Abstract
Purpose of review
Establishing an early, efficient diagnosis for cardiac amyloid (CA) is critical to avoiding adverse outcomes. We review current imaging tools that can aid early diagnosis, offer prognostic information, and possibly track treatment response in CA.
Recent findings
There are several current conventional imaging modalities that aid in the diagnosis of CA including electrocardiography, echocardiography, bone scintigraphy, cardiac computed tomography (CT), and cardiac magnetic resonance (CMR) imaging. Advanced imaging techniques including left atrial and right ventricular strain, and CMR T1 and T2 mapping as well as ECV quantification may provide alternative non-invasive means for diagnosis, more granular prognostication, and the ability to track treatment response.
Summary
Leveraging a multimodal imaging toolbox is integral to the early diagnosis of CA; however, it is important to understand the unique role and limitations posed by each modality. Ongoing studies are needed to help identify imaging markers that will lead to an enhanced ability to diagnose, subtype and manage this condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac amyloidosis (CA) is the result of deranged protein misfolding leading to amyloid fibril deposition into the extracellular space. This inevitably leads to a syndrome of progressive restrictive cardiomyopathy, myocardial conduction abnormalities, and clinical heart failure. The two main types of cardiac amyloidosis are categorized as either transthyretin amyloid (ATTR) or light chain amyloidosis (AL). ATTR CA is further subcategorized as wild type (ATTRwt) or hereditary (hATTR). The advent of disease-modifying therapies, including stabilizer and gene silencer therapies for ATTR and novel chemotherapeutics for AL amyloid have spurred an intense and increased interest in diagnosis. Importantly, prognosis hinges on a timely diagnosis to guide appropriate therapies. In this review, we will present the various non-invasive multimodal imaging tools available including current and novel approaches and techniques which can facilitate earlier diagnosis or show promise in helping to track the progression and response to therapy (Fig. 1, Table 1).
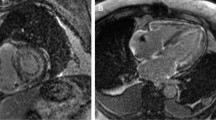
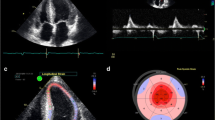
A ECG evaluation with low voltage is a commonly cited feature in CA (left panel); however, it is important to be cognizant of other ECG features such as voltage out of proportion to LV mass as well as pseudo-infarct pattern (right panel) and atrial fibrillation which is a common arrhythmia seen (right panel). B Echocardiographic assessment commonly shows LV thickening, occasionally with a pericardial effusion (top left), thickened and/or regurgitant valves (bottom left), while mitral inflow evaluation can show a restrictive diastolic pattern (top middle panel) and low tissue Doppler (bottom middle panel). RV evaluation with low TAPSE can suggest significant RV involvement and is a poor prognostic feature (top right). The characteristic global longitudinal strain pattern in CA is apical sparing which is also associated with poorer prognosis (bottom right). C Tc99m-PYP scanning showing SPECT/CT image on the left and planar imaging on the right with region of interest (ROI) drawn over the myocardium and ROI over the contralateral side in order to evaluate a heart/contralateral ratio. D Cardiac MRI images showing features consistent with CA. E LA strain has emerged as another means of evaluating for atrial myopathy as a result of amyloid infiltration and has been shown to have strong correlation with survival F ECV measurement by cardiac MRI.
Brief overview
Clinical suspicion for CA often hinges on a characteristic constellation of clinical signs and symptoms, often coupled with elevations in cardiac biomarkers such as NT pro BNP and/or troponin. A targeted diagnostic approach for CA rests on serum and urine immunofixation and serum-free light chains in conjunction with one or more of the imaging modalities reviewed below [1••]. Electrocardiography (ECG) is one of the most accessible diagnostic tools available for raising suspicion for CA, particularly when combined with a targeted review of systems and echocardiography [2]. The classic features of ECG are low voltage or voltage defined as < 5 mm in the limb leads or < 10 mm in the precordial leads. However, low voltage out of proportion to the degree of left ventricular (LV) mass can be another clue [3••, 4••]. This is attributed to amyloid deposition in the extracellular space which interferes with voltages generated. However, this is a nonspecific criterion and the absence of low voltage does not rule out CA as it is possible for patients with CA to even meet ECG criteria for left ventricular hypertrophy [3••, 4••, 5•]. Nonetheless, when present, low voltage does track with prognosis in CA [6, 7]. Other ECG clues include a pseudo-infarct pattern, conduction abnormalities, and atrial fibrillation [2, 3••, 4••].
Echocardiography
One of the first essential clues in raising suspicion for amyloidosis is echocardiography, as it is a mainstay in the evaluation of patients with dyspnea or heart failure symptoms. Increased LV wall thickness, particularly when combined with relative low voltage on ECG is a classic manifestation of CA. This is a direct consequence of amyloid infiltration rather than myocyte hypertrophy. Several echocardiographic defining features can often point towards the need for further work up including increased LV and right ventricular (RV) wall thickness, biatrial enlargement, interatrial septal thickening, thickened atrioventricular valves, pericardial effusions, and restrictive LV filling [1••, 8]. Pericardial effusions and valvular insufficiency, although nonspecific findings, are associated with a worse prognosis in CA [9].
Left ventricle
While echocardiography is not sufficient in distinguishing amyloid subtypes, it has been suggested that LV wall mass and thickness are typically higher in patients with ATTRwt as compared to hATTR or AL likely due to a more insidious disease process with longer exposure of amyloid aggregation and accumulation into the myocardial extracellular space [8, 10]. LV ejection fraction (EF) is often preserved despite a reduction in stroke volume. This is due to a reduction in left ventricular end-diastolic volume (LVEDV), and therefore, the ratio of stroke volume to LVEDV, which represents EF, is often normal. For this reason, EF is thought to poorly capture the pathological changes seen in CA, and it has been proposed that a more meaningful metric is myocardial contraction fraction (MCF), or the ratio of stroke volume to myocardial volume with normal values being 60–65% [11,12,13]. In fact, a reduced stroke volume despite normal or even “supra-normal” EF is associated with a higher risk of adverse outcome in CA [14,15,16,17]. However, systolic dysfunction can occur in more advanced stages of the disease due to cardiomyocyte damage and fibrotic changes over time [8]. Reduced EF at presentation is more common in the hereditary Val122Ile variant than in ATTRwt which commonly affects African American patients and is likely reflective of more advanced disease at diagnosis [18, 19].
Global longitudinal strain
Myocardial strain, which allows for a quantitative assessment of myocardial deformation, is a useful adjunct in the echocardiographic evaluation for CA. The characteristic LV longitudinal strain pattern in CA is sparing of the apical segment as compared to the impaired basal and mid myocardial segments and is often referred to as a “cherry on top” or “bull’s eye plot.” Although not diagnostic, an apical sparing pattern can be helpful in distinguishing CA from other etiologies such as hypertrophic cardiomyopathy, aortic stenosis, or hypertensive heart disease with a sensitivity of about 88% and specificity of 72% [5•, 20,21,22]. Importantly, decreased global longitudinal strain is an independent predictor of mortality irrespective of EF [1••, 8, 10, 23, 24]. Lower strain values are typically seen in AL CA as compared to ATTR CA for any given wall thickness which is thought to be secondary to direct light-chain myocardial toxicity and is strongly associated with worse outcomes [8, 10, 23].
Left atrial myopathy and left atrial strain
Historically, left atrial (LA) function has been inferred from size; however, other metrics for defining LA function are emerging as key in the assessment of overall cardiac performance and thromboembolic risk in various cardiac disorders [25•]. LA strain has important pathophysiologic, diagnostic, and prognostic implications in CA, however, has not been adopted into routine clinical practice outside of non-academic or research settings due to the need for operator input and time, making it impractical particularly in community settings. Nonetheless, LA function as measured by speckle-tracking echocardiography has been shown to be severely reduced in CA patients, both in AL and ATTR [26]. Bandera et al. utilized echo speckle-tracking strain imaging on a cohort of 906 pts with ATTR CA, and showed a substantial impairment of the 3 functions of the LA (reservoir, conduit, contraction) as well as increased atrial stiffness (the resistance to deformation of the LA), with increased LA stiffness correlating with worse prognosis [27••]. Their study also identified 20% of the study population as having loss of atrial contraction despite sinus rhythm (atrial electromechanical dissociation) and noted that this population had significantly poorer prognosis than patients with sinus rhythm who maintained effective mechanical contraction [27••].
Notably, CA patients have an incremental thromboembolic risk independent of atrial fibrillation (AF), with several studies suggesting atrial myopathy as the underlying etiology for this finding [27••, 28,29,30]. In fact, it has been a suggested practice to prophylactically offer anticoagulation to patients with CA and a diminutive A wave on mitral inflow evaluation or abnormal atrial strain suggestive of atrial mechanical failure [8, 27••]. Intracardiac thrombus has also been documented in patients with sinus rhythm, which might be explained by the previously mentioned concept of atrial electromechanical dissociation [27••, 31•, 32]. Prior studies have also demonstrated a high prevalence of intracardiac thrombus despite anticoagulation, with one study showing a 13.1% prevalence of intracardiac thrombus as detected on cardiac MRI (CMR) in an anticoagulated CA population with AF. Therefore, it is recommended on the basis of clinical experience at high-volume amyloid centers to routinely perform imaging with CMR or transesophageal echo (TEE) prior to attempts at direct current cardioversion regardless of anticoagulation status [30, 33•].
Right ventricle
The RV has a unique structure and function and differs from the LV with different myocardial fiber arrangement, cavity wall thickness, and structural make-up of the inflow and outflow tracts. A host of echocardiographic parameters, including dimension and function measures, has elucidated the impact of CA on the right heart. In a prior study comparing patients with CA vs. controls, those with CA showed increased RV size, RV basal diameter, IVC, RV wall thickness resulting in reduced total RV volume, and reduced systolic function as measured by tricuspid annular plane systolic excursion (TAPSE) [34]. Another study of AL patients investigated echocardiogram and doppler myocardial imaging (DMI) right heart parameters and found evidence of abnormal RV systolic function by TAPSE and DMI modalities, with a systolic strain of the basal segment of the RV free wall and TAPSE being most useful in distinguishing AL patients from controls [35]. Multiple studies have shown evidence of abnormal change in free wall RV strain correlating with worse prognosis in CA patients [35,36,37,38]. Additionally, TAPSE derived by both echocardiography and CMR has shown a strong correlation with worse prognosis in CA patients [15, 34, 39].
Bone scintigraphy
Contemporary diagnostic algorithms incorporate bone scintigraphy as part of the evaluation for CA, but only after laboratory screening evaluation to rule out AL amyloidosis using serum and urine immunofixation and serum-free light chains [40, 41••]. The most common radiotracer used in the USA for this purpose is technetium-99 m (Tc99m) which is bound to pyrophosphate (PYP). In the USA, PYP is the only available agent while other agents such as 3,3-diphosphono1,2-propanodicarboxylic acid are available in other parts of the world. The exact mechanism of uptake of tracer into the myocardium in the setting of CA is not fully understood but thought to be related to calcium deposition [42, 43••].
The rise in the use of bone scintigraphy as part of the evaluation for CA stems from the results of a large, multicenter study which evaluated patients with suspected CA. An abnormal bone scintigraphy study showing myocardial uptake in conjunction with a negative evaluation for a monoclonal gammopathy provided a specificity and positive predictive value of 100% for ATTR —paving the way for a nonbiopsy diagnosis of cardiac amyloidosis [1••]. Due to the diagnostic accuracy in identifying ATTR CA, this has led to less reliance on endomyocardial biopsy for confirmatory diagnosis, thus minimizing the potential for procedural-related risk to the patient. Furthermore, in a large analysis of Medicare beneficiaries, the incidence and prevalence of CA increased significantly between 2000 and 2012, particularly after 2006 [44, 45]. Therefore, bone scintigraphy has not only been practice changing in reducing the need for biopsy, but has also led to increased incidence of disease which has coincided with an increase in the availability of noninvasive imaging techniques to evaluate for CA, as endomyocardial biopsy is not widely available at all centers. However, biopsy continues to play an important role as the gold standard in settings in which there is discordant data or equivocal serologic or imaging workup.
Bone scintigraphy is performed by obtaining both planar and single photon emission computed tomography (SPECT) images after injection of the Tc99m-PYP tracer. The images are then assessed for the presence of a tracer in the myocardium. A quantitative evaluation based on planar images can be done in which the total counts are tabulated in a region of interest (ROI) over the heart and compared to a ROI over the contralateral lung. A ratio of ≥ 1.5 is considered positive for the presence of ATTR-CA using a 1-h protocol or ≥ 1.3 using a 3-h protocol [46••]. Additionally, a semiquantitative assessment is performed by comparing the uptake of the tracer in the heart as compared to nearby rib uptake. Myocardial uptake equal to (grade 2) or greater than rib uptake (grade 3) is considered suggestive of ATTR-CA [46••]. However, a key feature of interpretation relies on SPECT imaging for confirmation as described below.
A common pitfall in the interpretation of these tests is the presence of a tracer in the blood pool, or left ventricular cavity as opposed to the myocardium itself which may falsely elevate the counts in the ROI over the heart resulting in either an equivocal study or a false positive study when only planar imaging is used. Protocols include both a 1-h delay and a 3-h delay after injection. While a 1-h protocol can increase the throughput of patients, the 3-h protocol may help decrease the possibility of pooling of the tracer in the left ventricular cavity, known as blood pooling. Consequently, planar imaging alone is not enough to confirm the diagnosis of ATTR-CA, and therefore, SPECT is a key component for confirming myocardial uptake [47, 48•, 49•]. Several etiologies for potential false positive and false negative interpretation are reviewed in Table 2.
Radionuclide tracer uptake can vary by myocardial region and intensity. A recent study sought to determine the clinical impact of the intensity of tracer uptake. Despite the highly predictive nature of an abnormal scintigraphy scan, the degree of tracer uptake, as measured by the heart-to-contralateral lung ratio, did not impact clinical outcomes [50•]. However, while the precise mechanism of cardiac uptake is uncertain, more diffuse left ventricular tracer uptake may be associated with a worse prognosis [51••].
Once the diagnosis of CA is made typically patients are followed serially clinically, as there is no consensus on the use of serial bone scintigraphy imaging to assess disease progression. A small study involving 20 patients with ATTR-CA found no difference in the Tc99m-PYP scan at baseline and 1.5 years later [52]. In the clinical setting, therefore, these scans are not typically repeated once the diagnosis is made as their key role remains for the diagnosis of ATTR.
While the use of Tc99m-PYP scintigraphy has significantly facilitated the diagnosis of ATTR CA, evaluation for AL still requires tissue biopsy for evaluation. As a result, there is increased interest in noninvasive imaging techniques, specifically positron emission tomography (PET) and tracers that may help differentiate AL from ATTR. F18-labeled tracers have emerged as a possible answer to this problem. One such tracer, F18-florbetapir was shown to have increased uptake in patients with AL-CA as opposed to ATTR-CA [53]. The promise of these PET tracers is that they bind directly to the amyloid protein and may potentially provide a more sensitive way to detect early disease. Notably, serum amyloid P (SAP) scinitgraphy has been available in Europe to determine whole-body amyloid quantification, however, is not approved in the USA [54, 55]. However, there are other nuclear imaging radiotracers on the horizon including AT-01 (Attralus Inc., San Francisco, California), an amyloid-specific radiotracer that is capable of imaging all types of systemic amyloidosis through PET/CT imaging; however, published clinical trials supporting clinical utility and sensitivity are not yet available.
Cardiac magnetic resonance
Cardiovascular magnetic resonance (CMR) imaging is the gold-standard modality for assessing structure and function in all four chambers of the heart, but its key strength lies in myocardial tissue characterization [56]. Late gadolinium enhancement (LGE), typically used to identify replacement fibrosis in entities such as myocardial infarction or hypertrophic cardiomyopathy, has long been noted as a feature of cardiac amyloidosis as well [57]. A notable distinction is that in CA, LGE correlates not with replacement fibrosis, but with interstitial amyloid accumulation [58]. The typical LGE pattern of CA is global, predominantly subendocardial LGE. A closely associated feature, considered pathognomonic for CA, is the inability to null the myocardium on the inversion (TI) scout sequence performed in preparation for LGE imaging. In such scenarios, the myocardial extracellular matrix, expanded with amyloid fibrils, retains a similar amount of gadolinium as the blood pool, leading to indistinguishable T1 recovery curves. This, in turn, leads to difficulty in identifying the T1 null point of healthy myocardium, required for LGE techniques.
T1 mapping
Parametric mapping has allowed for additional CMR characterization of cardiac amyloid. Myocardial T1, which represents interstitial expansion that may be commonly related to myocardial edema or fibrosis, is also lengthened by the matrix expansion of myocardial amyloid deposition. The magnitude of T1 elevation is particularly large in CA, allowing for its distinction from other cardiomyopathies [59]. Importantly, so-called native T1 mapping provides a CMR mechanism to CA evaluation that does not require gadolinium contrast. A key consideration is the dependence of T1 values on magnetic field strength and individual sequence parameters, limiting the ability to standardize diagnostic thresholds [60••].
T1 mapping before and after gadolinium administration allows for quantification of the myocardial extracellular volume fraction (ECV), if the hematocrit is known, which tracks closely with collagen fraction on pathology in non-amyloidosis hearts [61•]. A meta-analysis, comparing the diagnostic and prognostic utility of ECV, found that it had a higher diagnostic odds ratio and hazard ratio for adverse events than LGE and native T1 mapping [62••]. ECV mapping has become an important tool in amyloid evaluation, as it may allow for the identification of early-stage cardiac amyloidosis that precedes the development of LGE [63•]. ECV is strongly prognostic in CA and may allow for tracking disease progression or response to CA therapies [32].
T2 mapping
T2 mapping also aids in the identification and prognostication of cardiac amyloidosis. T2 is sensitive to myocardial water content and state, and the edema associated with the myocyte toxicity of amyloid proteins leads to elevations in T2 times [64]. Myocardial T2 times appear to be prognostic and track response to treatment in AL, but not ATTR amyloid which is thought to be due to the direct cardiotoxicity of light chains themselves causing a greater degree of edema [65].
The stronger association between T2 and AL, compared with ATTR, has led to a recently proposed scoring system to distinguish AL from ATTR subtypes [66•]. The increased T2 with AL is also remarkable because most other markers of disease severity (LV mass, wall thickness, LGE transmural extent, RV involvement) are associated with ATTR amyloid [67]. Efforts to systematize the use of CMR criteria amyloid sub-typing are ongoing, but contemporary society recommendations do not rely on CMR for distinguishing TTR from AL [68•].
Diffusion tensor MRI
Another non-contrast magnetic resonance technique that has emerged for the characterization of CA is diffusion tensor MRI (DT-MRI). By tracking water diffusion through tissue, DT-MRI allows the evaluation of myocardial microstructure, quantifying fractional anisotropy (FA), mean diffusivity (MD), and myocardial sheetlet orientation. Early studies have demonstrated that MD tracks well with ECV estimates across amyloid subtypes, but that diastolic sheetlet changes may differ between ATTR and AL [69]. Additional validation studies and standardization of clinical protocols are the next important steps for this promising technique.
Cardiac computed tomography (CT)
It has become increasingly recognized that ATTR CA is common in patients with aortic stenosis (AS), particularly in the low-flow low-gradient aortic stenosis phenotype (LFLG AS) [70,71,72, 73•, 74]. Given the high prevalence of AS in an aging population and the increased use of transcatheter aortic valve replacement (TAVR) therapy in symptomatic patients, pre-procedural cardiac CT has emerged as a potential opportunistic screening tool for ATTR. Prior studies have evaluated patients with severe AS with the addition of ECV quantification to TAVR-planning CT and demonstrated significantly higher ECV values in patients with concomitant ATTR as confirmed by Tc99m-PYP scintigraphy. However, the ECV cutoff threshold needs further validation with larger-scale studies and validation across vendors as well as proposed algorithms for integrating into the current TAVR CT workflow. Importantly, identification of ATTR as a comorbid condition is crucial, as otherwise undiagnosed CA, may attenuate the long-term survival benefit of TAVR.
Imaging targets in treatment
The advent of rapidly expanding therapeutic options has revolutionized the landscape of CA and has led to increased interest in diagnosis [75, 76, 77••]. In the ATTR space, TTR stabilizers such as tafamidis (Vyndamax/Vydaqel, Pfizer) and TTR gene silencers such as patisiran (Onpattro, Alnylam Pharmaceuticals) and inotersen (Tegsedi, Akcea Therapeutics) are the two main disease-modifying therapies available. However, only tafamidis is approved for the treatment of CA while the silencers are approved for ATTR polyneuropathy with or without cardiac involvement [78, 79]. Treatments for AL, on the other hand, are targeted towards reduction in plasma cell and light chain burden with daratumumab becoming the first-line treatment in the current era [80].
The monitoring of response to therapy continues to be an area of intense research. Although novel treatment strategies have been shown to be clinically effective in reducing circulating biomarkers and improving functional status and symptoms, the translation to concrete imaging markers to tailor therapy is still lacking [81••]. Nonetheless, several studies have attempted to characterize the response to therapy via various imaging modalities, but are generally limited by small patient cohorts.
Echocardiography has been evaluated as a treatment-response monitoring strategy, although with mixed results. In a study evaluating 8 patients with hATTR and 7 patients with ATTRwt, stabilization of echocardiographic parameters including LV wall thickness, LV wall mass, and global longitudinal strain was also demonstrated in patients treated with inotersen [82, 83••]. A sub-study of the APOLLO trial evaluating a subpopulation of patients with CA involvement treated with patisiran showed improvements in echocardiographic markers such as LV wall thickness, LV end-diastolic volume, GLS, and cardiac output compared to placebo at 18 months [84]; however, in patients treated with inotersen, these echocardiographic variables did not differ between treatment and placebo after 15 months of treatment [79, 81••]. In AL disease, although some studies have shown improvement in strain ratio in chemotherapy responders as compared to nonresponders as well as LV size and stroke volume, other studies have failed to show a significant change in these parameters [81••, 85,86,87,88].
CMR has also been implemented for treatment monitoring in patients with different CA subtypes and various therapeutics. In a recent prospective study, 176 patients with AL CA were monitored with T1 and ECV mapping at diagnosis and serially at 6, 12, and 24 months after initiation of chemotherapy [89••]. The results of the study were promising and showed that ECV may possibly be used to track decreasing the myocardial amyloid burden and that change in ECV was an independent predictor of prognosis [89••]. Serial native T1 and ECV evaluation using CMR has also been used to monitor for stabilization of disease in ATTR CA patients treated with tafamidis over 12 months; however, large-scale prospective trials are still needed to support this in routine clinical practice [83••, 90]. With regard to gene silencer therapies, a prior study demonstrated a reduction in ECV in 16 patients treated with patisiran when retrospectively matched based on CMR results (adjusted mean difference between groups − 6.2% (95% CI: − 9.5 to − 3.0%); p = 0.001) [83••, 91••]. Furthermore, a study evaluating 33 ATTR patients treated with inotersen demonstrated mean LV mass reduction by 8.4% on CMR after 2 years of treatment and reduction by 11.4% at 3 years [83••, 92•].
Although tracking response to therapy is still evolving and current data has shown inconsistent results with regard to serial imaging, CMR-based quantification methods are promising candidates to be at the forefront of this progress given the elegant ability of magnetic resonance to provide high-resolution myocardial tissue characterization. However, the major limitation is cost and standardized protocols. Artificial intelligence (AI)-empowered imaging modalities are also emerging and may prove to be a powerful integration to better stratify patients both for diagnostic purposes and in monitoring response to therapy [83••].
Conclusions
Despite advances in treatment, CA remains an underdiagnosed and underappreciated condition. With ongoing advances in non-invasive imaging techniques, this diagnostic inertia may be overcome. Accurate diagnosis does not rest on any single technique but the reliance on a multimodal toolbox of integrated and targeted testing. The comprehensive use of current and emerging imaging techniques can help to augment risk stratification, monitoring response to therapy, and subsequent tailored therapy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–12. https://doi.org/10.1161/CIRCULATIONAHA.116.021612. Landmark study which defined the performance characteristics of bone scintigraphy in the diagnosis of ATTR cardiac amyloid, and thereby, created the framework for the integration of this imaging tool into the current diagnostic criteria.
Khedraki R. Cardiac amyloidosis: an update on diagnosis, current therapy, and future directions. Curr Opin Cardiol. 2022;37(3):272–84. https://doi.org/10.1097/HCO.0000000000000953.
•• Donnelly JP, Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84(12 Suppl 3):12–26. https://doi.org/10.3949/ccjm.84.s3.02. Consensus document which provides an algorithm for workup and management of cardiac amyloidosis.
•• Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7-e22. https://doi.org/10.1161/CIR.0000000000000792. Consensus document which provides an algorithm for work up and management of cardiac amyloidosis.
• Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872–2891. https://doi.org/10.1016/j.jacc.2019.04.003. State-of-the-art review on the diagnosis and treatment of ATTR amyloid with an in-depth review of noninvasive diagnostic testing.
Sperry BW, Vranian MN, Hachamovitch R, et al. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int J Cardiol. 2016;214:477–81. https://doi.org/10.1016/j.ijcard.2016.04.030.
Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114(7):1089–93. https://doi.org/10.1016/j.amjcard.2014.07.026.
Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging. 2020;13(6):1368–83. https://doi.org/10.1016/j.jcmg.2019.07.015.
Chacko L, Martone R, Bandera F, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020;41(14):1439–47. https://doi.org/10.1093/eurheartj/ehz905.
Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–9. https://doi.org/10.1161/CIRCULATIONAHA.113.006242.
King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40(2):325–9. https://doi.org/10.1016/s0735-1097(02)01944-7.
Matthews SD, Rubin J, Cohen LP, Maurer MS. Myocardial contraction fraction: a volumetric measure of myocardial shortening analogous to strain. J Am Coll Cardiol. 2018;71(2):255–6. https://doi.org/10.1016/j.jacc.2017.09.1157.
Maurer MS, Packer M. How should physicians assess myocardial contraction?: Redefining heart failure with a preserved ejection fraction. JACC Cardiovasc Imaging. 2020;13(3):873–8. https://doi.org/10.1016/j.jcmg.2019.12.021.
Shah S, Segar MW, Kondamudi N, et al. Supranormal left ventricular ejection fraction, stroke volume, and cardiovascular risk: findings from population-based cohort studies. JACC Heart Fail. 2022;10(8):583–594. https://doi.org/10.1016/j.jchf.2022.05.007.
Knight DS, Zumbo G, Barcella W, et al. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc Imaging. 2019;12(5):823–33. https://doi.org/10.1016/j.jcmg.2018.02.016.
Milani P, Dispenzieri A, Scott CG, et al. Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circ Cardiovasc Imaging. 2018;11(5):e006588. https://doi.org/10.1161/CIRCIMAGING.117.006588.
Martens P, Hanna M, Valent J, Estep JD, Tang WHW. Supra-normal left ventricular ejection fraction in cardiac amyloidosis. Clin Res Cardiol. 2022. https://doi.org/10.1007/s00392-022-02043-w.
Martyn T, Saef J, Dey AR, et al. Racial and genetic differences in presentation of transthyretin amyloid cardiomyopathy with impaired left ventricular function. JACC Heart Fail. 2022;10(9):689–91. https://doi.org/10.1016/j.jchf.2022.06.006.
Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. https://doi.org/10.1161/CIRCULATIONAHA.118.038169.
Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–8. https://doi.org/10.1136/heartjnl-2012-302353.
Liu D, Hu K, Niemann M, et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6(6):1066–72. https://doi.org/10.1161/CIRCIMAGING.113.000683.
Pagourelias ED, Mirea O, Duchenne J, et al. Echo parameters for differential diagnosis in cardiac amyloidosis. Circulation: Cardiovascular Imaging. 2017;10(3):e005588. https://doi.org/10.1161/CIRCIMAGING.116.005588.
Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3(4):333–42. https://doi.org/10.1016/j.jcmg.2009.11.013.
Senapati A, Sperry BW, Grodin JL, et al. Prognostic implication of relative regional strain ratio in cardiac amyloidosis. Heart. 2016;102(10):748–54. https://doi.org/10.1136/heartjnl-2015-308657.
• Bisbal F, Baranchuk A, Braunwald E, Bayes de Luna A, Bayes-Genis A. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol. 2020;75(2):222–232. https://doi.org/10.1016/j.jacc.2019.11.013. Important review article which defined the term atrial failure as a clinically relevant entity and the role of the atria in overall cardiac performance.
Nochioka K, Quarta CC, Claggett B, et al. Left atrial structure and function in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18(10):1128–37. https://doi.org/10.1093/ehjci/jex097.
•• Bandera F, Martone R, Chacko L, et al. Clinical importance of left atrial infiltration in cardiac transthyretin amyloidosis. JACC Cardiovasc Imaging. 2022;15(1):17–29. https://doi.org/10.1016/j.jcmg.2021.06.022. First study to perform a systematic assessment of left atrial function and structure in ATTR cardiac amyloidosis with echo speckle tracking and demonstrate the association of increased atrial stiffness with mortality. The study also characterized a distinct phenotype of atrial electromechanical dissociation, or the absence of atrial contraction despite sinus rhythm.
Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116(21):2420–6. https://doi.org/10.1161/CIRCULATIONAHA.107.697763.
Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119(18):2490–7. https://doi.org/10.1161/CIRCULATIONAHA.108.785014.
Martinez-Naharro A, Gonzalez-Lopez E, Corovic A, et al. High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol. 2019;73(13):1733–4. https://doi.org/10.1016/j.jacc.2019.01.035.
• Aquaro GD, Morini S, Grigoratos C, et al. Electromechanical dissociation of left atrium in patients with cardiac amyloidosis by magnetic resonance: prognostic and clinical correlates. Int J Cardiol Heart Vasc. 2020;31:100633. https://doi.org/10.1016/j.ijcha.2020.100633. This study evaluated the role of cardiac MRI in assessing left atrial dysfunction utilizing atrial emptying fraction and showed that severe impairment of left atrial contractile function was highly prevalent in cardiac amyloid and was associated with an independent risk for mortality.
Martinez-Naharro A, Abdel-Gadir A, Treibel TA, et al. CMR-Verified regression of cardiac al amyloid after chemotherapy. JACC Cardiovasc Imaging. 2018;11(1):152–4. https://doi.org/10.1016/j.jcmg.2017.02.012.
• El-Am EA, Dispenzieri A, Melduni RM, et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019;73(5):589–597. https://doi.org/10.1016/j.jacc.2018.10.079. Study showing the high rate of cancellation of direct current cardioversion for atrial fibrillation due to intracardiac thrombus when using transesophageal guidance, therefore confirming that all cardiac amyloid patients should undergo imaging guidance prior to any attempts at electrical cardioversion despite anticoagulation.
Cicco S, Solimando AG, Buono R, et al. Right heart changes impact on clinical phenotype of amyloid cardiac involvement: a single centre study. Life (Basel). 2020;10(10). https://doi.org/10.3390/life10100247.
Bellavia D, Pellikka PA, Dispenzieri A, et al. Comparison of right ventricular longitudinal strain imaging, tricuspid annular plane systolic excursion, and cardiac biomarkers for early diagnosis of cardiac involvement and risk stratification in primary systematic (AL) amyloidosis: a 5-year cohort study. Eur Heart J Cardiovasc Imaging. 2012;13(8):680–9. https://doi.org/10.1093/ehjci/jes009.
Motoki H, Borowski AG, Shrestha K, et al. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60(20):2074–81. https://doi.org/10.1016/j.jacc.2012.07.047.
Cappelli F, Porciani MC, Bergesio F, et al. Right ventricular function in AL amyloidosis: characteristics and prognostic implication. Eur Heart J Cardiovasc Imaging. 2012;13(5):416–22. https://doi.org/10.1093/ejechocard/jer289.
Fine NM, White JA, Jimenez-Zepeda V, Howlett JG. Determinants and prognostic significance of serial right heart function changes in patients with cardiac amyloidosis. Can J Cardiol. 2020;36(3):432–40. https://doi.org/10.1016/j.cjca.2020.01.020.
Bodez D, Ternacle J, Guellich A, et al. Prognostic value of right ventricular systolic function in cardiac amyloidosis. Amyloid. 2016;23(3):158–167. https://doi.org/10.1080/13506129.2016.1194264.
Witteles RM, Liedtke M. AL Amyloidosis for the cardiologist and oncologist. JACC: CardioOncology. 2019;1(1):117–130. https://doi.org/10.1016/j.jaccao.2019.08.002.
•• Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12(9):e006075. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006075. Expert consensus document highlighting the key aspects of diagnosis of ATTR cardiac amyloidosis.
Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol. 2016;25(5):413–7. https://doi.org/10.1016/j.carpath.2016.07.001.
•• Hanna M, Ruberg FL, Maurer MS, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol. 2020;75(22):2851–62. https://doi.org/10.1016/j.jacc.2020.04.022. This review highlights the diagnostic pitfalls in the scintigraphic evaluation of cardiac amyloidosis including highlighting the technical and clinical factors associated with false positive scans.
Gilstrap LG, Dominici F, Wang Y, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail. 2019;12(6):e005407. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005407.
Bourque JM, Schepart A, Bhambri R, et al. Temporal trends in diagnostic testing patterns for wild-type transthyretin amyloid cardiomyopathy in the medicare fee-for-service population. Am J Cardiol. 2022;167:98–103. https://doi.org/10.1016/j.amjcard.2021.11.048.
•• Dorbala S, Ando Y, Bokhari S, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol. 2019;26(6):2065–123. https://doi.org/10.1007/s12350-019-01760-6. This is a concise reference document for the evaluation of cardiac amyloidosis.
Sperry BW, Burgett E, Bybee KA, et al. Technetium pyrophosphate nuclear scintigraphy for cardiac amyloidosis: imaging at 1 vs 3 hours and planar vs SPECT/CT. J Nucl Cardiol. 2020;27(5):1802–7. https://doi.org/10.1007/s12350-020-02139-8.
• Asif T, Gomez J, Singh V, Doukky R, Nedeltcheva A, Malhotra S. Comparison of planar with tomographic pyrophosphate scintigraphy for transthyretin cardiac amyloidosis: perils and pitfalls. J Nucl Cardiol. 2021;28(1):104–111. https://doi.org/10.1007/s12350-020-02328-5. Study highlighting the importance of SPECT imaging in addition to planar evaluation in Tc-99m pyrophosphate scintigraphy in ATTR cardiac amyloidosis.
Regis C, Harel F, Martineau P, et al. Tc-99m-pyrophosphate scintigraphy for the diagnosis of ATTR cardiac amyloidosis: comparison of quantitative and semi-quantitative approaches. J Nucl Cardiol. 2020;27(5):1808–15. https://doi.org/10.1007/s12350-020-02205-1.OlderbutimportantstudyusinghistologicalvalidationofT1-derivedECVmappingtoestimateinterstitialchangesincardiacamyloidosis.
• Martyn T, Saef J, Hussain M, et al. The association of cardiac biomarkers, the intensity of Tc99 pyrophosphate uptake, and survival in patients evaluated for transthyretin cardiac amyloidosis in the early therapeutics era. J Card Fail. 2022. https://doi.org/10.1016/j.cardfail.2022.06.005. Retrospective study which showed lack of correlation between Tc-99m pyrophosphate uptake intensity and prognosis.
•• Sperry BW, Vranian MN, Tower-Rader A, et al. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):234–42. https://doi.org/10.1016/j.jcmg.2017.06.020. Study which showed that apical sparing can be seen on cardiac scintigraphy similar to echocardiographic global longitudinal strain, and showed that while intensity of uptake is not useful for prognostication, a diffuse pattern of uptake is associated with increase in mortality.
Castano A, DeLuca A, Weinberg R, et al. Serial scanning with technetium pyrophosphate ((99m)Tc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. 2016;23(6):1355–63. https://doi.org/10.1007/s12350-015-0261-x.
Park MA, Padera RF, Belanger A, et al. 18F-florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: autoradiography study. Circ Cardiovasc Imaging. 2015;8(8). https://doi.org/10.1161/CIRCIMAGING.114.002954.
Hazenberg BP, van Rijswijk MH, Lub-de Hooge MN, et al. Diagnostic performance and prognostic value of extravascular retention of 123I-labeled serum amyloid P component in systemic amyloidosis. J Nucl Med. 2007;48(6):865–72. https://doi.org/10.2967/jnumed.106.039313.
Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323(8):508–13. https://doi.org/10.1056/NEJM199008233230803.
Korthals D, Chatzantonis G, Bietenbeck M, Meier C, Stalling P, Yilmaz A. CMR-based T1-mapping offers superior diagnostic value compared to longitudinal strain-based assessment of relative apical sparing in cardiac amyloidosis. Sci Rep. 2021;11(1):15521. https://doi.org/10.1038/s41598-021-94650-2.
Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54. https://doi.org/10.1186/1532-429X-10-54.
Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155–64. https://doi.org/10.1016/j.jcmg.2009.09.023.
Liu JM, Liu A, Leal J, et al. Measurement of myocardial native T1 in cardiovascular diseases and norm in 1291 subjects. J Cardiovasc Magn Reson. 2017;19(1):74. https://doi.org/10.1186/s12968-017-0386-y.
•• Robinson AA, Chow K, Salerno M. Myocardial T1 and ECV measurement: underlying concepts and technical considerations. JACC Cardiovasc Imaging. 2019;12(11 Pt 2):2332–44. https://doi.org/10.1016/j.jcmg.2019.06.031. State-of-the-art review which illustrates the factors that can affect native T1 and ECV in cardiac MRI and describes the pitfalls and challenges as well as emerging techniques.
• White SK, Sado DM, Fontana M, et al. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013;6(9):955–62. https://doi.org/10.1016/j.jcmg.2013.01.011. Older but important study using histological validation of T1-derived ECV mapping to estimate interstitial changes in cardiac amyloidosis.
•• Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: a meta-analysis. JACC Cardiovasc Imaging. 2020;13(6):1299–310. https://doi.org/10.1016/j.jcmg.2020.03.010. This meta-analysis evaluating 31 studies is the clearest comparison of three different MRI features of cardiac amyloidosis: LGE, T1 mapping, and ECV mapping, suggesting that among these, ECV provides the highest diagnostic and prognostic utility.
• Fontana M, Pica S, Reant P, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. Oct 20 2015;132(16):1570–9. https://doi.org/10.1161/CIRCULATIONAHA.115.016567. This study demonstrated the important prognostic value of late gadolinium enhancement in cardiac amyloidosis.
Kotecha T, Martinez-Naharro A, Treibel TA, et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71(25):2919–31. https://doi.org/10.1016/j.jacc.2018.03.536.
O'Brien AT, Gil KE, Varghese J, Simonetti OP, Zareba KM. T2 mapping in myocardial disease: a comprehensive review. J Cardiovasc Magn Reson. 2022;24(1):33. https://doi.org/10.1186/s12968-022-00866-0.
• Slivnick JA, Tong MS, Nagaraja HN, et al. Novel cardiovascular magnetic resonance model to distinguish immunoglobulin light chain from transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging. 2021;14(1):302–4. https://doi.org/10.1016/j.jcmg.2020.07.030. This study proposes a novel cardiac MRI model which integrates age, right ventricular ejection fraction, and myocardial T2 for the discrimination of ATTR and AL cardiac amyloid.
Dungu JN, Valencia O, Pinney JH, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):133–42. https://doi.org/10.1016/j.jcmg.2013.08.015.
• Rapezzi C, Aimo A, Serenelli M, et al. Critical comparison of documents from scientific societies on cardiac amyloidosis: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(13):1288–303. https://doi.org/10.1016/j.jacc.2022.01.036. This state-of-the-art review compares the expert documents issued by 5 professional societies and highlights areas of agreement as well as areas of disagreement highlighting the need for further investigation in the areas of uncertainty.
Khalique Z, Ferreira PF, Scott AD, et al. Diffusion tensor cardiovascular magnetic resonance in cardiac amyloidosis. Circ Cardiovasc Imaging. 2020;13(5):e009901. https://doi.org/10.1161/CIRCIMAGING.119.009901.
Longhi S, Lorenzini M, Gagliardi C, et al. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2016;9(3):325–7. https://doi.org/10.1016/j.jcmg.2015.04.012.
Treibel TA, Fontana M, Gilbertson JA, et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9(8). https://doi.org/10.1161/CIRCIMAGING.116.005066.
Cavalcante JL, Rijal S, Abdelkarim I, et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson. 2017;19(1):98. https://doi.org/10.1186/s12968-017-0415-x.
• Scully PR, Treibel TA, Fontana M, et al. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71(4):463–464. https://doi.org/10.1016/j.jacc.2017.11.037. This was a prospective study in which patients with severe aortic stenosis undergoing cardiac CT for transcatheter aortic valve replacement also underwent cardiac scintigraphy to help corroborate ECV accuracy for detection of ATTR cardiac amyloidosis.
Castano A, Narotsky DL, Hamid N, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–87. https://doi.org/10.1093/eurheartj/ehx350.
Griffin JM, Rosenblum H, Maurer MS. Pathophysiology and Therapeutic approaches to cardiac amyloidosis. Circ Res. 2021;128(10):1554–75. https://doi.org/10.1161/CIRCRESAHA.121.318187.
Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. https://doi.org/10.1056/NEJMoa1805689.
•• Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR amyloidosis: current and emerging management strategies: JACC: cardiooncology state-of-the-art review. JACC CardioOncol. 2021;3(4):488–505. https://doi.org/10.1016/j.jaccao.2021.06.006. Important state-of-the-art review paper detailing current and future targeted therapies for ATTR cardiac amyloidosis.
Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. https://doi.org/10.1056/NEJMoa1716153.
Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen Treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31. https://doi.org/10.1056/NEJMoa1716793.
Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. https://doi.org/10.1056/NEJMoa2028631.
•• Patel RK, Fontana M, Ruberg FL. Cardiac amyloidosis: multimodal imaging of disease activity and response to treatment. Circ Cardiovasc Imaging. 2021;14(6):e009025. https://doi.org/10.1161/CIRCIMAGING.121.009025. This is a thorough review detailing the multimodal imaging tools available and their potential for tracking treatment response.
Benson MD, Dasgupta NR, Rissing SM, Smith J, Feigenbaum H. Safety and efficacy of a TTR specific antisense oligonucleotide in patients with transthyretin amyloid cardiomyopathy. Amyloid. 2017;24(4):219–225. https://doi.org/10.1080/13506129.2017.1374946.
•• Ioannou A, Patel R, Gillmore JD, Fontana M. Imaging-guided treatment for cardiac amyloidosis. Curr Cardiol Rep. 2022;24(7):839–50. https://doi.org/10.1007/s11886-022-01703-7. This review evaluates the role of multimodal imaging tools in tracking treatment response in both AL and ATTR cardiac amyloidosis.
Solomon SD, Adams D, Kristen A, et al. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis. Circulation. 2019;139(4):431–43. https://doi.org/10.1161/CIRCULATIONAHA.118.035831.
Tuzovic M, Kobayashi Y, Wheeler M, et al. Functional cardiac recovery and hematologic response to chemotherapy in patients with light-chain amyloidosis (from the Stanford University Amyloidosis Registry). Am J Cardiol. 2017;120(8):1381–6. https://doi.org/10.1016/j.amjcard.2017.07.025.
Pun SC, Landau HJ, Riedel ER, et al. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr. 2018;31(1):64–70. https://doi.org/10.1016/j.echo.2017.08.017.
Salinaro F, Meier-Ewert HK, Miller EJ, et al. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18(9):1057–64. https://doi.org/10.1093/ehjci/jew298.
Amano M, Izumi C, Nishimura S, et al. Predictors of prognosis in light-chain amyloidosis and chronological changes in cardiac morphology and function. Am J Cardiol. 2017;120(11):2041–8. https://doi.org/10.1016/j.amjcard.2017.08.024.
•• Martinez-Naharro A, Patel R, Kotecha T, et al. Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment. Eur Heart J. 2022. https://doi.org/10.1093/eurheartj/ehac363. This prospective study evaluated the role of cardiac MRI using ECV measurements to track treatment response in AL amyloidosis and the correlation of ECV as a metric of prognosis.
Shintani Y, Okada A, Morita Y, et al. Monitoring treatment response to tafamidis by serial native T1 and extracellular volume in transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2019;6(1):232–6. https://doi.org/10.1002/ehf2.12382.
•• Fontana M, Martinez-Naharro A, Chacko L, et al. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021;14(1):189–99. https://doi.org/10.1016/j.jcmg.2020.07.043. This is the first study to demonstrate regression of amyloidosis as measured by ECV using cardiac MRI in patients with hereditary ATTR cardiac amyloidosis treated with patisiran.
• Dasgupta NR, Rissing SM, Smith J, Jung J, Benson MD. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid. 2020;27(1):52–8. https://doi.org/10.1080/13506129.2019.1685487. This study enrolled patients with hereditary ATTR cardiomyopathy without peripheral neuropathy as well as wild-type ATTR in order to show that inotersen is well-tolerated and has potential to improve structural changes including wall thickness and left ventricular mass as measured by cardiac MRI.
Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–77. https://doi.org/10.1161/CIRCULATIONAHA.116.024438.
Guo X, Chen Z, Wan K, et al. Electrocardiogram characteristics and prognostic value in light-chain amyloidosis: a comparison with cardiac magnetic resonance imaging. Front Cardiovasc Med. 2021;8:751422. https://doi.org/10.3389/fcvm.2021.751422.
Zhao L, Li J, Tian Z, Fang Q. Clinical correlates and prognostic values of pseudoinfarction in cardiac light-chain amyloidosis. J Cardiol. 2016;68(5):426–30. https://doi.org/10.1016/j.jjcc.2015.11.004.
Kim D, Lee GY, Choi JO, Kim K, Kim SJ, Jeon ES. Associations of electrocardiographic parameters with left ventricular longitudinal strain and prognosis in cardiac light chain amyloidosis. Sci Rep. 2019;9(1):7746. https://doi.org/10.1038/s41598-019-44245-9.
Schrutka L, Anner P, Agibetov A, et al. Machine learning-derived electrocardiographic algorithm for the detection of cardiac amyloidosis. Heart. 2022;108(14):1137–47. https://doi.org/10.1136/heartjnl-2021-319846.
Goto S, Mahara K, Beussink-Nelson L, et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat Commun. 2021;12(1):2726. https://doi.org/10.1038/s41467-021-22877-8.
Koyama J, Ray-Sequin PA, Falk RH. Prognostic significance of ultrasound myocardial tissue characterization in patients with cardiac amyloidosis. Circulation. 2002;106(5):556–61. https://doi.org/10.1161/01.cir.0000023530.86718.b0.
Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2015;22(1):61–6. https://doi.org/10.3109/13506129.2014.994202.
Liu D, Hu K, Stork S, et al. Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS One. 2014;9(12):e115910. https://doi.org/10.1371/journal.pone.0115910.
Mohty D, Pibarot P, Dumesnil JG, et al. Left atrial size is an independent predictor of overall survival in patients with primary systemic amyloidosis. Arch Cardiovasc Dis. 2011;104(12):611–8. https://doi.org/10.1016/j.acvd.2011.10.004.
Mohty D, Petitalot V, Magne J, et al. Left atrial function in patients with light chain amyloidosis: a transthoracic 3D speckle tracking imaging study. J Cardiol. 2018;71(4):419–27. https://doi.org/10.1016/j.jjcc.2017.10.007.
Huntjens PR, Zhang KW, Soyama Y, Karmpalioti M, Lenihan DJ, Gorcsan J 3rd. Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC Cardiovasc Imaging. 2021;14(8):1508–19. https://doi.org/10.1016/j.jcmg.2021.01.016.
Petrescu A, Santos P, Orlowska M, et al. Velocities of naturally occurring myocardial shear waves increase with age and in cardiac amyloidosis. JACC Cardiovasc Imaging. 2019;12(12):2389–98. https://doi.org/10.1016/j.jcmg.2018.11.029.
Castano A, Haq M, Narotsky DL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1(8):880–9. https://doi.org/10.1001/jamacardio.2016.2839.
Conceicao I, Damy T, Romero M, et al. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid. 2019;26(1):3–9. https://doi.org/10.1080/13506129.2018.1556156.
Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4(6):659–70. https://doi.org/10.1016/j.jcmg.2011.03.016.
Singh V, Falk R, Di Carli MF, Kijewski M, Rapezzi C, Dorbala S. State-of-the-art radionuclide imaging in cardiac transthyretin amyloidosis. J Nucl Cardiol. 2019;26(1):158–73. https://doi.org/10.1007/s12350-018-01552-4.
•• Oda S, Kidoh M, Takashio S, et al. Quantification of myocardial extracellular volume with planning computed tomography for transcatheter aortic valve replacement to identify occult cardiac amyloidosis in patients with severe aortic stenosis. Circ Cardiovasc Imaging. 2020;13(5):e010358. https://doi.org/10.1161/CIRCIMAGING.119.010358. This retrospective study was the first to evaluate the utility of myocardial ECV by cardiac CT in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement and compared these findings to patients who had been referred for Tc-99m pyrophosphate evaluation to help define ECV cutoff values that identify ATTR cardiac amyloidosis.
Scully PR, Patel KP, Saberwal B, et al. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. JACC Cardiovasc Imaging. 2020;13(10):2177–89. https://doi.org/10.1016/j.jcmg.2020.05.029.
Wan K, Sun J, Han Y, et al. Right ventricular involvement evaluated by cardiac magnetic resonance imaging predicts mortality in patients with light chain amyloidosis. Heart Vessels. 2018;33(2):170–9. https://doi.org/10.1007/s00380-017-1043-y.
Bhatti S, Watts E, Syed F, et al. Clinical and prognostic utility of cardiovascular magnetic resonance imaging in myeloma patients with suspected cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2016;17(9):970–7. https://doi.org/10.1093/ehjci/jew101.
Lin L, Li X, Feng J, et al. The prognostic value of T1 mapping and late gadolinium enhancement cardiovascular magnetic resonance imaging in patients with light chain amyloidosis. J Cardiovasc Magn Reson. 2018;20(1):2. https://doi.org/10.1186/s12968-017-0419-6.
Martinez-Naharro A, Kotecha T, Norrington K, et al. Native T1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2019;12(5):810–9. https://doi.org/10.1016/j.jcmg.2018.02.006.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Khedraki declares that she has no conflict of interest. Dr. Robinson declares that he has no conflict of interest. Dr. Jordan declares that he has no conflict of interest. Dr. Grodin reports consulting fees from Pfizer, Eidos/BridgeBio, Astra-Zeneca, and Sarepta and research funding from Pfizer, Eidos/BridgeBio, Texas Health Resources Clinical Scholarship, and the NHLBI (1R01HL160892-01A1). Dr. Mohan reports personal fees from Pfizer, Alnylam, Akcea, and Eidos, outside the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Heart Failure
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khedraki, R., Robinson, A.A., Jordan, T. et al. A Review of Current and Evolving Imaging Techniques in Cardiac Amyloidosis. Curr Treat Options Cardio Med 25, 43–63 (2023). https://doi.org/10.1007/s11936-023-00976-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-023-00976-7