Abstract
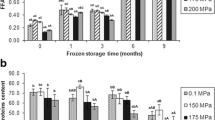
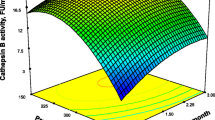
This research focuses on biochemical changes related to quality losses observed in Atlantic mackerel (Scomber scombrus) muscle stored under commercial frozen storage conditions (9 months, −18 °C) when subjected to high-hydrostatic pressure (HHP) treatments (125, 150, 175, and 200 MPa for 0 min) before freezing. After freezing, free fatty acid (FFA) formation (lipid hydrolysis assessment) showed a marked inhibition in HHP-treated fish and during frozen storage of samples treated at 175 MPa. Fluorescence ratio (FR) assessment of tertiary lipid oxidation showed a partial inhibitory effect during the 0–9-month period for samples treated at 175 and 200 MPa. After a 3-month storage of samples treated at these pressure levels, one-dimensional SDS-PAGE analysis of the sarcoplasmic protein fraction revealed the disappearance of a band; additionally, samples treated at 150 MPa showed the same effect at month 9. After gel excision, trypsin digestion, tandem mass spectrometry (MS/MS), and sequence database analysis, the band was identified as phosphoglycerate mutase 2 (28.7 kDa). On the other hand, HHP processing did not show a significant effect on trimethylamine (TMA) values, primary and secondary lipid oxidation, PUFA levels, 1-D myofibril protein pattern, and the activity of acid phosphatase and cathepsins B and D. Biochemical quality indices such as FFA, TMA, and FR and the activity of acid phosphatase and cathepsin B showed a progressive increase throughout the frozen storage of all samples.



Similar content being viewed by others
References
Ashie, I., Smith, J., & Simpson, B. (1996). Spoilage and shelf-life extension of fresh fish and shellfish. Critical Reviews in Food Science and Nutrition, 36, 87–121.
Aubourg, S. (1999). Review: recent advances in assessment of marine lipid oxidation by using fluorescence. Journal of the American Oil Chemists’ Society, 76, 409–419.
Aubourg, S, Medina, I., & Pérez-Martín, R. (1996). Polyunsaturated fatty acids in tuna phospholipids: distribution in the sn-2 location and changes during cooking. Journal of Agricultural and Food Chemistry, 44, 585-589.
Aubourg, S., Rodríguez, A., & Gallardo, J. (2005). Rancidity development during frozen storage of mackerel (Scomber scombrus): effect of catching season and commercial presentation. European Journal of Lipid Science and Technology, 107, 316–323.
Aubourg, S., Torres, J. A., Saraiva, J., Guerra-Rodríguez, E., & Vázquez, M. (2013). Effect of high-pressure pretreatments applied before freezing and frozen storage on the functional and sensory properties of Atlantic mackerel (Scomber scombrus). Food Science and Technology (LWT), 53, 100–106.
Bligh, E., & Dyer, W. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Brutti, A., Rovere, P., Cavallero, S., D’Amelio, S., Danesi, P., & Arcangeli, G. (2010). Inactivation of Anisakis simplex larvae in raw fish using high hydrostatic pressure treatments. Food Control, 21, 331–333.
Buckow, R., Truong, B., & Versteeg, C. (2010). Bovine cathepsin D activity under high pressure. Food Chemistry, 120, 474–481.
Campus, M. (2010). High pressure processing of meat, meat products and seafood. Food Engineering Reviews, 2, 256–273.
Chapman, R., & McKay, J. (1949). The estimation of peroxides in fats and oils by the ferric thiocyanate method. Journal of the American Oil Chemists’ Society, 26, 360–363.
Chéret, R., Chapleau, N., Delbarre-Ladrat, C., Vérrez-Bagnis, V., & De Lamballerie, M. (2005). Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. Journal of Food Science, 70, E477–E483.
Chéret, R., Hernández-Andrés, A., Delbarre-Ladrat, C., de Lamballerie, M., & Vérrez-Bagnis, V. (2006). Proteins and proteolytic activity changes during refrigerated storage in sea bass (Dicentrarchus labrax L.) muscle after high-pressure treatment. European Food Research and Technology, 222, 527–535.
Chevalier, D., Le Bail, A., Chourot, J., & Chantreau, P. (1999). High pressure thawing of fish (whiting): influence of the process parameters on drip losses. Lebensmittel-Wissenschaft und -Technologie, 32, 25–31.
Chevalier, D., Sequeira-Muñoz, A., Le Bail, A., Simpson, B., & Ghoul, M. (2000). Effect of pressure shift freezing, air-blast freezing and storage on some biochemical and physical properties of turbot (Scophthalmus maximus). Lebensmittel-Wissenschaft und -Technologie, 33, 570–577.
Fidalgo, L. G., Saraiva, J. A., Aubourg, S. P., Vázquez, M., & Torres, J. A. (2014). Effect of high-pressure pre-treatments on enzymatic activities of Atlantic mackerel (Scomber scombrus) during frozen storage. Innovative Food Science & Emerging Technologies, 23, 18–24.
Gallardo, J. M., Carrera, M., & Ortea, I. (2013). Proteomics in food science. In A. Cifuentes (Ed.), Foodomics: advanced mass spectrometry in modern food science and nutrition (pp. 125–165). Hoboken: John Wiley & Sons, Inc.
Groβ, M., Auerbach, G., & Jaenicke, R. (1993). The catalytic activities of monomeric enzymes show complex pressure dependence. FEBS Letters, 321, 256–260.
Han, J.-Z., & Wang, Y.-B. (2008). Proteomics: present and future in food science and technology. Trends in Food Science and Technology, 19, 26–30.
Harris, P., & Tall, J. (1994). Rancidity in fish. In J. Allen, & R. Hamilton (Eds.), Rancidity in foods (pp. 256–272). London: Chapman and Hall.
Laemmli, U. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lakshmanan, R., Pigott, J., & Paterson, A. (2003). Potential applications of high pressure for improvement in salmon quality. Trends in Food Science and Technology, 14, 354–363.
Lakshmanan, R., Miskin, D., & Piggott, J. R. (2005). Quality of vacuum packed cold-smoked salmon during refrigerated storage as affected by high-pressure processing. Journal of the Science of Food and Agriculture, 85, 655–661.
Lowry, R., & Tinsley, I. (1976). Rapid colorimetric determination of free fatty acids. Journal of the American Oil Chemists’ Society, 53, 470–472.
Mackie, I. M. (1993). The effects of freezing on flesh proteins. Food Reviews International, 9, 575–610.
Montero, P., Giménez, B., Pérez-Mateos, M., & Gómez-Guillén, M. C. (2005). Oxidation stability of muscle with quecetin and rosemary during thermal and high-pressure gelation. Food Chemistry, 93, 17–23.
Mújica-Paz, H., Valdez-Fragoso, A., Tonello Samson, C., Welti-Chanes, J., & Torres, J. A. (2011). High-pressure processing technologies for the pasteurization and sterilization of foods. Food and Bioprocess Technology, 4, 969–985.
Norton, T., & Sun, D.-W. (2008). Recent advances in the use of high pressure as an effective processing technique in the food industry. Food and Bioprocess Technology, 1, 2–34.
Ohmori, T., Shigehisa, T., Taji, S., & Hayashi, R. (1992). Biochemical effects of high hydrostatic pressure on the lysosome and proteases involved in it. Bioscience, Biotechnology, and Biochemistry, 56, 1285–1288.
Ohshima, T., Nakagawa, T., & Koizumi, C. (1992). Effect of high hydrostatic pressure on the enzymatic degradation of phospholipids in fish muscle during storage. In E. Bligh (Ed.), Seafood science and technology, chapter 8 (pp. 64–75). Oxford: Fishing News Books.
Ortea, I., Rodríguez, A., Tabilo-Munizaga, G., Pérez-Won, M., & Aubourg, S. (2010). Effect of hydrostatic high-pressure treatment on proteins, lipids and nucleotides in chilled farmed salmon (Oncorhynchus kisutch) muscle. European Food Research and Technology, 230, 925–934.
Ortiz, J., Larraín, MªA., Vivanco, J., & Aubourg, S. (2009). Rancidity development during the frozen storage of farmed coho salmon (Oncorhynchus kisutch): effect of antioxidant composition supplied in the diet. Food Chemistry, 115, 143-148.
Pazos, M., Pereira da Rocha, A., Roepstorff, P., & Rogowska-Wrzesinska, A. (2011). Fish proteins as targets of ferrous-catalyzed oxidation: identification of protein carbonyls by fluorescent labeling on two-dimensional gels and MALDI-TOF/TOF mass spectrometry. Journal of Agricultural and Food Chemistry, 59, 7962–7977.
Pazos, M., Méndez, L., Gallardo, J. M., & Aubourg, S. (2014). Selective-targeted effect of high pressure processing on proteins related to quality: a proteomics evidence in Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 7, 2342–2353.
Refsgaard, H., Brockhoff, P., & Jensen, B. (2000). Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. Journal of Agricultural and Food Chemistry, 48, 3280–3285.
Saeed, S., & Howell, N. (2001). 12-lipoxygenase activity in the muscle tissue of Atlantic mackerel (Scomber scombrus) and its prevention by antioxidants. Journal of the Science of Food and Agriculture, 81, 745–750.
Senturk, T., & Alpas, H. (2013). Effect of high hydrostatic pressure treatment (HHPT) on quality and shelf life of Atlantic mackerel (Scomber scombrus). Food and Bioprocess Technology, 6, 2306–2318.
Shewfelt, R. (1981). Fish muscle lipolysis—a review. Journal of Food Biochemistry, 5, 79–100.
Sikorski, Z., & Kolakowska, A. (1994). Changes in protein in frozen stored fish. In Z. Sikorski, B. Sun Pan, & F. Shahidi (Eds.), Seafood proteins (pp. 99–112). New York: Chapman and Hall.
Sikorski, Z., & Kolakowski, E. (2000). Endogenous enzyme activity and seafood quality: influence of chilling, freezing, and other environmental factors. In N. Haard, & B. Simpson (Eds.), Seafood enzymes (pp. 451–487). New York: Marcel Dekker.
Smith, P., Krohn, R., Hermanson, G., Mallia, A., Gartner, F., Provenzano, M., Fujimoto, E., Goeke, N., Olson, B., & Klenk, D. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150, 76–85.
Tironi, V., Tomás, M., & Añón, M. C. (2002). Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malondialdehyde (RI). Journal of Food Science, 67, 930–935.
Tironi, V., de Lamballerie, M., & Le Bail, A. (2010). Quality changes during the frozen storage of sea bass (Dicentrarchus labrax) muscle after pressure shift freezing and pressure assisted thawing. Innovative Food Science & Emerging Technologies, 11, 565–573.
Torres, A., Vázquez, M., Saraiva, J., Gallardo, J., & Aubourg, S. (2013). Lipid damage inhibition by previous high pressure processing in white muscle of frozen horse mackerel. European Journal of Lipid Science and Technology, 115, 1454–1461.
Tozawa, H., Erokibara, K., & Amano, K. (1971). Proposed modification of Dyer’s method for trimethylamine determination in codfish. In R. Kreuzer (Ed.), Fish inspection and quality control (pp. 187–190). London: Fishing News Books Ltd.
Vázquez, M., Torres, J. A., Gallardo, J., Saraiva, J., & Aubourg, S. (2013). Lipid hydrolysis and oxidation development in frozen mackerel (Scomber scombrus): effect of a high hydrostatic pressure pre-treatment. Innovative Food Science & Emerging Technologies, 18, 24–30.
Vyncke, W. (1970). Direct determination of the thiobarbituric acid value in trichloracetic acid extracts of fish as a measure of oxidative rancidity. Fette Seifen Anstrichmittel, 72, 1084–1087.
Acknowledgments
The Xunta de Galicia and the European Social Fund are thankfully recognized for the financial support of the postdoctoral “Isidro Parga Pondal” contract to M. P. The Spanish Ministry of Science and Innovation is also gratefully acknowledged for the doctoral fellowship to L. M. The authors thank Dr. María Lavilla (AZTI Tecnalia, Derio, Spain), Dr. Barbara Teixeira (IPMA, Lisbon, Portugal), Mr. Marcos Trigo, and Mrs. Lorena Barros for their help in carrying out the present study. This work was supported by the Secretaría Xeral de I + D from the Xunta de Galicia (Galicia, Spain) through the Research Project 10TAL402001PR (2010–2012), by Fundação para a Ciência e a Tecnologia (FCT Portugal), European Union, QRN, FEDER, COMPETE through founding of the Organic Chemistry Research Unit (QOPNA) (project PEst-C/QUI/UI0062/2013; FCOMP-01-0124-FEDER-037296), and by Formula Grants no. 2011-31200-06041 and 2012-31200-06041 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pazos, M., Méndez, L., Fidalgo, L. et al. Effect of High-Pressure Processing of Atlantic Mackerel (Scomber scombrus) on Biochemical Changes During Commercial Frozen Storage. Food Bioprocess Technol 8, 2159–2170 (2015). https://doi.org/10.1007/s11947-015-1567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1567-z