Abstract
In order to search for a new method for the anti-browning of food products, a novel hydroxypyridinone (HPO) derivative with a formyl group was evaluated for its anti-tyrosinase property. This compound was found to exhibit potent tyrosinase inhibition on the monophenolase activity of mushroom tyrosinase with an IC50 value of 1.33 μM, indicating that this HPO derivative was 12-fold stronger than kojic acid (IC50 15.89 μM). This molecule can inhibit tyrosinase via two action modes, namely copper reduction and chelation, and the formation of a Schiff’s base with the amino group at the active site of the enzyme. A synergistic effect of these two action modes to enhance the inhibitory activity was observed. This compound was also investigated for the inhibitory effect on diphenolase activity of mushroom; the inhibitory mechanism was found to be reversible and of competitive-uncompetitive mixed-type inhibition. This hydroxypyridinone was demonstrated to effectively control the browning of vegetable products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many foods readily turn brown during processing and storage. This causes a serious decrease in the quality and commercial value of the food, shortening shelf life. The prevention of such browning is a problem which needs to be solved urgently in the food industry. Browning includes non-enzymatic browning and enzymatic browning. Browning of fruits, vegetables, and shrimp is mainly caused by the latter. Tyrosinase is known to be one of the main enzymes causing the browning of fruits, vegetables, and shrimp (Friedman 1996; Tang and Newton 2004; Nirmal and Benjakul 2011). The tissue damage, peeling and cutting of fruits and vegetables (such as apples, bananas, and potatoes), can cause the rupture of cell membrane, resulting in the contact of phenolic compounds with enzymes, thereby initiating the occurrence of enzymatic browning under the aerobic conditions. Tyrosinase inhibitors can in principle, delay or prevent the browning of fruits, vegetables, and shrimp (Dai et al. 2016; Radhakrishnan et al. 2016).
Tyrosinase (EC 1.14.18.1), also called polyphenoloxidase, is a metalloenzyme, the active site of which includes two copper ions (Siegbahn 2003; Sendovski et al. 2011). It is widely distributed in animals, plants and microorganisms (Zhao et al. 2016). Tyrosinase is known to be a key and rate-limiting enzyme in the biosynthesis of melanin pigment (Chen et al. 2015). It catalyzes two distinct reactions involving molecular oxygen in the hydroxylation of monophenols to o-diphenols (monophenolase activity), as well as the subsequent oxidation of o-diphenols to o-quinones (diphenolase activity) (Li et al. 2013). Thereafter, o-quinones can polymerize spontaneously to form melanin in a series of reaction pathways (Seo et al. 2003). Furthermore, the reaction of amines, amino acids, peptides, and proteins with quinones causes the destruction of essential amino acids, decreasing the digestibility of the food. This is also an associated loss in proteolytic and glycolytic enzymic activity, thereby leading to the loss of nutritional quality (Kim and Uyama 2005).
A large number of naturally occurring and synthetic compounds possessing tyrosinase inhibitory properties have been reported (Kim and Uyama 2005; Parvez et al. 2007), but only a few find application because of their general limitation with regards to toxicity, activity, and stability. Hydroxypyridinones (HPOs), a class of iron chelators with high iron(III) affinity, have many applications in medicinal area. One HPO ligand, 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone), was approved for clinical use by the EMEA in 1999 and by the FDA in 2011, for the treatment of β-thalassaemia major and other iron overload associated diseases (Barman Balfour and Foster 1999). Kojic acid, a metabolic product of many species of Aspergillus and Penicillium molds, is a well-known tyrosinase inhibitor with an action mode of chelating copper at the active site of the enzyme. The potency of newly developed tyrosinase inhibitors are typically evaluated by comparison with kojic acid. HPOs bind copper with superior affinity to that of kojic acid. Thus, in principle, HPOs are predicted to possess tyrosinase inhibitory activity by binding copper ions coordinated in the active site of the enzyme. Indeed, in our previous work some HPO-amino acid conjugates were demonstrated to possess stronger anti-tyrosinase activity than kojic acid (Li et al. 2013; Zhao et al. 2016). Recently, a novel HPO derivative containing a formyl group, 1,4-dihydro-5-hydroxy-1-octyl-4-oxopyridine-2-carbaldehyde (1, Fig. 1) was synthesized (Zhao 2017). Aldehydes isolated from plants, such as cinnamaldehyde and cuminaldehyde, are reported to inhibit tyrosinase activity by reacting with the amino groups in the active site of tyrosinase to form Schiff’s bases, which prevent the binding of normal substrate to the enzyme (Kim and Uyama 2005). Thus, it was anticipated that compound 1 would inhibit tyrosinase efficiently. In the present study, we investigate the possibility of mushroom tyrosinase being inhibited by two different action modes which could act in a synergistic manner. In order to evaluate the potential application of compound 1 in the food industry, it was further investigated for its anti-browning effect on vegetable such as potato. A Lab color model was employed to evaluate the color change of potato during storage.
Materials and Methods
Chemicals
Compound 1, 5-hydroxy-2-(hydroxymethyl)-1-octylpyridin-4(1H)-one (2) and 5-(benzyloxy)-1,4-dihydro-1-octyl-4-oxopyridine-2-carbaldehyde (3) was synthesized starting from kojic acid (4) as described by Zhao (2017). Compound 1 was found to readily absorb water to form a hydrate, having a geminal diol structure. The ratio of 1 to its hydrate was calculated to be 1.85:1 from the 1H NMR spectra. Although compound 1 used in present study was a mixture with its hydrate, 1H NMR demonstrated that compound 1 was transformed to its hydrate in the presence of water (for convenience we described the tested HPO as compound 1 in the following text).
Tyrosinase from mushroom (lyophilized powder, ≥1000 unit/mg solid) was purchased from Sigma. Kojic acid, l-tyrosine (l-Tyr), l-3,4-dihydroxyphenylalanine (l-DOPA), and bathocuproine were purchased from Aladdin Chemical Reagents Co. (Shanghai, China). Other reagents were of AR grade and used without any further purification, and redistilled and ion-free water was used throughout the experiments. Ion-free water was obtained using a laboratory water purification system (ELGA PURELAB Ultra).
Effects of Inhibitors on the Mushroom Tyrosinase Activity
The monophenolase activity and the diphenolase activity assays were performed according to a previous report with a slight modification (Kim et al. 2004). l-Tyrosine (2 mM) was used as the substrate for the monophenolase activity assay, and l-DOPA (2 mM) was used as the substrate for the diphenolase activity assay. Different concentrations of inhibitors (0.1 mL in DMSO) was first mixed with the substrate solution (1 mL) and phosphate buffer (pH 6.8, 1.8 mL, 50 mM), then a portion of tyrosinase solution (0.1 mL) was added to this mixture. This solution was incubated at 30 °C for 10 min. Absorption and kinetic measurements were undertaken on a UV-3600 spectrophotometer at 475 nm, the final concentration of DMSO was 3.33%. Controls, without inhibitors, were routinely monitored. Each treatment was performed in triplicate. The inhibitory activity of the sample was expressed as the concentration that inhibits 50% of the enzyme activity (IC50).
Determination of the Inhibition Type and Inhibition Constant
The inhibition type was assayed by the Lineweaver–Burk plot based on the results of inhibitory effect on the diphenolase activity of tyrosinase, and the inhibition constant was determined by the secondary plots of the apparent K m/V m or 1/V m versus the concentration of the inhibitor, as described by Chen et al. (2003).
Determination of pK a and Copper Affinity Constants
The automatic titration system used in this study consists of a Metrohm 765 Dosimat autoburette, a Mettler Toledo MP230 pH meter with SENTEK pH electrode (P11), and an HP 8453 UV-visible spectrophotometer with a Hellem quartz flow cuvette being circulated through a Gilson Mini-plus #3 pump-speed capability (20 mL/min). A potassium chloride electrolyte solution (0.1 M) was used to maintain the ionic strength. The temperature of the test solutions was maintained in a thermostatic jacketed titration vessel at 25 °C (± 0.1 °C) using a Fisherbrand Isotemp water bath. The pH electrodes were calibrated using GLEE (Gans and O’Sullivan 2000) with data obtained by titrating a volumetric standard HCl (0.1 M) in KCl (0.1 M) with KOH (0.1 M) under an argon gas atmosphere in the vessel. The solution under investigation was stirred vigorously during the experiment. For pK a determinations, a quartz cuvette path length of 10 mm was used, while for metal stability constant determinations a quartz cuvette path length of 50 mm was used. All instruments were interfaced to a computer and controlled by an in-house program. An automated titration adopted the following strategy: the pH of a solution was increased by 0.1 pH unit by the addition of potassium hydroxide solution (0.1 M) from the autoburette. The pH readings were judged to be stable if their values varied by less than 0.01 pH unit after a set incubation period. For pK a determinations, an incubation period of 1.5 min was adopted; for copper stability constant determinations, an incubation period of 3 min was adopted. The cycle was repeated until the defined end point pH value was achieved. Because the copper complexes of 1 possess high lipophilicity, the corresponding stability constants in aqueous solution were determined through the extrapolation from titrations in three different composition solutions, molar ratio of DMSO/H2O = 0.4:1, 0.3:1, and 0.2:1. All the titration data were analyzed with the HypSpec2014 program (Gans et al. 1996, 1999) (http://www.hyperquad.co.uk/). The speciation plot was calculated with the HYSS program (Alderighi et al. 1999).
Assay of Copper-Reducing Ability
The ability of sample to reduce cupric copper to cuprous copper was determined according to the reported methods with minor modification (Andrawis and Kahn 1990; Chen et al. 1991). Different concentrations of the sample solution (1 mL in DMSO) were first mixed with CuSO4 solution (0.5 mL, 0.4 mM) in a centrifuge tube, and the mixture was incubated in a 25 °C water bath for 10 min, then bathocuproine (0.5 mL, 4 mM) was added to this blend. The absorbance of the solution was measured at 483 nm after 20 min incubation at 25 °C. Kojic acid was used as a positive control for comparison.
Analysis of Browning Degree of Potatoes
Preparation of Potato Samples
Potatoes were purchased from the vegetable market located in Hangzhou of Zhejiang Province, China. The potatoes were selected from the same batch with similar shapes and maturity and without injuries, diseases, or pests. First, the potatoes were cut into 5 mm thick slices, and those with a diameter of about 3–4 cm were chosen and washed with cool running tap water for 1 min. The potato slices were divided into two groups for different treatments: the treatment group, in which the samples were soaked in a solution of compound 1 (0.5 mg/mL) for 1 min; the control group, in which the samples were soaked in redistilled and ion-free water without any anti-browning treatment. After dipping, the slices were gravity drained for 1 min, immediately packed into individual polypropylene film bags and stored at 4 °C until use. The slices were measured for the color changes at an interval of 2 days.
Color Measurement
The color change of the surface of potato samples was monitored by measuring the CIE Lab values (Abbott 1999) (L*, lightness, a low number (0–50) indicates dark and a high number (51–100) indicates light; a*, redness and greenness, a positive number indicates red and a negative number indicates green; and b*, yellowness and blueness, a positive number indicates yellow and a negative number indicates blue) using a CR-400 Minolta chronometer instrument (Konica Minolta, Osaka, Japan), which had been calibrated against a standard white plate provided by the manufacture. Readings of L*, a*, and b* were recorded for three pieces per sample. As the color of the potato surface is homogeneous, L*, a*, and b* values were measured for the central area of the potato surface for each potato sample at 2-day interval during the storage of 10 days. All measurements were performed in triplicate. Total color difference (ΔE) was also used to evaluate the anti-browning potential of different treatments. ΔE was calculated as follows:
where L 0 *, a 0 *, and b 0 * values represented the readings at day 0, and L*, a*, and b* values represented the data during storage.
Analysis of the Browning Index
The browning index was measured as previously reported method with slight modification (Jung et al. 2008). The sample (2 g) of each group was crushed and fully mixed in redistilled water (40 mL) and trichloroacetic acid (10%, 10 mL), then incubated for 2 h at 35 °C in a constant temperature incubator. After the incubation, the medium was filtered. The absorbance of the filtrate was measured at 420 nm on a spectrophotometer.
Statistical Analysis
All the experiments were conducted in triplicate. The data were statistically analyzed by variance test (SPSS 16 Software, SPSS Inc., Chicago, IL, USA). The treatments were considered to be significantly different at P < 0.05. Significant differences between the treatments were examined by Duncan’s multiple range test. The data values were expressed as the means ± SD (n = 3).
Results and Discussion
Inhibitory Effect of Compound 1 on Monophenolase Activity of Tyrosinase
The effect of the HPO derivative 1 on the monophenolase activity of mushroom tyrosinase was investigated using l-tyrosine as a substrate. The inhibitory effect on mushroom tyrosinase increased with increasing concentration of the inhibitor (P < 0.05) (Fig. 2). The IC50 value of compound 1 was calculated to be 1.33 μM, indicating that it was 11.9-fold more active than kojic acid (IC50 = 15.89 μM).
In order to explore the synergistic effect of these two proposed action modes, compound 2 containing 5-hydroxyl-4-oxo group, and compound 3 with a formyl group at position-2 and protected 5-hydroxyl group were investigated for their inhibitory effect on monophenolase activity in comparison with 1 (Fig. 1). According to the compound structures, it was expected that compound 2 could inhibit tyrosinase via chelation of copper at the enzyme active site, while formation of a Schiff’s base with a primary amino group in the enzyme would be the inhibitory mechanism for compound 3. As shown in Fig. 2, both 2 and 3 exhibited comparable inhibitory effects to that of kojic acid, with IC50 values of 19.12 μM and 13.08 μM, respectively. Thus, 1 is 14.4-fold more active than 2 and 9.8-fold more active than 3. Compound 2 and 3 inhibit tyrosinase by one action mode. In contrast, compound 1 is assumed to be able to inhibit the enzyme by two action modes due to its structure containing both a 5-hydroxyl-4-oxo moiety and a formyl group. This significant increase in inhibitory activity could be attributed to the synergistic effect of these two action modes, namely copper chelation and the formation of a Schiff’s base.
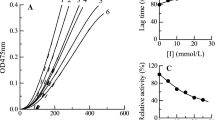
The kinetic course of the oxidation of l-Tyr by mushroom tyrosinase in the presence of different concentrations of compound 1 is presented in Fig. 3a; the reaction system reached a steady state after an initial time lag. Subsequently, the progress of product accumulation increased linearly with increasing reaction time. When using compound 1 as an inhibitor, both the steady-state rate and the lag time were influenced by increasing concentration. With the increase of inhibitor concentration, the steady-state rate of monophenolase activity decreased distinctly and dose-dependently, decreasing to 38.0% of the uninhibited rate at an inhibitor concentration of 33.33 μM (Fig. 3b, curve I); while lag time of the enzyme reaction showed an increase, the lag time changing from 35 s in its absence to 105.3 s in the presence of 1 (16.67 μM) as shown in Fig. 3b (curve II). Similar results were obtained in the investigation of mushroom tyrosinase inhibition by other inhibitors, such as p-methoxybenzaldehyde thiosemicarbazone (MBT) and some alpha-substituted derivatives of cinnamaldehyde (Chen et al. 2012; Cui et al. 2015). The increase in lag time indicates an inhibition of monophenolase activity, which subsequently led to a reduction in total tyrosinase activity (Anish and Adinpunya 2014).
Inhibitory effects of 1 on monophenolase activity of mushroom tyosinase. a Progress curves for the inhibition of mushroom tyrosinase by compound 1. b Effect of compound 1 on the steady-state rate (curve I) and on the lag time (curve II) of mushroom tyrosinase for the oxidation of l-tyrosine. The concentrations of 1 for curves 1–6 were 0.000, 4.17, 8.33, 16.67, 25.00, and 33.33 μM, respectively
Inhibitory Effect of 1 on Diphenolase Activity of Mushroom Tyrosinase
Inhibition Kinetics
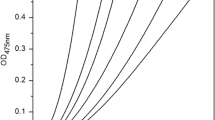
The inhibitory effect of compound 1 on diphenolase activity of mushroom tyrosinase was evaluated using l-DOPA as a substrate. The kinetic course of the oxidation of l-DOPA by mushroom tyrosinase in the presence of different concentrations of 1 was investigated. The formation of o-quinone was concentration dependent and increased with time (Fig. 4a), but the increasing rate of o-quinone formation slowed down with increasing reaction time, indicating that the inhibitory effect decreased. In addition, on consideration of the slope of the straight line, there was no intersection between the tangent of the curve and the abscissa (Fig. 4a), suggesting that no lag period existed in the progress of oxidation of l-DOPA catalyzed by diphenolase activity of tyrosinase.
Inhibition of inhibitor 1 on diphenolase activity of tyrosinase. a Inhibition kinetics. b Relative activity of tyrosinase in the presence of different concentration of 1. c Inhibitory effects at different enzyme concentrations. The concentrations of inhibitor for curves 1–6 in a and c were 0.00, 4.17, 8.33, 16.67, 25.00, and 33.33 μM, respectively
As indicated in Fig. 4b, the relative activity of enzyme decreased rapidly at the beginning and then decreased slowly with increasing concentration of 1. The IC50 value of compound 1 on diphenolase activity of mushroom tyrosinase was calculated to be 7.83 μM.
Inhibition Mechanism
Investigation of the relationship between enzyme activity and its concentration in the presence of 1 indicated that the plots of the remaining enzyme activity versus the concentration of enzyme at different inhibitor concentrations gave a family of straight lines (Fig. 4c), which all passed through the origin. This result is similar to that reported by Chen et al. (2012). An increase of inhibitor concentration resulted in decrease of the slope of the line, indicating that the presence of inhibitor did not reduce the quantity of enzyme, but just resulted in the inhibition of enzyme activity. Thus, the inhibition of HPO 1 on diphenolase activity of tyrosinase was reversible.
The Lineweaver–Burk double-reciprocal plots (Li et al. 2010) for the inhibition of tyrosinase by 1 were obtained at variable concentrations of 1 and the substrate (Fig. 5a). The plots of 1/v versus 1/[S] gave a group of straight lines with different slopes that intercept in the second quadrant, indicating that 1 was a competitive-uncompetitive mixed-type inhibitor, which binds with both the free enzyme and the enzyme-substrate complexes (ES). The equilibrium constant of inhibitor for binding with free enzyme (K I) was obtained from a plot of slope (K m/V m) versus the concentration of the inhibitor (Fig. 5b), and the equilibrium constant with enzyme-substrate complex (K IS) was obtained from a plot of the vertical intercept (1/V m) versus the concentration of the inhibitor (Fig. 5c). The K I and K IS values of 1 were determined to be 11.37 and 20.01 μM, respectively. The K IS value is larger than the K I value, indicating that the affinity of the inhibitor for free enzyme was greater than that for the enzyme-substrate complex.
Lineweaver–Burk plots for the inhibition of 1 on mushroom tyrosinase for the oxidation of l-DOPA. The concentrations of 1 for curves 1–5 were 0.00, 8.33, 16.67, 25.00, and 33.33 μM, respectively. Inset b represents the plot of slope versus the concentration of 1 for determining the inhibition constants K I. Inset c represents the plot of intercept versus the concentration of 1 for determining the inhibition constant K IS
Determination of the Copper Binding and Reducing Abilities of Compound 1
To further explore the inhibitory mechanism of 1 on tyrosinase, its affinity for copper(II) and its copper-reducing ability were investigated. In order to evaluate the copper chelating ability of 1, its pK a values were firstly determined. The UV spectra of compound 1 is pH dependent (Fig. 6a). With the increase of pH from 2.04 to 11.24, the maximum absorbance of 1 shifted from 265 nm to 245 nm, indicating that the changes in its protonation status at the different pH. Using the spectrophotometric titration method, the pK a values of 1 were determined to be 2.6 and 7.7. As shown in Fig. 6b, in the presence of copper ions, the absorbance of 1 solution at 400 nm increased with increasing pH value from 2.38 to 4.0, suggesting the increase in formation of the copper(II)-1 complex. As a bidentate ligand, 1 can form two different copper complexes, namely CuL+ and CuL2 (El-Jammal et al. 1994). The distribution of these two complexes varies with the pH (Fig. 6c). The stability constants of these two metal-ligand complexes can be expressed as follows:
where L− represents deprotonated 1 (ligand).
Abilities of 1 in binding and reducing the copper. a UV spectra of 1. [1] = 97.23 μM (started in 16.630 mL of 0.1 M KCl), pH was changed from 2.04 to 11.24 by the addition of KOH at 25 °C. b UV spectra of 1 in the presence of copper. [1] = 346.2 μM, [Cu2+] = 101.5 μM (in 18.685 mL of 0.1 M KCl), pH was changed from 2.38 to pH 4.00 by the addition of KOH at 25 °C. c Speciation plot of 1 in the presence of copper, [1]total = 10 μM and [Cu2+]total = 1 μM. d Copper-reducing capacity of compound 1 in comparison with kojic acid
As indicated in Fig. 6c, at pH 2.0, the content of complex CuL+ was more than 90%, but almost no complex CuL2 was formed, under these conditions, the amount of free cupric ions in the solution was about 5%. However, the amount of CuL+ and copper ions decreased with increasing pH, while the amount of CuL2 increased. At pH 3.0, almost no free copper ions existed in the solution. At the pH of about 3.3, the amount of CuL+ and CuL2 were equal. At pH above 5.0, almost all CuL+ converted to CuL2. From spectrophotometric titrations, the accumulated log stability constants of the two copper complexes, log β 1 (CuL+) and log β 2 (CuL2), were determined to be 12.7 and 22.3, respectively, which are higher than those of kojic acid (log β 1 = 6.6, log β 2 = 11.7) (Jones et al. 1958).
The ability of 1 and kojic acid to reduce cupric ion to cuprous ion is shown in Fig. 6d. The increase in absorbance value at 483 nm indicated the formation of cuprous ion. The absorbance value increased rapidly with increasing concentration of compound 1 up to 0.09 mM, after which increased slowly with the increase of concentration until 0.15 mM, and thereafter the absorbance remained almost unchanged, indicating that all cupric ions were reduced to cuprous ions. In the case of kojic acid, the absorbance at 483 nm fleetingly increased with the increase of its concentration until 0.3 mM, after which the increasing rate rapidly slowed, reaching a maximum value. Thus, compound 1 possessed a stronger copper-reducing ability than kojic acid. It is well-known that tyrosinase exists in three isoforms, namely, oxy-tyrosinase [Cu(II)Cu(II)O2], met-tyrosinase [Cu(II)Cu(II)], and deoxy-tyrosinase [Cu(I)Cu(I)] (Likhitwitayawuid 2008). Met-tyrosinase is reduced by reductant to deoxy-tyrosinase, which is then oxidized by oxygen, forming oxy-tyrosinase capable of catalyzing mono- or diphenol oxidation (Lubien et al. 1981). Copper in the active site of tyrosinase plays a key role in browning reaction. Reduction of cupric ion to cuprous ion at the active site of tyrosinase could convert tyrosinase into the deoxy form. Therefore, 1 could inhibit tyrosinase activity by chelating the copper(II) and by reducing met-tyrosinase to deoxy-tyrosinase.
Anti-browning Effect of 1 on the Fresh-Cut Potato Slices
In general, L*, a*, b*, and ΔE (total color difference) have been used as the most crucial parameters for measuring the extent of browning in fruits and vegetables. A decrease in L* and an increase in a*, b*, and ΔE mean the occurrence of browning (Hou et al. 2014). The changes in the surface browning degree of fresh-cut potato slices during the storage are shown in Fig. 7. The L* values decreased with storage time in both test and control groups (Fig. 7a). During the whole test period (0–10 days), the descent rate of L* value of the control group was significantly higher than that of group treated with 1 (P < 0.05), although the descent rate of L* value of control group decreased slowly during the latter half test period (6–10 days). On the 10th day, L* values of the control group and 1-treated group were 44.562 ± 0.669 and 49.294 ± 1.218, respectively, which indicated that the browning degree of the treatment group was lower than that of the control group (P < 0.05). As indicated in Fig. 7b, c, a* and b* values of both groups tended to increase with increasing storage time, while the a* and b* values of the treatment group were lower than those of control group (P < 0.05). As shown in Fig. 7d, the results of ΔE values displayed the same trend as that of the L* values; thus, 1 was effective in maintaining the appearance of the potato slices.
Effect of 1 in controlling the browning of fresh-cut potato slices during the storage for 10 days at 4 °C. a–c Measurement of L*, a*, and b* values of fresh-cut potato slices. d The total color difference (ΔE) of fresh-cut potato slices. e Changes in the browning index of fresh-cut potato slices. Means ± SD (n = 3) with different letters are significantly different at 5% level
Browning Index
The degree of browning can be also represented as the absorbance of filtrate containing browning products of each group. As presented in Fig. 7e, during the measurement period (0–10 days), the browning degree of the control group increased quickly, whereas that of the treatment groups slowly increased during the former half test period (0–4 days) then increased slightly faster in the next 6 days. On the 10th day, the absorbance value of the 1-treated group control group at 420 nm was measured to be 0.363 ± 0.017, which was much lower than that of control group (0.459 ± 0.032), indicating a significantly lower browning index (P < 0.05). Thus, 1 could effectively inhibit browning reaction, and prolong the shelf life of potato slices.
Although compound 1 is anticipated to possess a relatively low toxicity, as judged by related HPOs, and the residue of 1 in potato slices will be extremely low when compared to the dose of deferiprone adopted in the treatment of β-thalassaemia patients (75 mg kg−1 day−1), the toxicity of 1 in food stuffs will need to be extensively investigated.
Conclusion
The HPO 1 possesses potent inhibitory activity against mushroom tyrosinase. It is capable of inhibiting tyrosinase via two modes of action, copper chelation and reduction, and the formation of a Schiff’s base with the enzyme. The synergistic effect of these two modes of action leads to a significant increase in tyrosinase inhibitory activity. Compound 1 exhibited reversible and mixed-type inhibition on mushroom tyrosinase. It has also been demonstrated that 1 impedes the browning of potatoes by decreasing the formation of the intermediate browning product, the o-quinone. We are continuing to investigate the potential of this compound for food preservation.
References
Abbott, J. A. (1999). Quality measurement of fruits and vegetables. Postharvest Biology and Technology, 15(3), 207–225.
Alderighi, L., Gans, P., Ienco, A., Peters, D., Sabatini, A., & Vacca, A. (1999). Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coordination Chemistry Reviews, 184(1), 311–318.
Andrawis, A., & Kahn, V. (1990). Ability of various chemicals to reduce copper and to inactivate mushroom tyrosinase. Journal of Food Biochemistry, 14(2), 103–115.
Anish, K., & Adinpunya, M. (2014). Evaluating tyrosinase (monophenolase) inhibitory activity from fragrant roots of Hemidesmus indicus for potent use in herbal products. Industrial Crops and Products, 52, 394–399.
Barman Balfour, J. A., & Foster, R. H. (1999). Deferiprone: a review of its clinical potential in iron overload in beta-thalassaemia major and other transfusion-dependent diseases. Drugs, 58(3), 553–578.
Chen, C. Y., Lin, L. C., Yang, W. F., Bordon, J., & Wang, H. M. D. (2015). An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Current Organic Chemistry, 19(1), 4–18.
Chen, J. S., Wei, C. I., & Marshall, M. R. (1991). Inhibition mechanism of kojic acid on polyphenol oxidase. Journal of Agricultural and Food Chemistry, 39(11), 1897–1901.
Chen, L. H., Hu, Y. H., Song, W., Song, K. K., Liu, X., Jia, Y. L., Zhuang, J. X., & Chen, Q. X. (2012). Synthesis and antityrosinase mechanism of benzaldehyde thiosemicarbazones: novel tyrosinase inhibitors. Journal of Agricultural and Food Chemistry, 60(6), 1542–1547.
Chen, Q., Chen, Q. X., Qiu, L., Song, K. K., & Huang, H. (2003). Inhibitory effect of 4-cyanobenzaldehyde and 4-cyanobenzoic acid on mushroom (agaricus bisporus) tyrosinase. The Protein Journal, 22(7), 607–612.
Cui, Y., Liang, G., Hu, Y. H., Shi, Y., Cai, Y. X., Gao, H. J., Chen, Q. X., & Wang, Q. (2015). Alpha-substituted derivatives of cinnamaldehyde as tyrosinase inhibitors: inhibitory mechanism and molecular analysis. Journal of Agricultural and Food Chemistry, 63(2), 716–722.
Dai, X. Y., Zhang, M. X., Wei, X. Y., Hider, R. C., & Zhou, T. (2016). Novel multifunctional hydroxypyridinone derivatives as potential shrimp preservatives. Food and Bioprocess Technology, 9(7), 1079–1088.
El-Jammal, A., Howell, P. L., Turner, M. A., Li, N. Y., & Templeton, D. M. (1994). Copper complexation by 3-hydroxypyridin-4-one iron chelators: structural and iron competition studies. Journal of Medicinal Chemistry, 37(4), 461–466.
Friedman, M. (1996). Food browning and its prevention: an overview. Journal of Agricultural and Food Chemistry, 44(3), 631–653.
Gans, P., & O’Sullivan, B. (2000). GLEE, a new computer program for glass electrode calibration. Talanta, 51(1), 33–37.
Gans, P., Sabatini, A., & Vacca, A. (1996). Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta, 43(10), 1739–1753.
Gans, P., Sabatini, A., & Vacca, A. (1999). Determination of equilibrium constants from spectrophotometric data obtained from solutions of known pH: the program pHab. Annali di Chimica, 89(1–2), 45–49.
Hou, Z., Feng, Y., Wei, S., & Wang, Q. (2014). Effects of curing treatment on the browning of fresh-cut potatoes. American Journal of Potato Research, 91(6), 655–662.
Jones, J. G., Poole, J. B., Tomkinson, J. C., & Williams, R. J. P. (1958). The relationship between proton dissociation constants and the stability constants of complex ions. Journal of the Chemical Society, 2001–2009.
Jung, J. Y., Shin, S. H., Choi, J. H., & Jeong, M. C. (2008). Browning and quality changes of fresh-cut iceberg lettuce by gas flushing packagings. Korean Journal of Horticultural Science & Technology, 26(4), 406–412.
Kim, H., Choi, J., Cho, J. K., Kim, S. Y., & Lee, Y. S. (2004). Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorganic & Medicinal Chemistry Letters, 14(11), 2843–2846.
Kim, Y. J., & Uyama, H. (2005). Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cellular and Molecular Life Sciences, 62(15), 1707–1723.
Li, D. F., Hu, P. P., Liu, M. S., Kong, X. L., Zhang, J. C., Hider, R. C., & Zhou, T. (2013). Design and synthesis of hydroxypyridinone-l-phenylalanine conjugates as potential tyrosinase inhibitors. Journal of Agricultural and Food Chemistry, 61(27), 6597–6603.
Li, Z. C., Chen, L. H., Yu, X. J., Hu, Y. H., Song, K. K., Zhou, X. W., & Chen, Q. X. (2010). Inhibition kinetics of chlorobenzaldehyde thiosemicarbazones on mushroom tyrosinase. Journal of Agricultural and Food Chemistry, 58(23), 12537–12540.
Likhitwitayawuid, K. (2008). Stilbenes with tyrosinase inhibitory activity. Current Science, 94(1), 44–52.
Lubien, C. D., Winkler, M. E., Thamann, T. J., Scott, R. A., Co, M. S., Hodgson, K. O., & Solomon, E. I. (1981). Chemical and spectroscopic properties of the binuclear copper active site in Rhus laccase: direct confirmation of a reduced binuclear type 3 copper site in type 2 depleted laccase and intramolecular coupling of the type 3 to the type 1 and type 2 copper sites. Journal of the American Chemical Society, 103, 7014–7016.
Nirmal, N. P., & Benjakul, S. (2011). Inhibitory effect of mimosine on polyphenoloxidase from cephalothoraxes of Pacific white shrimp (Litopenaeus vannamei). Journal of Agricultural and Food Chemistry, 59(18), 10256–10260.
Parvez, S., Kang, M., Chung, H. S., & Bae, H. (2007). Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytotherapy Research, 21(9), 805–816.
Radhakrishnan, S. K., Shimmon, R. G., Conn, C., & Baker, A. T. (2016). Inhibitory kinetics of azachalcones and their oximes on mushroom tyrosinase: a facile solid-state synthesis. Chemistry & Biodiversity, 13(5), 531–538.
Sendovski, M., Kanteev, M., Ben-Yosef, V. S., Adir, N., & Fishman, A. (2011). First structures of an active bacterial tyrosinase reveal copper plasticity. Journal of Molecular Biology, 405(1), 227–237.
Seo, S. Y., Sharma, V. K., & Sharma, N. (2003). Mushroom tyrosinase: recent prospects. Journal of Agricultural and Food Chemistry, 51(10), 2837–2853.
Siegbahn, P. E. (2003). A comparison of the thermodynamics of O[bond]O cleavage for dicopper complexes in enzymes and synthetic systems. Journal of Biological Inorganic Chemistry, 8(5), 577–585.
Tang, W., & Newton, R. J. (2004). Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.) Plant Science, 167(3), 621–628.
Zhao, D. Y. (2017). Design, synthesis and biological actives of multifunctional hydroxypyridinone derivatives and their application in shrimp preservation. Master thesis, Zhejiang Gongshang University, China.
Zhao, D. Y., Zhang, M. X., Dong, X. W., Hu, Y. Z., Dai, X. Y., Wei, X. Y., Hider, R. C., Zhang, J. C., & Zhou, T. (2016). Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorganic & Medicinal Chemistry Letters, 26(13), 3103–3108.
Acknowledgements
The authors thank the Zhejiang Provincial Natural Science Foundation of China (No. LY17B020001) and Food Science and Engineering—the most important discipline of Zhejiang Province (2017SIAR216) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, K., Zhao, DY., Chen, YL. et al. A Novel Inhibitor Against Mushroom Tyrosinase with a Double Action Mode and Its Application in Controlling the Browning of Potato. Food Bioprocess Technol 10, 2146–2155 (2017). https://doi.org/10.1007/s11947-017-1976-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1976-2