hydroxybenzoic acid, protocatechuic acid, caffeic acid, syringic acid, and ferulic acid), previously solubilised in absolute methanol, using the SupelcosilTM LC-18 column at the following operating conditions (Hu et al., 2011): mobile phase, 18 mL n-butanol (solvent A)/1.5 mL 50% v/v acetic acid (solvent B); flow rate, 0.6 mL/min; isocratic flow; wavelength, 214 nm, 275 nm, and 310 nm; temperature, 25 °C; and running time, 60 min. The assay was performed in triplicate.
Antioxidant Activity
The assay has been performed by using the ferric reducing antioxidant potential (FRAP) method, agreeing with the literature (Stratil et al., 2008). The FRAP reagent was prepared by mixing 2.5 mL of 0.01 M of TPTZ in HCl 40 mM, 2.5 mL of aqueous solution of 0.02 M FeCl3, and 25 mL of 0.2 M of sodium acetate buffer (0.2 M sodium acetate/0.2 M acetic acid). A FRAP volume of 900 µL was mixed with 100 µL of sample and incubated at 37 °C for 30 min. Also, a blank solution was prepared with the dilution solvent. The absorbance was measured at 593 nm using a Varian Carry 50 Bio UV/Vis spectrophotometer. The results were expressed as mg of Trolox equivalent per g of sample (mg TE/g), and the assay was performed in triplicate.
Determination of the Oxidative Stability
Rapeseed and sunflower EWS at two different concentrations (5 and 15%) have been mixed with soybean, rapeseed, and sunflower oils for 20 min using an orbital shaker. After a sonication treatment for 2 min (4 intervals of 30 s), the samples were centrifuged for 10 min at 4 °C at 2370 g. Then, the oil supernatants were recovered, and the exhausted powders were subjected to another cycle of extraction. Both oil supernatants obtained were mixed and used to assess the oxidative stability through the official Rancimat method (AOCS, 2012), according to the procedure previously described (Tinello et al., 2018). As controls, the same oils without antioxidants (C) and oils containing BHT at 0.02% w/w were introduced in the Rancimat assay. A quantity of 3 g of samples (control or oil supernatants) was weighted in the Rancimat apparatus (Metrohm, model 743, Herisau, Switzerland), and subjected to a stream of air at the rate of 20 L/h kept at a constant temperature of 110 °C, causing an accelerated oxidation process. The oxidative stability was expressed as the induction time (IT) corresponding to the time (h) at which the water conductivity (μS/min) starts increasing because of the production of compounds involved in the lipid oxidation. The antioxidant activity index (AAI) was calculated by the following equation:
Statistical Analysis
The data presented the mean ± standard deviation of three independent experiments (n = 3) by using an analysis of variance (ANOVA) one way, followed by the post-hoc Duncan test (p < 0.05) using Statgraphics Centurion XVI (StatPoint Inc., Rockville, MD, USA)
Results and Discussion
Quantification and Identification of Phenolic Compounds
The sunflower EWS showed significant (p < 0.05) more TPC (119.39 ± 1.13a mg GA/g), than the rapeseed one (103.44 ± 5.94b mg GA/g). Previous data have revealed the presence of phenols in sunflower and rapeseed meals (Laguna et al., 2018); this study shows the remaining concentration of these compounds after the ethanol-washing, as reported previously (Georgiev et al., 2021). However, the number of bioactive compounds inside the meals is not homogeneous due to the factors involved during the oil extraction at an industrial level, such as the duration of the heat treatment and the organic solvents used (Kalaydzhiev et al., 2020a, b). This condition as well as the kind of cultivar used produces a high variability in the quality of the meal obtained (Ivanova et al., 2016). Ethanol has been recognised as a suitable solvent in the extraction of TPC since it is environmentally friendly (Cisneros-Yupanqui et al., 2020a), especially at concentrations between 50 and 80% (Sun-Waterhouse et al., 2013a, b), their recovery being dependant on their nature (Khiari et al., 2009). In addition, Fig. 1S and 2S show the main polyphenols found in both EWS, hydroxybenzoic acid and protocatechuic acid, being the most representative in the rapeseed EWS (179.35 ± 43.01a mg/L and 2.27 ± 0.31 mg/L, respectively). On the other hand, the phenolic profile of sunflower EWS seemed to be more complex with pyrogallol, syringic acid, caffeic acid, and ferulic acid, besides the hydroxybenzoic acid (106.62 ± 1.33b mg/L). However, the amount of this latter was significantly higher (p < 0.05) in the rapeseed EWS probably due to it is the main representative compound present in rapeseed oil (Laguna et al., 2018). The concentration of hydroxybenzoic and protocatechuic acid in rapeseed, and caffeic acid and pyrogallol in sunflower EWS is comparable with the values reported in their respective meals (Laguna et al., 2018; Zhu et al., 2018), which present several functional properties due to the remaining bioactive compounds (Wang et al., 2015). Furthermore, these phenolic acids exhibit many benefits including antibacterial, antimicrobial, and antioxidant activities that could give bioactivities for health to both EWS (Laguna et al., 2019).
Antioxidant Activity
The sunflower EWS showed the highest antioxidant activity (193.97 ± 9.77a mg TE/g), in comparison to the rapeseed EWS (89.51 ± 3.17b mg TE/g). The result may be due to the high yield of polyphenols and bioactive compounds of this waste. Several authors have previously demonstrated the high value and the possible exploitation of rapeseed and sunflower meals as a source of antioxidants (Kreps et al., 2014; Lomascolo et al., 2012), being able to scavenge not only radicals such as DPPH but also superoxide and hydroxyl radical (Pan et al., 2011). Among the possible compounds responsible for this bioactivity, it has been widely demonstrated that polyphenols exhibit a marked antioxidant activity, due to their chemical structure (Bors & Michel, 2002). Their aromatic features and highly conjugated system with multiple hydroxyl groups make these compounds good electron and hydrogen atoms donor, neutralising free radicals and other reactive oxygen species (Zhang & Tsao, 2016). In particular, various authors have shown the strong antioxidant properties of caffeic acid (Sato et al., 2011) and pyrogallol (Ozturk Sarikaya, 2015), both present in the sunflower EWS. Beyond the polyphenolic fractions, other bioactive compounds were not only attributed to exert antioxidant activity in ethanolic extracts (Bidchol et al., 2011), but also detected previously in the sunflower and rapeseed. Among these compounds, vitamin C and E were the most promising due to they can work synergically with the polyphenols, contributing to the antioxidant capacity of both EWS (Kreps et al., 2014; Pal et al., 2014).
Determination of the Oxidative Stability
The oxidative stability of the rapeseed and sunflower EWS-supplemented soybean, rapeseed, and sunflower oil was evaluated by measuring their ITs with the Rancimat apparatus and compared to the same oils without antioxidants (C) and those containing BHT. The results obtained are shown in Fig. 3 and, according to them, the addition of the rapeseed EWS at 15% had comparable effects to BHT in soybean oil or even higher in the case of the rapeseed oil, where the AAI of the rapeseed EWS-supplemented oil was 1.18 while the BHT one was 1.09 (which means 18% and 9% more compared to the control, respectively). On the other hand, the sunflower EWS at 5% obtained similar AAI to the BHT samples in all the oils assessed. Moreover, the sunflower EWS at 15% exhibited the best antioxidant capacity with a delay of oxidation in sunflower and soybean oil. The replacement of the BHT use with the EWS may have some benefits. Several studies have underscored the probable toxicity of this synthetic antioxidant that exerts a potential teratogen behaviour on rats (Babich, 1982; Ito et al., 1985). These EWS could be a potential source of functional components, developing a higher value to the food products, due to their polyphenols content (Hashmi & Satwadhar, 2010). From the practical point of view, using sonication in the preparation of the oil systems could improve the transfer of antioxidants from both EWS to the oils. In this regard, several authors have reported the enhancement of the antioxidant capacity and the preservation of bioactive compounds in the matrix (Abid et al., 2014; Guerrouj et al., 2016; Riciputi et al., 2018; Rombaut et al., 2020) when using this emerging technology, because it allows obtaining a more homogeneous distribution of bioactive compounds inside to samples thanks to the formation of bubbles during the cavitation process (Amirante et al., 2017; Shanmugam & Ashokkumar, 2015). In addition, the polyphenol compounds extracted from a sonication process have shown a higher antioxidant potential (Dzah et al., 2020). Moreover, the use of sonication has been related to the improvement of the oxidative stability in peanuts (Wambura et al., 2011, 2012), showing the advantages of using this technology in the present study. However, the influence of this technology on the oil samples with the EWS needs further investigation.
(a) Induction time (IT) and (b) antioxidant activity index (AAI) of ethanol-wash solute (EWS) supplemented oils. Different letters within the same type of oil indicate statistically significant differences (p < 0.05), according to ANOVA (one-way) and the Duncan test. C control, RS-EWS rapeseed-EWS, SF-EWS sunflower-EWS
Previous research has reported the use of several products and by-products from the food industry in oxidation stability. For instance, it was demonstrated the efficiency of grape pomace in delaying the corn oil oxidation (Cisneros-Yupanqui et al., 2020b), the use of red chicory extract to prevent lipid oxidation (Lante et al., 2011), and the use of sesame seed extract to increase the oxidative stability of sunflower oil (Hussain et al., 2018). Therefore, the results from this study could suggest that preliminary, both EWS may contribute to control lipid peroxidation in different types of oils. However, this is the first time that the application of EWS as antioxidants in different oil systems has been assessed. So, further research should focus on the optimisation of the ethanolic phenolic extraction, assessing different parameters.
Conclusions
The preliminary characterisation of rapeseed and sunflower EWS shows a higher TPC and antioxidant activity in the last one, pyrogallol and caffeic acid being the most representative phenols. According to the phenolic profile, more compounds are detected in the sunflower EWS. Based on these outcomes, both EWS (especially the sunflower one) may be useful for the different types of oil stabilisation and fortification, having a comparable lipid oxidation inhibition to the traditional synthetic additive BHT. Rapeseed EWS seem to be more efficient when used in rapeseed oil as well as the sunflower EWS in their respective oil and when applied in soybean oil. In addition, the utilisation of these products contributes to extend the life cycle of these agricultural wastes and according to the principles of the circular economy. Taking into account these considerations, further studies are in progress regarding the optimisation of the bioactive compounds extraction parameters and the evaluation in other types of oil, in order to keep assessing the valorisation of both EWS.
Abbreviations
- EWS:
-
ethanol-wash solutes
- TPC:
-
Total phenolic compounds
- BHT:
-
Butylated hydroxytoluene
- TPTZ:
-
2,3,5-Triphenyltetrazolium chloride
References
Abid, M., Jabbar, S., Wu, T., Hashim, M. M., Hu, B., Lei, S., & Zeng, X. (2014). Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrasonics Sonochemistry, 21(1), 93–97. https://doi.org/10.1016/j.ultsonch.2013.06.002
Adem, H. N., Tressel, R. P., Pudel, F., Slawski, H., & Schulz, C. (2014). Rapeseed use in aquaculture. OCL - Oilseeds and Fats, 21(1), 105. https://doi.org/10.1051/ocl/2013041
Amaral, A., Solva, M., & Lannes, S. (2018). Lipid oxidation in meat: Mechanisms and protective factors - a review. Food Science and Technology, 38, 1–15. https://doi.org/10.1590/fst.32518
Amirante, R., Distaso, E., Tamburrano, P., Paduano, A., Pettinicchio, D., & Clodoveo, M. L. (2017). Acoustic cavitation by means ultrasounds in the extra virgin olive oil extraction process. Energy Procedia, 126, 82–90. https://doi.org/10.1016/j.egypro.2017.08.065
AOCS. (2012). Official methods and recommended practices of the American Oil Chemists’ Society. Champaign, IL. https://scholar.google.com/scholar_lookup?title=Official+Methods+and+Recommended+Practices+of+the+AOCS&author=AOCS&publication_year=2012. Accessed 17 March 2021.
Azuma, K., Ippoushi, K., Ito, H., Higashio, H., & Terao, J. (1999). Evaluation of antioxidative activity of vegetable extracts in linoleic acid emulsion and phospholipid bilayers. Journal of the Science of Food and Agriculture, 79(14), 2010–2016. https://doi.org/10.1002/(SICI)1097-0010(199911)79:14%3c2010::AID-JSFA471%3e3.0.CO;2-U
Babich, H. (1982). Butylated hydroxytoluene (BHT): A review. Environmental Research, 29(1), 1–29. https://doi.org/10.1016/0013-9351(82)90002-0
Bidchol, A. M., Wilfred, A., Abhijna, P., & Harish, R. (2011). Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food and Bioprocess Technology, 4(7), 1137–1143. https://doi.org/10.1007/s11947-009-0196-9
Bors, W., & Michel, C. (2002). Chemistry of the antioxidant effect of polyphenols. Annals of the New York Academy of Sciences, 957(1), 57–69. https://doi.org/10.1111/j.1749-6632.2002.tb02905.x
Bouallegue, K., Allaf, T., Younes, R. Ben., & Allaf, K. (2016). Texturing and instant cooling of rapeseed as pretreatment prior to pressing and solvent extraction of oil. Food and Bioprocess Technology, 9, 1521–1534. https://doi.org/10.1007/s11947-016-1734-x
Chernova, A., Gubaev, R., Mazin, P., Goryunova, S., Demurin, Y., Gorlova, L., et al. (2019). UPLC–MS triglyceride profiling in sunflower and rapeseed seeds. Biomolecules, 9(1). https://doi.org/10.3390/biom9010009
Choe, E., & Min, D. B. (2009). Mechanisms of antioxidants in the oxidation of foods. Comprehensive Reviews in Food Science and Food Safety, 8(4), 345–358. https://doi.org/10.1111/j.1541-4337.2009.00085.x
Cisneros-Yupanqui, M., Zagotto, A., Alberton, A., Lante, A., Zagotto, G., Ribaudo, G., & Rizzi, C. (2020a). Monitoring the antioxidant activity of an eco-friendly processed grape pomace along the storage. Natural Product Research, 1–4. https://doi.org/10.1080/14786419.2020.1815741
Cisneros-Yupanqui, M., Zagotto, A., Alberton, A., Lante, A., Zagotto, G., Ribaudo, G., & Rizzi, C. (2020b). Study of the phenolic profile of a grape pomace powder and its impact on delaying corn oil oxidation. Natural Product Research, 1–5. https://doi.org/10.1080/14786419.2020.1777414
Dzah, C. S., Duan, Y., Zhang, H., Wen, C., Zhang, J., Chen, G., & Ma, H. (2020). The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Bioscience, 35, 100547. https://doi.org/10.1016/j.fbio.2020.100547
Gandova, V., Ivanova, P., Kalaydzhiev, H., Perifanova-Nemska, M., & Chalova, V. I. (2021). Dissolution and surface tension properties of ethanol-wash solute obtained from industrial sunflower meal. Biointerface Research in Applied Chemistry, 11(4), 11284–11292. https://doi.org/10.33263/BRIAC114.1128411292
Georgiev, R., Ivanov, I. G., Ivanova, P., Tumbarski, Y., Kalaydzhiev, H., Dincheva, I. N., et al. (2021). Phytochemical profile and bioactivity of industrial rapeseed meal ethanol-wash solutes. Waste and Biomass Valorization, 1, 3. https://doi.org/10.1007/s12649-021-01373-6
Guerrouj, K., Sánchez-Rubio, M., Taboada-Rodríguez, A., Cava-Roda, R. M., & Marín-Iniesta, F. (2016). Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food and Bioproducts Processing, 99, 20–28. https://doi.org/10.1016/j.fbp.2016.03.007
Hashmi, S., & Satwadhar, P. (2010). Rapeseed meal nutraceuticals. Journal of Oilseed Brassica, 1, 43–54.
Hu, J., Guo, Z., Glasius, M., Kristensen, K., Xiao, L., & Xu, X. (2011). Pressurized liquid extraction of ginger (Zingiber officinale Roscoe) with bioethanol: An efficient and sustainable approach. Journal of Chromatography A, 1218(34), 5765–5773. https://doi.org/10.1016/j.chroma.2011.06.088
Hussain, S. A., Hameed, A., Ajmal, I., Nosheen, S., Suleria, H. A. R., & Song, Y. (2018). Effects of sesame seed extract as a natural antioxidant on the oxidative stability of sunflower oil. Journal of Food Science and Technology, 55(10), 4099–4110. https://doi.org/10.1007/s13197-018-3336-2
Ito, N., Fukushima, S., & Tsuda, H. (1985). Carcinogenicity and modification of the carcinogenic response by bha, bht, and other antioxidants. Critical Reviews in Toxicology, 15(2), 109–150. https://doi.org/10.3109/10408448509029322
Ivanova, P., Chalova, V., Koleva, L., & Pishtiyski, I. (2013). Amino acid composition and solubility of proteins isolated from sunflower meal produced in Bulgaria. International Food Research Journal, 20(6), 2995–3000.
Ivanova, P., Vesela, C., & Uzunova, G. (2016). Biochemical characterization of industrially produced rapeseed meal as a protein source in food industry | Elsevier Enhanced Reader. Agriculture and Agricultural Science Procedia, 10, 52–62.
Jang, S. A., Lim, G. O., & Song, K. Bin. (2011). Preparation and mechanical properties of edible rapeseed protein films. Journal of Food Science, 76(2), C218–C223. https://doi.org/10.1111/j.1750-3841.2010.02026.x
Kalaydzhiev, H., Georgiev, R., Ivanova, P., Stoyanova, M., Silva, C. L. M., & Chalova, V. I. (2020a). Enhanced solubility of rapeseed meal protein isolates prepared by sequential isoelectric precipitation. Foods, 9(6). https://doi.org/10.3390/foods9060703
Kalaydzhiev, H., Ivanova, P., Stoyanova, M., Pavlov, A., Rustad, T., Silva, C. L. M., & Chalova, V. I. (2020b). Valorization of rapeseed meal: Influence of ethanol antinutrients removal on protein extractability, amino acid composition and fractional profile. Waste and Biomass Valorization, 11(6), 2709–2719. https://doi.org/10.1007/s12649-018-00553-1
Karefyllakis, D., Altunkaya, S., Berton-Carabin, C. C., van der Goot, A. J., & Nikiforidis, C. V. (2017). Physical bonding between sunflower proteins and phenols: Impact on interfacial properties. Food Hydrocolloids, 73, 326–334. https://doi.org/10.1016/j.foodhyd.2017.07.018
Khiari, Z., Makris, D. P., & Kefalas, P. (2009). An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food and Bioprocess Technology, 2(4), 337–343. https://doi.org/10.1007/s11947-007-0044-8
Kirpluks, M., Kalnbunde, D., Walterova, Z., & Cabulis, U. (2017). Rapeseed oil as feedstock for high functionality polyol synthesis. Journal of Renewable Materials, 5, 3–4. https://doi.org/10.7569/JRM.2017.634116
Klinchongkon, K., Bunyakiat, T., & Khuwijitjaru, P. (2019). Ethanol precipitation of mannooligosaccharides from subcritical water-treated coconut meal hydrolysate. Food and Bioprocess Technology, 1197–1204.
Koski, A., Pekkarinen, S., Hopia, A., Wähälä, K., & Heinonen, M. (2003). Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. European Food Research and Technology, 217(2), 110–114. https://doi.org/10.1007/s00217-003-0721-4
Kreps, F., Schmidt, S., Kreps, F., Vrbiková, L., & Schmidt, Š. (2014). Industrial rapeseed and sunflower meal as source of antioxidants complex utilization of bark extractives View project BIOFOODS-Complex utilization of plant biomass in biofoods with added value View project Industrial Rapeseed and Sunflower Meal as. Source, 4(2), 45–54.
Laguna, O., Barakat, A., Alhamada, H., Durand, E., Baréa, B., Fine, F., et al. (2018). Production of proteins and phenolic compounds enriched fractions from rapeseed and sunflower meals by dry fractionation processes. Industrial Crops and Products, 118, 160–172. https://doi.org/10.1016/j.indcrop.2018.03.045
Laguna, O., Odinot, E., Bisotto, A., Baréa, B., Villeneuve, P., Sigoillot, J. C., et al. (2019). Release of phenolic acids from sunflower and rapeseed meals using different carboxylic esters hydrolases from Aspergillus niger. Industrial Crops and Products, 139, 111579. https://doi.org/10.1016/j.indcrop.2019.111579
Lante, A., Nardi, T., Zocca, F., Giacomini, A., & Corich, V. (2011). Evaluation of red chicory extract as a natural antioxidant by pure lipid oxidation and yeast oxidative stress response as model systems. Journal of Agricultural and Food Chemistry, 59(10), 5318–5324. https://doi.org/10.1021/jf2003317
Lee, J., Kim, J. W., & Nyachoti, C. M. (2021). Standardized total tract digestibility of phosphorus in high-protein sunflower meal fed to growing pigs with or without phytase supplementation. Animal Feed Science and Technology, 274, 114853. https://doi.org/10.1016/j.anifeedsci.2021.114853
Li, W., Zhang, Y., Nie, W., & Zhan, J. (2017). Optimization of conditions of concentrating protein rapeseed meal by ultrasonic-assisted alcohol washing. Agricultural Biotechnology, 6(5), 66–68.
Lomascolo, A., Uzan-Boukhris, E., Sigoillot, J. C., & Fine, F. (2012). Rapeseed and sunflower meal: A review on biotechnology status and challenges. Applied Microbiology and Biotechnology, 95(5), 1105–1114. https://doi.org/10.1007/s00253-012-4250-6
Medina-Meza, I. G., Barnaba, C., & Barbosa-Cánovas, G. V. (2014). Effects of high pressure processing on lipid oxidation: A review. Innovative Food Science and Emerging Technologies, 22, 1–10. https://doi.org/10.1016/j.ifset.2013.10.012
Moreno-González, M., & Ottens, M. (2021). A structured approach to recover valuable compounds from agri-food side streams. Food and Bioprocess Technology. Springer. https://doi.org/10.1007/s11947-021-02647-6
Ozturk Sarikaya, S. B. (2015). Acethylcholinesterase inhibitory potential and antioxidant properties of pyrogallol. Journal of Enzyme Inhibition and Medicinal Chemistry, 30(5), 761–766. https://doi.org/10.3109/14756366.2014.965700
Pal, U. S., Patra, R. K., Sahoo, N. R., Bakhara, C. K., & Panda, M. K. (2014). Effect of refining on quality and composition of sunflower oil. Journal of Food Science and Technology, 52(7), 4613–4618. https://doi.org/10.1007/s13197-014-1461-0
Pan, M., Jiang, T. S., & Pan, J. L. (2011). Antioxidant activities of rapeseed protein hydrolysates. Food and Bioprocess Technology, 4(7), 1144–1152. https://doi.org/10.1007/s11947-009-0206-y
Pickardt, C., Eisner, P., Kammerer, D. R., & Carle, R. (2015). Pilot plant preparation of light-coloured protein isolates from de-oiled sunflower (Helianthus annuus L.) press cake by mild-acidic protein extraction and polyphenol adsorption. Food Hydrocolloids, 44, 208–219. https://doi.org/10.1016/j.foodhyd.2014.09.020
Rauf, S., Jamil, N., Tariq, S. A., Khan, M., Kausar, M., & Kaya, Y. (2017). Progress in modification of sunflower oil to expand its industrial value. Journal of the Science of Food and Agriculture, 97(7), 1997–2006. https://doi.org/10.1002/jsfa.8214
Ribeiro, J. S., Santos, M. J. M. C., Silva, L. K. R., Pereira, L. C. L., Santos, I. A., da Silva Lannes, S. C., & da Silva, M. V. (2019). Natural antioxidants used in meat products: A brief review. Meat Science, 148, 181–188. https://doi.org/10.1016/j.meatsci.2018.10.016
Riciputi, Y., Diaz-de-Cerio, E., Akyol, H., Capanoglu, E., Cerretani, L., Caboni, M. F., & Verardo, V. (2018). Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chemistry, 269, 258–263. https://doi.org/10.1016/j.foodchem.2018.06.154
Rodrigues, I. M., Carvalho, M. G. V., & Rocha, J. M. (2017). Increase of protein extraction yield from rapeseed meal through a pretreatment with phytase. Journal of the Science of Food and Agriculture, 97(8), 2641–2646. https://doi.org/10.1002/jsfa.8087
Rombaut, N., Chave, T., Nikitenko, S. I., Maâtaoui, M. El, Fabiano-Tixier, A. S., & Chemat, F. (2020). Modification of olive leaves’ surface by ultrasound cavitation. Correlation with polyphenol extraction enhancement. Applied Sciences, 11(1), 232. https://doi.org/10.3390/app11010232
Santos-Sánchez, N. F., Salas-Coronado, R., Valadez-Blanco, R., Hernández-Carlos, B., & Guadarrama-Mendoza, P. C. (2017). Natural antioxidant extracts food preservatives. Acta Scientiarum Polonorum Technologia Alimentaria, 16(4), 361–370. https://doi.org/10.17306/J.AFS.2017.0530
Sato, Y., Itagaki, S., Kurokawa, T., Ogura, J., Kobayashi, M., Hirano, T., et al. (2011). In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. International Journal of Pharmaceutics, 403(1–2), 136–138. https://doi.org/10.1016/j.ijpharm.2010.09.035
Savoire, R., Lanoisellé, J.-L., & Vorobiev, E. (2013). Mechanical continuous oil expression from oilseeds: a review. Food and Bioprocess Technology, 6, 1–16. https://doi.org/10.1007/s11947-012-0947-x
Shanmugam, A., & Ashokkumar, M. (2015). Characterization of ultrasonically prepared flaxseed oil enriched beverage/carrot juice emulsions and process-induced changes to the functional properties of carrot juice. Food and Bioprocess Technology, 8(6), 1258–1266. https://doi.org/10.1007/s11947-015-1492-1
Stratil, P., Kubáň, V., & Fojtová, J. (2008). Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech Journal of Food Sciences, 26(4), 242–253. https://doi.org/10.17221/1119-cjfs
Sun-Waterhouse, D., Luberriaga, C., Jin, D., Wibisono, R., Wadhwa, S. S., & Waterhouse, G. I. N. (2013a). Juices, fibres and skin waste extracts from white, pink or red-fleshed apple genotypes as potential food ingredients: A comparative study. Food and Bioprocess Technology, 6(2), 377–390. https://doi.org/10.1007/s11947-011-0692-6
Sun-Waterhouse, D., Wang, W., Waterhouse, G. I. N., & Wadhwa, S. S. (2013b). Utilisation potential of feijoa fruit wastes as ingredients for functional foods. Food and Bioprocess Technology, 6(12), 3441–3455. https://doi.org/10.1007/s11947-012-0978-3
Thiyam, U., Claudia, P., Jan, U., & Alfred, B. (2009). De-oiled rapeseed and a protein isolate: Characterization of sinapic acid derivatives by HPLC-DAD and LC-MS. European Food Research and Technology, 229(5), 825–831. https://doi.org/10.1007/s00217-009-1122-0
Tinello, F., Lante, A., Bernardi, M., Cappiello, F., Galgano, F., Caruso, M. C., & Favati, F. (2018). Comparison of OXITEST and RANCIMAT methods to evaluate the oxidative stability in frying oils. European Food Research and Technology, 244(4), 747–755. https://doi.org/10.1007/s00217-017-2995-y
Tinello, F., Zannoni, S., & Lante, A. (2020). Antioxidant properties of soybean oil supplemented with ginger and turmeric powders. Applied Sciences (Switzerland), 10(23), 1–14. https://doi.org/10.3390/app10238438
Upadhyay, R., & Mishra, H. N. (2015). Classification of sunflower oil blends stabilized by oleoresin rosemary (Rosmarinus officinalis L.) Using Multivariate Kinetic Approach. Journal of Food Science, 80(8), E1746–E1754. https://doi.org/10.1111/1750-3841.12966
Verardo, V., Messia, M. C., Marconi, E., & Caboni, M. F. (2020). Effect of different egg products on lipid oxidation of biscuits. Foods, 9(11), 1714. https://doi.org/10.3390/foods9111714
Vieira, S. A., Zhang, G., & Decker, E. A. (2017). Biological implications of lipid oxidation products. JAOCS, Journal of the American Oil Chemists’ Society, 94(3), 339–351. https://doi.org/10.1007/s11746-017-2958-2
Wambura, P., Tegete, H., & Verghese, M. (2012). Application of high-power ultrasound to improve adhesion of honey on roasted peanuts to improve oxidative stability. Food and Bioprocess Technology, 5(5), 2012–2016. https://doi.org/10.1007/s11947-010-0467-5
Wambura, P., Yang, W., & Mwakatage, N. R. (2011). Effects of sonication and edible coating containing rosemary and tea extracts on reduction of peanut lipid oxidative rancidity. Food and Bioprocess Technology, 4(1), 107–115. https://doi.org/10.1007/s11947-008-0150-2
Wang, C. Y. (2020). A review on the potential reuse of functional polysaccharides extracted from the by-products of mushroom processing. Food and Bioprocess Technology, 13(2), 217–228. https://doi.org/10.1007/s11947-020-02403-2
Wang, Z., Ju, X., He, R., Yuan, J., & Wang, L. (2015). The effect of rapeseed protein structural modification on microstructural properties of peptide microcapsules. Food and Bioprocess Technology, 8, 1305–1318. https://doi.org/10.1007/s11947-015-1472-5
Wroniak, M., Rekas, A., Siger, A., & Janowicz, M. (2016). Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT - Food Science and Technology, 68, 634–641. https://doi.org/10.1016/j.lwt.2016.01.013
Zhang, H., & Tsao, R. (2016). Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science, 8, 33–42. https://doi.org/10.1016/j.cofs.2016.02.002
Zhu, J., Zhang, D., Tang, H., & Zhao, G. (2018). Structure relationship of non–covalent interactions between phenolic acids and arabinan–rich pectic polysaccharides from rapeseed meal. International Journal of Biological Macromolecules, 120, 2597–2603. https://doi.org/10.1016/j.ijbiomac.2018.09.036
Acknowledgements
We acknowledge Stefania Zannoni for technical assistance. Nicole Pagan is gratefully acknowledged for skilful support in laboratory work.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement. This work was supported by the Bulgarian National Science Fund, project no. KП-06-H37/21 “An integrated approach for efficient utilisation of by-products of vegetable oil producing industry: Sunflower and rapeseed meals” and by the University of Padova prot. DOR 2032990.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cisneros-Yupanqui, M., Chalova, V.I., Kalaydzhiev, H.R. et al. Preliminary Characterisation of Wastes Generated from the Rapeseed and Sunflower Protein Isolation Process and Their Valorisation in Delaying Oil Oxidation. Food Bioprocess Technol 14, 1962–1971 (2021). https://doi.org/10.1007/s11947-021-02695-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02695-y