Abstract
Purpose of Review
To provide a comprehensive overview of the spectrum of large and medium vessel vasculitis in adults with primary vasculitides, arthritides, connective tissue, and fibroinflammatory diseases as well as vasculitis mimics, for an efficient differential diagnosis and initial diagnostic approach.
Recent Findings
Imaging has had a tremendous impact on the diagnosis of medium to large vessel vasculitis, now often replacing histopathologic confirmation and identifying new disease manifestations (e.g., intracranial disease in giant cell arteritis; vascular manifestations of IgG4-related disease). Novel diseases or syndromes involving blood vessels have been described (e.g., VEXAS-Syndrome with polychondritis). The use of the terms “medium” or “large” vessel varies considerably between medical specialties.
Summary
The differential diagnosis of large and medium vessel vasculitis is becoming increasingly complex as new entities or disease manifestations of known inflammatory rheumatic diseases are regularly identified. A more precise and widely recognized definition of the vessel sizes would make future research more comparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This review is intended as a comprehensive guide for the differential diagnosis of suspected medium to large vessel vasculitis (MVV, LVV) in adults. Primary vasculitides and vasculitis in arthritides, connective tissue, and fibroinflammatory diseases are discussed. MVV and LVV in the context of primary immunodeficiency, autoinflammatory, neoplastic, or infectious diseases, as well as drug induced vasculitis, are considered elsewhere [1••]. The complex topic of single organ vasculitis (SOV) was recently reviewed in this journal [2]. The first part of the article is dedicated to a discussion of the difficulties in classifying vasculitis by vessel size (Table 1). The individual diseases associated with MVV and LVV are discussed subsequently. Thereafter, a brief overview of vasculitis mimics is provided, as consideration of these disorders is essential in the diagnostic process. We only consider diseases without inflammation of the vessel wall as vasculitis mimics (Table 2). Table 3 provides additional details and pearls important for diagnosis of individual diseases causing MVV and LVV.
Traditionally, vasculitides are grouped according to the affected vessel size (small, medium, and large). This can be helpful in approaching the diagnosis in an individual patient. The allocation of a disease to the categories LVV, MVV, and small vessel vasculitis (SVV) is based on the typical vessel size affected. In reality, many diseases can affect vessels of any size, and even typical LVVs often involve primarily medium-sized vessels [3]. The division of vessels into these categories seems simple at first glance, but the terms are used very inconsistently in the literature, e.g., a small artery in neuroradiology would be defined as a medium-sized artery in dermatology. The presence of a vasa vasorum vasculitis (a SVV) in the wall of larger vessels (MVV or LVV) even further complicates the topic. The definitions are strongly influenced by different diagnostic approaches: in dermatology by histology, in neuroradiology by imaging, in other fields by a combination of these approaches [3–6]. Because there is no universal classification system for vessel size, it is understandable that different medical specialties approach this classification in the way that best serves their clinical practice. This makes the comparison of publications challenging. Table 1 briefly compares examples of published categorizations by authors of a selection of different specialties and shows the differing definitions. Because Chapel Hill’s nomenclature remains ambiguous, for this review we have classified vessels according to our daily rheumatological practice following the Chapel Hill nomenclature, but also attempting to define large vessels more clearly: distributing vessels proximal to the elbow, knee, and dura mater are included in this category [3].
The Primary Vasculitides
Most cases of MVV and LVV will fall into this category. Due to the tendency to use imaging for diagnostic confirmation, other types of vasculitides and mimics should be carefully considered in every patient.
Giant Cell Arteritis
Giant cell arteries (GCA) is the most frequent disease causing MVV or LVV. Apart from classic polymyalgic and cranial symptoms, GCA should be considered in a variety of other presentations, including patients with constitutional symptoms with elevated C-reactive protein (CRP) and/or erythrocyte sedimentation rate [14•]. Most arteries, especially any elastic large and large medium-sized arteries above the clavicles, but not capillaries, venules, or arterioles, can be affected [8–12]. Since SVV is found in the eye (e.g., cilioretinal artery) and in temporal artery (TA) biopsies, the possibility of SVV should be considered in other organs [117]. If the typical histology is found unexpectedly in resected organs, e.g., uterus, a SOV must be considered. In postulated isolated aortitis, GCA needs to be screened for thoroughly [2]. While GCA was assumed not to cause central nervous system (CNS) vasculitis, vessel-wall MRI changed that paradigm; usually it represents extension of extradural disease and involves the proximal intracranial arteries [13••].
Takayasu Arteritis
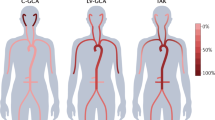
Especially outside Asia, Takayasu arteries (TAK) is an extremely rare disease of mostly young women [7]. Symptoms are often vague, but characteristic findings such as arterial bruits aid in its identification [19]. Several diseases have been found to coexist with TAK or to cause “TAK-like” LVV (e.g., common variable immunodeficiency, systemic lupus erythematosus (SLE), inflammatory bowel disease, spondyloarthritis, sarcoidosis) [17, 18]. Whether it is a co-occurrence of two diseases or just one disease with a “TAK-like” appearance is a difficult question and subject of debate, especially for spondyloarthritis and inflammatory bowel disease a co-occurrence seems to be likely [1••]. TAK causes mainly LVV and MVV of the larger medium-sized arteries, including the CNS [19, 21, 22]. Because retinal and skin vasculitis can occur, rare SVV manifestations should be considered [11, 118].
Polyarteritis Nodosa
A severe, necrotizing vasculitis, with propensity for aneurysms of visceral medium-sized arteries [28]. The definition of polyarteritis nodosa (PAN) has changed considerably in the past, and by definition neither capillaries nor venules are involved [3, 119••]. While classic PAN is the idiopathic form, in secondary or non-classical PAN, another disease manifests in a “PAN-like” fashion: infections (e.g., HBV, HCV or HIV), autoinflammatory or immunodeficiency diseases (e.g., familial Mediterranean fever; adenosine-deaminase-2-deficiency), blood dyscrasias (e.g., hairy-cell leukemia, myelodysplastic and myeloproliferative syndromes), and systemic rheumatic diseases [1••, 28, 119••]. Therefore, in the case of a necrotizing ANCA-negative MVV, both "PANlike" disorders and mimics (e.g., segmental arterial mediolysis) need to be differentiated. The clinical manifestations of classical PAN range from severe systemic to localized presentations, where differentiation of systemic PAN from SOV is necessary (e.g., cutaneous-limited forms or isolated involvement of testicles) [29, 120]. Pulmonary capillaritis is not compatible with PAN and prominent CNS affection, usually a rare and late feature, should trigger consideration of adenosine deaminase-2 deficiency [1••, 23, 30]. Any small- to medium-sized artery can potentially be affected; LVV is extremely uncommon [24–27].
Kawasaki Disease
A typical childhood disease that can also occur in young adults [31•]. The presentation is similar to children, with dreaded cardiac (esp. coronary aneurysm) and neurological sequelae [36]. Being so rare in adults, infectious mimics of its clinical presentation (fever, rash etc.) and other diseases causing coronary aneurysms need to be evaluated [1••, 10, 31•, 36]. Apart from the coronaries, involvement of other large and distributing medium-sized arteries has been described in autopsies of children. A similar distribution of vessel inflammation is expected in adults, but data are limited [32–35].
Anti-neutrophil Cytoplasmic Antibody(ANCA)-Associated Vasculitis
The anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) are subdivided into granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA), with EGPA set apart in terms of frequency (very rare) and clinical presentation [3]. There is no “usual” presentation, any organ can be affected, and myriads of symptoms and signs are possible. EGPA almost exclusively presents in patients with asthma and the ANCA-negative subtype is difficult to differentiate from other eosinophilic diseases [45, 121]. It is unclear whether necrotizing vasculitis occurs in hypereosinophilic syndrome or whether it is just an anti-neutrophil cytoplasmic antibody (ANCA)-negative EGPA. MPA and GPA partially overlap in clinical presentation, but only the later has a tendency for destructive mass lesions [46]. Most manifestations of AAV are due to SVV, and MVV/LVV are very rare (approximately 1–5%) [38–40, 41••, 42]. Cerebral MVV was occasionally described [43].
Immune Complex Vasculitides
Typically, immune complexes cause SVV. For urticaria vasculitis or anti-glomerular basement membrane disease, no cases with MVV or LVV could be identified by our literature search. For the few cases of IgA-vasculitis with possible MVV or LVV, a definite etiological link was not demonstrated [122]. Cryoglobulinemic Vasculitis(CV): CV is predominantly secondary to infections (esp. HCV), lymphoproliferative or autoimmune diseases [48•]. Manifestations of occlusive vasculopathy (Raynaud’s or cold-induced acral necrosis) can occur with high, especially monoclonal, cryoglobulin levels. CV usually presents as SVV; rarely and mainly in HCV-positive patients, it can additionally cause “PAN-like” necrotizing MVV [5•, 49, 52, 53]. A single case with a clinical presentation like cranial GCA was identified; histology showed an adventitial SVV with an otherwise unremarkable TA [54]. LVV is a rarity as only two cases with HCV infection could be identified (histologically a vasa vasorum vasculitis) [50, 51].
Cogan’s Syndrome
A very rare disease without a clear definition [57, 58•]. The diagnosis requires the presence of eye inflammation and inner-ear dysfunction, classically interstitial keratitis and Menière-like symptoms [57]. In the majority of patients, either ocular or vestibulocochlear symptoms are initially present and within approximately 3–6 months the other manifestation occurs [58•]. The division into “typical” (interstitial keratitis and Menière-like symptoms occurring within less than 2 years) and “atypical” (other ocular inflammation and Menière-like symptoms occurring within less than 2 years or interstitial keratitis and Menière-like symptoms occurring more than 2 years apart) CS is mostly historical [123]. Vasculitis can be identified in about 20% of patients and can affect any vessel size. SVV of the inner ear, eyes (retinal vasculitis), and skin was described [124]. The typical manifestation is a MVV to LVV with similar manifestations like in PAN or TAK, respectively [58•, 60]. TA affection was not identified by our search. Whether intracranial arteries are affected remains unclear [125].
Behçet’s Syndrome
Behcet’s syndrome (BS) is of unknown etiology. Its manifold clinical manifestations include the affection of arteries and veins of all sizes in 5–40%; thus, BS is regarded as a “variable vessel vasculitis” [3, 62•]. Certain features of BS, i.e., the response to colchicine or the recurrent and self-limiting nature of some manifestations, imply a polygenic autoinflammatory pathogenesis [126]. The major symptom of BS is the presence of recurrent oral ulcerations; while this symptom alone is non-specific, its absence makes the diagnosis of BS unlikely. Young men are generally more severely affected than women and more commonly show vascular manifestations. Affected vessels have a strong tendency for thrombosis, but the risk of embolism is low due to the strong wall adherence of thrombi. Selected thrombotic manifestations in BS (e.g., Budd-Chiari syndrome and aneurysms of the pulmonary arteries, including Hughes-Stovin syndrome) are associated with a high mortality rate [63].
Vasculitis in Connective Tissue Diseases and Arthritis
In general, SVV is more common in these disorders, but MVV and LVV do occur [73]. Vasculitis usually manifests in patients with established disease and is rarely one of the initial manifestations. In systemic sclerosis, vasculopathy does not reveal the histological characteristics of vasculitis. The numerous published cases of MVV and LVV tend to represent overlapping diseases (esp. AAV or CV) rather than true systemic sclerosis associated vasculitis [73]. In mixed connective tissue disease, SVV of the retina, bowel, or skin can occur and a vasculopathy like in systemic sclerosis can affect the arteries of the distal extremities. No definite case of MVV or LVV could be identified, but in overlapping syndromes with other connective tissue disease, more diverse vasculitic presentations are expected.
Systemic Lupus Erythematosus
Lupus vasculitis rarely manifests as MVV or LVV, the most common presentation is SVV affecting the skin in 90% of cases (usually leukocytoclastic). Less frequently, also the lungs, liver, pancreas, bowel, and nervous system can be involved [64, 69, 70, 73]. Lupus vasculitis rarely presents in a “PAN-like” fashion with necrotizing vasculitis in multiple organs, predominantly of small arteries and occasionally with MVV and development of aneurysms [64, 69]. Especially mesenteric vasculitis is characteristic for SLE with conventional angiography often showing normal findings but occasionally demonstrating typical findings of MVV with aneurysms. Endoscopic biopsies usually do not show the culprit vessel [71, 72]. LVV is very rare; histopathology reveals lymphoplasmacytic infiltration of the adventitia and media or vasa vasorum vasculitis with fibrinoid necrosis. In the occasionally described “TAK-like” disease in patients with SLE, it usually remains unclear whether it is a manifestation of SLE or two different diseases (i.e., TAK and SLE concomitantly). A case report revealing histologic evidence of vasa vasorum vasculitis of an iliac artery suggests that SLE can cause LVV with a “TAK-like” morphology without TAK being present concomitantly [66–68, 92••].
Sjogren’s Syndrome
Vasculitis usually occurs as a late manifestation in Sjogren’s (median 10 years after diagnosis), with SVV presentation in >95% of cases (usually leukocytoclastic, occasionally lymphocytic) and MVV in <5%, which might be underreported and usually coexists with SVV of the skin [76, 77, 74•]. Since cryoglobulins are common in Sjogren’s, CV should always be considered [74•]. Rarely, a “PAN-like” necrotizing vasculitis of small medium-sized arteries (without aneurysms) occurs in internal organs [74•, 77]. Rarely, based solely on angiographic findings, MVV of the CNS has been reported, but a definite etiologic association of these findings with Sjogren’s syndrome was not proven [78]. Periaortitis or aortitis, diagnosed by FDG-PET, was also described in a few patients, also without a clear direct etiological link to the primary disease [74•, 75].
Idiopathic Inflammatory Myopathies
Apart from SVV of the skin and muscle in dermatomyositis (DM), inflammatory myopathies only rarely present with vasculitis. In DM, especially juvenile DM, a severe gastrointestinal vasculitis with possible MVV was described [73]. Apart from pulmonary capillaritis, a rare MVV of pulmonary arteries was demonstrated in “polymyositis” in older autopsy cases [79]. In anti-PL-12-anti-synthetase-syndrome and in DM, MVV of the CNS was demonstrated (in angiographically MVV, histologically a SVV was detected) [80, 81]. Cases with LVV could not be identified.
Relapsing Polychondritis
Relapsing polychondritis (RP) primarily affects cartilaginous and proteoglycan-rich (eyes, blood vessel, or inner ears etc.) tissues and is either idiopathic or secondary to systemic diseases (approximately 30%). Since secondary RP is frequently due to primary vasculitides, the clear differentiation to vasculitis as a manifestation of idiopathic RP is difficult and the interpretation of the literature challenging [84]. Any vessel size can be affected, as supradiaphragmatic vessels more commonly than infradiaphragmatic [85]. SVV can affect the inner ear, eye, or the skin [84]. LVV occurs in approximately 4–18% of idiopathic RP, the thoracic aorta being most commonly affected; MVV usually occurs in addition to LVV, but sometimes only MVV may be present [84–86•]. In any male patient with RP, especially in elderly subjects with a “PAN-like” manifestation (uncommon in idiopathic RP) and macrocytosis, the recently described VEXAS syndrome should be considered [1••, 88••]. SVV and MVV of the CNS are extremely rare [87].
Rheumatoid Vasculitis in Rheumatoid Arthritis
Rheumatoid vasculitis (RV) has become rare today, most likely due to better disease control [89–91]. It typically appears 10–15 years after the onset of arthritis in patients with erosive disease and high titers of rheumatoid factor or anti-cyclic citrullinated peptide (anti-CCP) antibodies; rarely it antedates arthritis. RV can occur without clinical activity of the arthritis itself but often with extraarticular disease like nodulosis, Felty-syndrome, or rheumatoid eye disease [89, 91]. While LVV does occur, RV typically involves medium-sized and small vessels and can manifest in a “PAN-like” fashion without microaneurysms. Coronaritis and aortitis occur extremely rarely, often diagnosed postmortem [92••]. CNS vasculitis is also very rare and needs to be differentiated from rheumatoid meningitis [91].
Spondyloarthritis
Aortitis in spondyloarthritis is rare and is described less frequently today than before the advent of biologics [94, 95•]. Histologically, lymphoplasmocytic infiltrates and fibrosis are typical, with the fibrosis being particularly pronounced in the region of the aortic sinuses and aortic valve with possible involvement of the mitral valve and the interventricular septum [92••, 95•]. Several cases of reactive or psoriasis arthritis with similar manifestations were published, but ankylosing spondylitis is the most common subtype associated with LVV [94, 95•]. MVV has not been described in patients with spondyloarthritis; for the few cases with coronary aneurysms, it remains unclear whether they were caused by vasculitis [94].
Fibroinflammatory Diseases
In these disorders, excessive fibrosis dominates the histological picture and it is accompanied by chronic inflammation. IgG4-related disease (IgG4-RD) can be considered as the classical fibroinflammatory disease.
IgG4-Related Disease
The concept of IgG4-RD exists since approximately 2003. Its typical presentation is insidious; acute or highly inflammatory manifestations usually do not occur [96]. It can be a focal, multifocal, or systemic disease and typically leads to fibrosis and mass effect in the affected organs [96–98]. The involvement of veins alone is rare, but arterial involvement is common; the aorta is usually affected alone or in addition to other vessels in approx. 10–35% of cases. Primary affection of the vessel wall itself (mainly adventitia) occurs less frequently than secondary involvement with perivascular disease (typically periaortitis/retroperitoneal fibrosis). Inflammation of the vessel wall is associated with a tendency to aneurysm formation and possible dissection and rupture whereas periaortal inflammation less frequently leads to luminal changes [96–98]. Vessel stenosis can also occur due to encasing lesions (e.g., superior vena cava, pulmonary or coronary arteries) [97, 98, 127•]. Single cases with cerebral MVV with stenosis or dilatation were reported [128, 129].
Chronic Periaortitis
Retroperitoneal fibrosis (RPF) is characterized by chronic lymphoplasmocellular infiltrate and fibrosis of retroperitoneal structures with typical ureteral obstruction; it includes idiopathic (75%) and “secondary” forms (25%: malignancies, infections, drugs, carcinoid syndrome, AAV, SLE, Erdheim-Chester etc.) [103•]. Since the disease process typically develops in the adventitia, the majority of idiopathic RPF are located around large arteries (abdominal aorta/iliac arteries > thoracic aorta), where the term chronic periaortitis (CP) is most appropriate [103•, 104]. CP can be subdivided into the entities “periaortic RPF” (no aneurysm; ureteral involvement), “inflammatory abdominal aortic aneurysm” (no ureteral involvement), and “perianeurysmal retroperitoneal fibrosis” (with ureteral involvement) [103•]. The idiopathic forms are difficult to differentiate from IgG4-RD without a biopsy, which usually is only performed if there is evidence for a neoplastic/infectious process or an atypical localization (i.e., not peri-arterial) [101, 103•]. Affection of visceral medium-sized vessel can occur [103•, 104].
Miscellaneous Inflammatory Diseases
Sarcoidosis
Sarcoid vasculitis can manifest at any time during the disease. It can involve any vessel size—arteries and veins alike—especially the aorta and the pulmonary vessels (possibly with secondary pulmonary hypertension) [105, 106]. It can also manifest as “PAN-like” MVV, including development of aneurysms, or SVV, especially of the skin, peripheral nerves, and lungs [105, 108, 130]. “TAK-like” LVVs are described, and because granulomatous inflammation is present in TAK and sarcoidosis, it is difficult to differentiate whether the two diseases are concomitantly present or only sarcoidosis [92••, 107, 110]. With the use of vessel-wall imaging, CNS vasculitis is more commonly identified: small perforating arteries and periventricular veins are most often affected, but occasionally also medium-sized cerebral arteries or dural sinuses [109•].
Thrombangiitis Obliterans or Buerger’s Disease
This manifests as inflammatory vasculopathy of medium and small arteries and veins with typical corkscrew collaterals. Because of the main histologic feature, i.e., a highly cellular intraluminal thrombus with comparatively little involvement of the vessel wall itself, and because the therapy is not immunosuppressive, TO is clearly distinct from other types of vasculitis. Thrombangiitis obliterans (TO) is only exceptionally found in non-smokers and it typically affects infrapopliteal and infrabrachial arteries and superficial veins, but other medium-sized vessels can be involved [112–116].
Juvenile Temporal Arteritis
This very rare SOV has an imprecise definition. It is more common in males and manifests < 40–50 years of age with a non-tender or painful swelling in the temples, headache (50%), mild eosinophilia (30%), and generally without systemic symptoms or CRP elevation. Histology shows in 90% of cases an eosinophilic infiltrate with perivascular expansion and occasionally some giant cells. There are no known complications or progression to systemic disease. Kimura disease or angiolymphoid hyperplasia with eosinophilia has overlapping features (especially perivascular germinal centers); a clear differentiation from juvenile temporal arteritis can be difficult [131]. IgG4-RD and AAV can demonstrate eosinophil-rich infiltrates of the TA and should be considered.
Isolated aortitis
This by definition is an SOV, but likely a group of disorders with the same clinical manifestation rather than a common pathophysiology [2, 132]. Isolated aortitis can be found incidentally with the use of cross-sectional imaging (often in the diagnostic process of fever or inflammation of unknown origin) or on histopathology after surgical resection due to aneurysmal dilation or dissection (often without elevation of acute phase reactants prior to the operation) [92••, 132]. Before aortitis can be classified as “isolated,” a comprehensive workup regarding the many diseases that can cause aortitis is advisable, including an FDG-PET/CT if possible. The main differential diagnosis is GCA [133].
Vasculitis Mimics and Important Other Disorders
It is mostly the similarity of imaging findings (especially stenoses on angiography) and sometimes the clinical manifestations, but much less the histopathological presentation that can mimic vasculitis. The grouping of mimics into partially overlapping categories of vascular manifestations, as well as the size of the affected vessels, should be helpful in compiling a list of differential diagnoses for the individual patient (Table 2). A few selected mimics are discussed individually below. A detailed discussion about vasculitis mimics can be found elsewhere [134•, 135].
Transient Perivascular Inflammation of the Carotid Artery (TIPIC) Syndrome
This is a rare entity of unknown etiology affecting adults. Presentations is with acute unilateral anterior neck pain with an enhancing periadventitial soft tissue alteration of the carotid bifurcation in 100% and a soft intimal plaque in approximately 50% [136]. Histology can show inflammation and fibrosis [137]. Rarely mild fever, myalgias, dysesthesia, or paresthesia can occur. CRP is rarely mildly elevated. Spontaneously or with the use of nonsteroidal anti-inflammatory drugs, symptoms resolve within a couple of weeks, but slight perivascular thickening persists for months, and relapses can occur [136]. Before transient perivascular inflammation of the carotid artery (TIPIC) is diagnosed, arterial dissection needs to be considered. The persistence of symptoms or marked CRP elevation should raise the suspicion of another disease.
Erdheim-Chester Disease
Erdheim-Chester disease (ECD) is a rare histiocytosis with a BRAF-V600E mutation in approximately 50%. Diagnosis can be delayed for many years due to its insidious onset. Infiltration of multiple organs is typical: bone (> 95%; osteosclerotic lesions, pain in 50%), pituitary (diabetes insipidus, hypogonadism), orbital (exophthalmos), retroperitoneal (“hairy kidney”), CNS (25–50%; pachymeningitis, parenchymal lesions), skin (xanthelasma), or heart (pseudotumor) [138]. Adventitial and periadventitial infiltration leads to its characteristic “coated” aspect. Typically, the aorta but potentially all its large- and medium-sized branches and pulmonary, femoro-popliteal, basilar, and middle cerebral arteries can be affected. Stenosis usually occurs only in medium-sized arteries (e.g., coronary, renal, mesenteric), and vessel dilatation is very rare [139].
Acute aortic Pathology
LVV can rarely present with chest pain, with consequent suspicion of acute coronary or aortic syndromes. Also, imaging findings of aortic intramural hematoma and dissection can sometimes mimic LVV and vice versa [140, 141].
Radiation-Induced Vascular Disease
Radiation typically results in inhomogeneous and calcified atherosclerotic changes especially of large arteries, including the aorta, and is limited to the field of radiation. Because the wall alterations can be relatively long, smooth, and concentric, they can mimic LVV [142].
Conclusion
Considering the multitude of possible etiologies of MVV and LVV as well as their mimics discussed in the present article and other reviews, the correct diagnosis remains a challenge even for experts [1••, 2]. As new entities are constantly described, the astute clinician will reevaluate the diagnosis of any MVV or LVV from time to time, especially in atypical cases [88••]. While it is now common practice to omit biopsy if imaging findings are compatible with MVV or LVV, caution is advised if this approach is used in patients with low to medium pretest-probabilities, because multiple vasculitides and mimics can show similar findings [143]. A thorough clinical examination, medical history and laboratory examination of each patient is mandatory and guides the generation of a differential diagnosis, the choice of imaging modality and whether a biopsy is needed. We clearly advise investing a lot of resources early in the diagnostic process, as the diagnostic accuracy of laboratory tests and imaging rapidly decreases once therapy is started. The diagnosis often remains unclear if investigations were initially performed too superficially. However, even with the most detailed evaluation, there will be situations where an exact entity cannot be identified. Isolated aortitis without the possibility to biopsy is an example.
Regarding the unsatisfactory definitions of vessel sizes, a more precise, consensus-based, and universally accepted definition is desirable. We would be inclined to limit the term “small vessel” to capillaries, venules, and arterioles, since the disease processes and clinical manifestations are rather clearly defined (pulmonary hemorrhage, glomerulonephritis, diffuse purpura of the skin etc.) and the delineation of small muscular arteries from medium-sized muscular arteries seems an impossible task. Defining a “large vessel” is even more difficult but could be approached with a minimum diameter of the artery (i.e., 5 mm), an exhaustive list or by abandoning this principle altogether by defining a new category of medium to large vessel vasculitis. This would make future research studies more comparable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lötscher F, Pop R, Seitz P, Recher M, Seitz L. Spectrum of large and medium-vessel vasculitis in adults: neoplastic, infectious, drug-induced, autoinflammatory and primary immunodeficiency diseases. Curr Rheumatol Rep. 2022. https://doi.org/10.1007/s11926-022-01083-5. Comprehensive review of diseases with potential vasculitis of medium and large vessels that are not covered by the present review.
Martins-Martinho J, Dourado E, Khmelinskii N, Espinosa P, Ponte C. Localized forms of vasculitis. Curr Rheumatol Rep. 2021;23(7):49. https://doi.org/10.1007/s11926-021-01012-y.
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. https://doi.org/10.1002/art.37715.
Stone JR (2016). Diseases of small and medium-sized blood vessels. cardiovascular pathology: 4th Edition, 125–168. Academic Press. https://doi.org/10.1016/B978-0-12-420219-1.00004-5.
Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol. 2008;9(2):71–92. https://doi.org/10.2165/00128071-200809020-00001. Excellent and detailed review of all aspects of cutaneous vasculitis. Detailed information on histopathological findings and differential diagnoses.
Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, et al. Distal thrombectomy summit group. thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. 2020;51(9):2872–84. https://doi.org/10.1161/STROKEAHA.120.028956.
Watts RA, Hatemi G, Burns JC, Mohammad AJ. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2021;1:1–13. https://doi.org/10.1038/s41584-021-00718-8.
Kermani TA, Diab S, Sreih AG, Cuthbertson D, Borchin R, Carette S, et al. Vasculitis clinical research consortium. arterial lesions in giant cell arteritis: a longitudinal study. Semin Arthritis Rheum. 2019;48(4):707–13. https://doi.org/10.1016/j.semarthrit.2018.05.002.
Ren J, Liu J, Su J, Zhang J, Zhao J. Systemic vasculitis involving the breast: a case report and literature review. Rheumatol Int. 2019;39(8):1447–55. https://doi.org/10.1007/s00296-019-04279-8.
Khanna S, Garikapati K, Goh DSL, Cho K, Lo P, Bhojaraja MV, Tarafdar S. Coronary artery vasculitis: a review of current literature. BMC Cardiovasc Disord. 2021;21(1):7. https://doi.org/10.1186/s12872-020-01813-6.
Elbendary A, Abdel-Halim MRE, Ragab G. Updates in cutaneous manifestations of systemic vasculitis. Curr Opin Rheumatol. 2022;34(1):25–32. https://doi.org/10.1097/BOR.0000000000000847.
Soowamber M, Weizman AV, Pagnoux C. Gastrointestinal aspects of vasculitides. Nat Rev Gastroenterol Hepatol. 2017;14(3):185–94. https://doi.org/10.1038/nrgastro.2016.179.
Beuker C, Wankner MC, Thomas C, Strecker JK, Schmidt-Pogoda A, Schwindt W, et al. Characterization of extracranial giant cell arteritis with intracranial involvement and its rapidly progressive subtype. Ann Neurol. 2021;90(1):118–29. https://doi.org/10.1002/ana.26101. Demonstrates the affection of the proximal intracranial arteries in GCA, which is probably underrecognized.
Fayyaz B, Rehman HJ. The spectrum of pericardial involvement in giant cell arteritis and polymyalgia rheumatica: a systematic review of literature. J Clin Rheumatol. 2021;27(1):5–10. https://doi.org/10.1097/RHU.0000000000001140. Important review that highlights the relevance of GCA as an etiology of pericarditis in the elderly.
Martins P, Teixeira V, Teixeira FJ, Canastro M, Palha A, Fonseca JE, Ponte C. Giant cell arteritis with normal inflammatory markers: case report and review of the literature. Clin Rheumatol. 2020;39(10):3115–25. https://doi.org/10.1007/s10067-020-05116-1. Important overview about a frequent clinical question (GCA in patients without elevated inflammatory markers).
Ilan Y, Ben-Chetrit E. Liver involvement in giant cell arteritis. Clin Rheumatol. 1993;12(2):219–22. https://doi.org/10.1007/BF02231530.
Sy A, Khalidi N, Dehghan N, Barra L, Carette S, Cuthbertson D, et al. Vasculitis Clinical Research Consortium (VCRC); Canadian Vasculitis Network (CanVasc). Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum. 2016;45(4):475–82. https://doi.org/10.1016/j.semarthrit.2015.07.006.
Esatoglu SN, Ok AM, Ucar D, Celik AF, Ugurlu S, Hamuryudan V, et al. Takayasu’s arteritis: associated inflammatory diseases. Clin Exp Rheumatol. 2020;38(Suppl 124(2)):61–8.
Schmidt J, Kermani TA, Bacani AK, Crowson CS, Cooper LT, Matteson EL, Warrington KJ. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc. 2013;88(8):822–30. https://doi.org/10.1016/j.mayocp.2013.04.025.
Goel R, Gribbons KB, Carette S, Cuthbertson D, Hoffman GS, Joseph G, et al. Derivation of an angiographically based classification system in Takayasu’s arteritis: an observational study from India and North America. Rheumatology (Oxford). 2020;59(5):1118–27. https://doi.org/10.1093/rheumatology/kez421. Very large study providing a data-derived angiographic classification system of Takayasu arteritis subtypes.
Silveira LH. Cardiovascular manifestations of systemic vasculitides. Curr Rheumatol Rep. 2020;22(10):72. https://doi.org/10.1007/s11926-020-00952-1.
Bond KM, Nasr D, Lehman V, Lanzino G, Cloft HJ, Brinjikji W. Intracranial and extracranial neurovascular manifestations of Takayasu arteritis. AJNR Am J Neuroradiol. 2017;38(4):766–72. https://doi.org/10.3174/ajnr.A5095.
Gupta V, Chinchure SD, Goe G, Jha AN, Malviya S, Gupta R. Coil embolization of intracranial aneurysm in polyarteritis nodosa. A case report and review of the literature. Interv Neuroradiol. 2013;19(2):203–8. https://doi.org/10.1177/159101991301900209.
Fonseka CL, Galappaththi SR, Abeyaratne D, Tissera N, Wijayaratne L. A Case of polyarteritis nodosa presenting as rapidly progressing intermittent claudication of right Leg. Case Rep Med. 2017;2017:4219718. https://doi.org/10.1155/2017/4219718.
Stanson AW, Friese JL, Johnson CM, McKusick MA, Breen JF, Sabater EA, Andrews JC. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics. 2001;21(1):151–9. https://doi.org/10.1148/radiographics.21.1.g01ja16151.
Iino T, Eguchi K, Sakai M, Nagataki S, Ishijima M, Toriyama K. Polyarteritis nodosa with aortic dissection: necrotizing vasculitis of the vasa vasorum. J Rheumatol. 1992;19(10):1632–6.
Martin de Frémont G, Gimenez de Mestral S, Jubault V, Chauchard M, Mekinian A, Fain O. La périartérite noueuse, un diagnostic alternatif à l’artérite gigantocellulaire devant une artérite temporale [Polyarteritis nodosa, an alternative diagnosis of giant cell arteritis in cases of temporal arteritis]. Rev Med Interne. 2019;40(8):533–5. French. https://doi.org/10.1016/j.revmed.2019.05.012.
Puéchal X. Polyarteritis nodosa: state of the art. Joint Bone Spine. 2021;89(4):105320. https://doi.org/10.1016/j.jbspin.2021.105320.
Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, et al. French Vasculitis Study Group. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010;62(2):616–26. https://doi.org/10.1002/art.27240.
de Boysson H, Guillevin L. Polyarteritis nodosa neurologic manifestations. Neurol Clin. 2019;37(2):345–57. https://doi.org/10.1016/j.ncl.2019.01.007.
Fraison JB, Sève P, Dauphin C, Mahr A, Gomard-Mennesson E, Varron L, CRI and the French Vasculitis Study Group, et al. Kawasaki disease in adults: observations in France and literature review. Autoimmun Rev. 2016;15(3):242–9. https://doi.org/10.1016/j.autrev.2015.11.010. Important review about Kawasaki disease from an adult perspective.
Takahashi K, Oharaseki T, Yokouchi Y, Hiruta N, Naoe S. Kawasaki disease as a systemic vasculitis in childhood. Ann Vasc Dis. 2010;3(3):173–81. https://doi.org/10.3400/avd.sasvp01003.
Sato W, Yokouchi Y, Oharaseki T, Asakawa N, Takahashi K. The pathology of Kawasaki disease aortitis: a study of 37 cases. Cardiovasc Pathol. 2021;51:107303. https://doi.org/10.1016/j.carpath.2020.107303.
Yeom JS, Cho JY, Woo HO. Understanding the importance of cerebrovascular involvement in Kawasaki disease. Korean J Pediatr. 2019;62(9):334–9. https://doi.org/10.3345/kjp.2019.00143.
Bonté I, Mahr A, Laroche L, Guillevin L, Robineau M. Peripheral gangrene in adult-onset Kawasaki disease. Scand J Rheumatol. 2005;34(1):71–3. https://doi.org/10.1080/03009740410011235.
Rife E, Gedalia A. Kawasaki disease: an update. Curr Rheumatol Rep. 2020;22(10):75. https://doi.org/10.1007/s11926-020-00941-4.
Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011;30(12):1022–6. https://doi.org/10.1097/INF.0b013e31822d4f56.
Ozaki T, Maeshima K, Kiyonaga Y, Torigoe M, Imada C, Hamasaki H, et al. Large-vessel involvement in granulomatosis with polyangiitis successfully treated with rituximab: a case report and literature review. Mod Rheumatol. 2017;27(4):699–704. https://doi.org/10.3109/14397595.2015.1021950.
Logar D, Rozman B, Vizjak A, Ferluga D, Mulder AH, Kallenberg CG. Arteritis of both carotid arteries in a patient with focal, crescentic glomerulonephritis and anti-neutrophil cytoplasmic autoantibodies. Br J Rheumatol. 1994;33(2):167–9. https://doi.org/10.1093/rheumatology/33.2.167.
Coattrenec Y, Muller YD, Spoerl D, Lobrinus JA, Seebach JD. Prevalence of large vessel vasculitis in ANCA-associated vasculitis: a retrospective cohort study. Rheumatol Int. 2021;41(12):2147–56. https://doi.org/10.1007/s00296-021-04993-2.
Delaval L, Samson M, Schein F, Agard C, Tréfond L, Deroux A, French Vasculitis Study Group and French Study Group for Giant Cell Arteritis, et al. Temporal arteritis revealing antineutrophil cytoplasmic antibody-associated vasculitides: a case-control study. Arthritis Rheum. 2021;73(2):286–94. https://doi.org/10.1002/art.41527. Reminder, that vasculitis of the temporal arteries is not equal to giant cell arteritis.
Kim YK, Chekka P, Mysore M, Childress J, Alfaraidhy M, Thomas A, et al. Isolated antineutrophil cytoplasmic antibody-associated coronary vasculitis and valvulitis. JACC Case Rep. 2021;3(2):309–13. https://doi.org/10.1016/j.jaccas.2020.12.007.
Ghinoi A, Zuccoli G, Pipitone N, Salvarani C. Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis involving the central nervous system: case report and review of the literature. Clin Exp Rheumatol. 2010;28(5):759–66.
Abissegue Y, Lyazidi Y, Arache W, Ouldsalek E, Chtata HT, Taberkant M. Multiple visceral artery aneurysms: an uncommon manifestation of antineutrophil cytoplasmic antibody vasculitis. Ann Vasc Surg. 2016;34:271.e9–271.e13. https://doi.org/10.1016/j.avsg.2016.01.013.
Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–81. https://doi.org/10.1002/art.37721.
Pagnoux C. Updates in ANCA-associated vasculitis. Eur J Rheumatol. 2016;3(3):122–33. https://doi.org/10.5152/eurjrheum.2015.0043.
Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suárez LF, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol. 2017;13(11):683–92. https://doi.org/10.1038/nrrheum.2017.140.
Roccatello D, Saadoun D, Ramos-Casals M, Tzioufas AG, Fervenza FC, Cacoub P, et al. Cryoglobulinaemia. Nat Rev Dis Primers. 2018;4(1):11. https://doi.org/10.1038/s41572-018-0009-4. Excellent starting point for anything connected to cryoglobulins.
Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379(9813):348–60. https://doi.org/10.1016/S0140-6736(11)60242-0.
Fukunaga N, Fujiwara H, Nasu M, Okada Y. Aortic dissection caused by aortitis associated with hepatitis C virus-related cryoglobulinemia. J Thorac Cardiovasc Surg. 2010;140(5):e81–2. https://doi.org/10.1016/j.jtcvs.2010.07.021.
Lenhart A, Meighani A, Hassan M, Gordon S. Hepatitis C virus-associated aortitis caused by type i cryoglobulins. ACG Case Rep J. 2017;4:e114. https://doi.org/10.14309/crj.2017.114.
de La Peña Garcia Lefebvre P, Mouthon L, Cohen P, Lhote F, Guillevin L. Polyarteritis nodosa and mixed cryoglobulinaemia related to hepatitis B and C virus coinfection. Ann Rheum Dis. 2001;60(11):1068–9. https://doi.org/10.1136/ard.60.11.1068.
Tada M, Naruse S, Arai A, Sato A, Tanaka K, Piao YS, et al. An autopsy case of systemic vasculitis associated with hepatitis C virus-related mixed cryoglobulinemia presenting severe peripheral neuropathy. Rinsho Shinkeigaku. 2004;44(10):686–90.
Généreau T, Martin A, Lortholary O, Nöel V, Guillevin L. Temporal arteritis symptoms in a patient with hepatitis C virus associated type II cryoglobulinemia and small vessel vasculitis. J Rheumatol. 1998;25(1):183–5.
Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86(6):707–13. https://doi.org/10.1016/j.jbspin.2019.01.016.
Terrier B, Marie I, Lacraz A, Belenotti P, Bonnet F, Chiche L, et al. Non HCV-related infectious cryoglobulinemia vasculitis: results from the French nationwide CryoVas survey and systematic review of the literature. J Autoimmun. 2015;65:74–81. https://doi.org/10.1016/j.jaut.2015.08.008.
Mazlumzadeh M, Matteson EL. Cogan’s syndrome: an audiovestibular, ocular, and systemic autoimmune disease. Rheum Dis Clin N Am. 2007;33(4):855–74, vii-viii. https://doi.org/10.1016/j.rdc.2007.07.015.
Grasland A, Pouchot J, Hachulla E, Blétry O, Papo T, Vinceneux P, Study Group for Cogan’s Syndrome. Typical and atypical Cogan’s syndrome: 32 cases and review of the literature. Rheumatology (Oxford). 2004;43(8):1007–15. https://doi.org/10.1093/rheumatology/keh228. Large case series and literature review about Cogan Syndrome. Describes the systemic symptoms.
Vavricka SR, Greuter T, Scharl M, Mantzaris G, Shitrit AB, Filip R, et al. ECCO CONFER investigators. Cogan’s syndrome in patients with inflammatory bowel disease--a case series. J Crohns Colitis. 2015;9(10):886–90. https://doi.org/10.1093/ecco-jcc/jjv128. Good discussion of this little-known disease association.
Livingston JZ, Casale AS, Hutchins GM, Shapiro EP. Coronary involvement in Cogan’s syndrome. Am Heart J. 1992;123(2):528–30. https://doi.org/10.1016/0002-8703(92)90674-k.
Vollertsen RS, McDonald TJ, Younge BR, Banks PM, Stanson AW, Ilstrup DM. Cogan’s syndrome: 18 cases and a review of the literature. Mayo Clin Proc. 1986;61(5):344–61. https://doi.org/10.1016/s0025-6196(12)61951-x.
Yazici H, Seyahi E, Hatemi G, Yazici Y. Behçet syndrome: a contemporary view. Nat Rev Rheumatol. 2018;14(2):107–19. https://doi.org/10.1038/nrrheum.2017.208. Excellent review and starting point for further reading in Behçet syndrome.
Seyahi E. Behçet’s disease: How to diagnose and treat vascular involvement. Best Pract Res Clin Rheumatol. 2016;30(2):279–95. https://doi.org/10.1016/j.berh.2016.08.002.
Ramos-Casals M, Nardi N, Lagrutta M, Brito-Zerón P, Bové A, Delgado G, et al. Vasculitis in systemic lupus erythematosus: prevalence and clinical characteristics in 670 patients. Medicine (Baltimore). 2006;85(2):95–104. https://doi.org/10.1097/01.md.0000216817.35937.70.
Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. 2019;96:1–13. https://doi.org/10.1016/j.jaut.2018.11.001.
Akebo H, Sada R, Matsushita S, Ishimaru H, Minoda S, Miyake H, et al. lupus aortitis successfully treated with moderate-dose glucocorticoids: a case report and review of the literature. Intern Med. 2020;59(21):2789–95. https://doi.org/10.2169/internalmedicine.4964-20.
Schwarz-Eywill M, Rinaldi N, Barth T, Nusslein HG. Seltene Manifestation einer Grossgefassvaskulitis bei systemischem Lupus erythematodes. Z Rheumatol. 2000;59(5):330–3. Interesting report with histological demonstration of vasa vasorum vasculitis of large arteries causing a Takayasu-like presentation in SLE.
Saxe PA, Altman RD. Takayasu’s arteritis syndrome associated with systemic lupus erythematosus. Semin Arthritis Rheum. 1992;21(5):295–305. https://doi.org/10.1016/0049-0172(92)90023-7.
Calle-Botero E, Abril A. Lupus vasculitis. Curr Rheumatol Rep. 2020;22(10):71. https://doi.org/10.1007/s11926-020-00937-0.
Nishigaichi A, Oiwa H, Hosokawa Y, Hayashi M, Mine N, Nomura E, Yamawaki T. A case of systemic lupus erythematosus associated with cerebral arteritis: a case report and case-based literature review. Nagoya J Med Sci. 2020;82(4):807–14. https://doi.org/10.18999/nagjms.82.4.807.
Ju JH, Min JK, Jung CK, Oh SN, Kwok SK, Kang KY, et al. Lupus mesenteric vasculitis can cause acute abdominal pain in patients with SLE. Nat Rev Rheumatol. 2009;5(5):273–81. https://doi.org/10.1038/nrrheum.2009.53.
Ashouri JF, Davis JL, Farkas A, Durack JC, Ramachandran R, Dall’Era M. A young woman with systemic lupus erythematosus and extensive mesenteric vasculitis involving small and medium vessels. Arthritis Care Res. 2012;64(12):1928–33. https://doi.org/10.1002/acr.21833.
Sharma A, Dhooria A, Aggarwal A, Rathi M, Chandran V. Connective tissue disorder-associated vasculitis. Curr Rheumatol Rep. 2016;18(6):31. https://doi.org/10.1007/s11926-016-0584-x.
Argyropoulou OD, Tzioufas AG. Common and rare forms of vasculitis associated with Sjögren’s syndrome. Curr Opin Rheumatol. 2020;32(1):21–8. https://doi.org/10.1097/BOR.0000000000000668. Good review for vasculitis in Sjogren’s Syndrome.
Loricera J, Blanco R, Hernández JL, Carril JM, Martínez-Rodríguez I, Canga A, et al. Non-infectious aortitis: a report of 32 cases from a single tertiary centre in a 4-year period and literature review. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S-19–31.
Ramos-Casals M, Anaya JM, García-Carrasco M, Rosas J, Bové A, Claver G, et al. Cutaneous vasculitis in primary Sjögren syndrome: classification and clinical significance of 52 patients. Medicine (Baltimore). 2004;83(2):96–106. https://doi.org/10.1097/01.md.0000119465.24818.98.
Tsokos M, Lazarou SA, Moutsopoulos HM. Vasculitis in primary Sjögren’s syndrome. Histologic classification and clinical presentation. Am J Clin Pathol. 1987;88(1):26–31. https://doi.org/10.1093/ajcp/88.1.26.).
Unnikrishnan G, Hiremath N, Chandrasekharan K, Sreedharan SE, Sylaja PN. Cerebral large-vessel vasculitis in sjogren’s syndrome: utility of high-resolution magnetic resonance vessel wall imaging. J Clin Neurol. 2018;14(4):588–90. https://doi.org/10.3988/jcn.2018.14.4.588.
Lakhanpal S, Lie JT, Conn DL, Martin WJ 2nd. Pulmonary disease in polymyositis/dermatomyositis: a clinicopathological analysis of 65 autopsy cases. Ann Rheum Dis. 1987;46(1):23–9. https://doi.org/10.1136/ard.46.1.23.
Shipa M, Di Cicco M, Roussou E. CNS vasculitis in anti-synthetase syndrome. Mediterr J Rheumatol. 2020;30(4):220–3. https://doi.org/10.31138/mjr.30.4.220.
Regan M, Haque U, Pomper M, Pardo C, Stone J. Central nervous system vasculitis as a complication of refractory dermatomyositis. J Rheumatol. 2001;28(1):207–11.
Stuhlmüller B, Schneider U, González-González JB, Feist E. Disease specific autoantibodies in idiopathic inflammatory myopathies. Front Neurol. 2019;10:438. https://doi.org/10.3389/fneur.2019.00438.
Conticini E, d’Alessandro M, Al Khayyat SG, D’Alessandro R, D’Ignazio E, Pata AP, et al. Inflammatory muscle involvement in systemic vasculitis: a systematic review. Autoimmun Rev. 2021;21(3):103029. https://doi.org/10.1016/j.autrev.2021.103029.
Mathian A, Miyara M, Cohen-Aubart F, Haroche J, Hie M, Pha M, et al. Relapsing polychondritis: A 2016 update on clinical features, diagnostic tools, treatment and biological drug use. Best Pract Res Clin Rheumatol. 2016;30(2):316–33. https://doi.org/10.1016/j.berh.2016.08.001.
Tomelleri A, Campochiaro C, Sartorelli S, Papa M, De Luca G, Cavalli G, et al. Large-vessel vasculitis affecting the aorta and its branches in relapsing polychondritis: case series and systematic review of the literature. J Rheumatol. 2020;47(12):1780–4. https://doi.org/10.3899/jrheum.190862.
Erdogan M, Esatoglu SN, Hatemi G, Hamuryudan V. Aortic involvement in relapsing polychondritis: case-based review. Rheumatol Int. 2021;41(4):827–37. https://doi.org/10.1007/s00296-019-04468-5. Excellent overview of case series on primary relapsing polychondritis with large vessel vasculitis.
Almackenzie M, Alharbi A, Alhassan S, Cook E, Altorok N. Successful treatment of central nervous system vasculitis associated with relapsing polychondritis with cyclophosphamide. Am J Med Sci. 2017;353(5):495–7. https://doi.org/10.1016/j.amjms.2016.11.023.
Ferrada MA, Sikora KA, Luo Y, Wells KV, Patel B, Groarke EM, et al. Somatic Mutations In Uba1 Define A Distinct Subset Of Relapsing Polychondritis Patients with VEXAS. Arthritis Rheum. 2021;73(10):1886–95. https://doi.org/10.1002/art.41743. Definition of a subtype with relapsing polychondritis in the recently described VEXAS-Syndrome.
Kishore S, Maher L, Majithia V. Rheumatoid Vasculitis: A diminishing yet devastating menace. Curr Rheumatol Rep. 2017;19(7):39. https://doi.org/10.1007/s11926-017-0667-3.
Watts RA, Scott DG. Vasculitis and inflammatory arthritis. Best Pract Res Clin Rheumatol. 2016;30(5):916–31. https://doi.org/10.1016/j.berh.2016.10.008.
Makol A, Crowson CS, Wetter DA, Sokumbi O, Matteson EL, Warrington KJ. Vasculitis associated with rheumatoid arthritis: a case-control study. Rheumatology (Oxford). 2014;53(5):890–9. https://doi.org/10.1093/rheumatology/ket475.
Stone JR, Bruneval P, Angelini A, Bartoloni G, Basso C, Batoroeva L, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol. 2015;24(5):267–78. https://doi.org/10.1016/j.carpath.2015.05.001. Comprehensive overview of histological changes in inflammatory aortic disease.
Verweij KE, van Well AM, Vd Sluijs JW, Dees A. Late onset takayasu arteritis and rheumatoid arthritis. Case Rep Med. 2012;2012:523218. https://doi.org/10.1155/2012/523218.
Palazzi C, Salvarani C, D’Angelo S, Olivieri I. Aortitis and periaortitis in ankylosing spondylitis. Joint Bone Spine. 2011;78(5):451–5. https://doi.org/10.1016/j.jbspin.2010.11.003.
Bulkley BH, Roberts WC. Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation. 1973;48(5):1014–27. https://doi.org/10.1161/01.cir.48.5.1014. Excellent autopsy series defining the aortic manifestations in ankylosing spondylitis.
Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK, Stone JH. ACR/EULAR IgG4-RD Classification Criteria Committee. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78(3):406–12. https://doi.org/10.1136/annrheumdis-2018-214603.
Ozawa M, Fujinaga Y, Asano J, Nakamura A, Watanabe T, Ito T, et al. Clinical features of IgG4-related periaortitis/periarteritis based on the analysis of 179 patients with IgG4-related disease: a case-control study. Arthritis Res Ther. 2017;19(1):223. https://doi.org/10.1186/s13075-017-1432-8.
Perugino CA, Wallace ZS, Meyersohn N, Oliveira G, Stone JR, Stone JH. Large vessel involvement by IgG4-related disease. Medicine (Baltimore). 2016;95(28):e3344. https://doi.org/10.1097/MD.0000000000003344.
Fragoulis GE, Evangelatos G, Tektonidou MG. Vasculitis beyond aortitis in IgG4-related disease (IgG4-RD): case report and review of the literature. Clin Rheumatol. 2021;40(3):1167–73. https://doi.org/10.1007/s10067-020-05302-1.
Ferfar Y, Charlotte F, Cacoub P, Saadoun D. Temporal arteritis in IgG4 related disease. Joint Bone Spine. 2021;88(2):105087. https://doi.org/10.1016/j.jbspin.2020.105087.
Maritati F, Rocco R, Accorsi Buttini E, Marvisi C, Nicastro M, Urban ML, et al. clinical and prognostic significance of serum IgG4 in chronic periaortitis. an analysis of 113 patients. Front Immunol. 2019;10:693. https://doi.org/10.3389/fimmu.2019.00693.
Stone JH, Brito-Zerón P, Bosch X, Ramos-Casals M. Diagnostic approach to the complexity of IgG4-related disease. Mayo Clin Proc. 2015;90(7):927–39. https://doi.org/10.1016/j.mayocp.2015.03.020. Excellent discussion about the pearls and pitfalls in diagnosing IgG4-related disease.
Fenaroli P, Maritati F, Vaglio A. Into clinical practice: diagnosis and therapy of retroperitoneal fibrosis. Curr Rheumatol Rep. 2021;23(3):18. https://doi.org/10.1007/s11926-020-00966-9. Comprehensive review on the evolving topic of retroperitoneal fibrosis and the term fibroinflammatory diseases.
Palmisano A, Urban ML, Corradi D, Cobelli R, Alberici F, Maritati F, et al. Chronic periaortitis with thoracic aorta and epiaortic artery involvement: a systemic large vessel vasculitis? Rheumatology (Oxford). 2015;54(11):2004–9. https://doi.org/10.1093/rheumatology/kev225.
Fernandes SR, Singsen BH, Hoffman GS. Sarcoidosis and systemic vasculitis. Semin Arthritis Rheum. 2000g;30(1):33–46. https://doi.org/10.1053/sarh.2000.8364.
Takemura T, Matsui Y, Saiki S, Mikami R. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol. 1992;23(11):1216–23. https://doi.org/10.1016/0046-8177(92)90288-e.
Weiler V, Redtenbacher S, Bancher C, Fischer MB, Smolen JS. Concurrence of sarcoidosis and aortitis: case report and review of the literature. Ann Rheum Dis. 2000;59(11):850–3. https://doi.org/10.1136/ard.59.11.850.
Levy MH, Margo CE. Temporal artery biopsy and sarcoidosis. Am J Ophthalmol. 1994;117(3):409–10. https://doi.org/10.1016/s0002-9394(14)73159-9.
Bathla G, Abdel-Wahed L, Agarwal A, Cho TA, Gupta S, Jones KA, et al. Vascular involvement in neurosarcoidosis: early experiences from intracranial vessel wall imaging. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1063. https://doi.org/10.1212/NXI.0000000000001063Demonstration of the diagnostic potential of vessel wall imaging in sarcoidosis of the central nervous system.
Chapelon-Abric C, Saadoun D, Marie I, Comarmond C, Desbois AC, Domont F, et al. Sarcoidosis with Takayasu arteritis: a model of overlapping granulomatosis. A report of seven cases and literature review. Int J Rheum Dis. 2018;21(3):740–5. https://doi.org/10.1111/1756-185X.13137.
Ramos-Casals M, Retamozo S, Sisó-Almirall A, Pérez-Alvarez R, Pallarés L, Brito-Zerón P. Clinically-useful serum biomarkers for diagnosis and prognosis of sarcoidosis. Expert Rev Clin Immunol. 2019;15(4):391–405. https://doi.org/10.1080/1744666X.2019.1568240.
Puéchal X, Fiessinger JN. Thromboangiitis obliterans or Buerger’s disease: challenges for the rheumatologist. Rheumatology (Oxford). 2007;46(2):192–9. https://doi.org/10.1093/rheumatology/kel388.
Le Joncour A, Soudet S, Dupont A, Espitia O, Koskas F, Cluzel P, et al. French Buerger’s network. long-term outcome and prognostic factors of complications in thromboangiitis obliterans (Buerger’s Disease): A Multicenter Study of 224 Patients. J Am Heart Assoc. 2018;7(23):e010677. https://doi.org/10.1161/JAHA.118.010677. Very large and recent case-series with detailed laboratory and clinical data as well as description of treatment effects (amputations, smoking cessation).
Kindem S, Sanmartin O, Llombart B, Requena C, Serra-Guillén C, Nagore E, Guillén C. Temporal artery involvement as the presenting sign of thromboangiitis obliterans. J Clin Rheumatol. 2013;19(3):158–9. https://doi.org/10.1097/RHU.0b013e318289df15.
Fisher CM. Cerebral thromboangiitis obliterans. Medicine (Baltimore). 1957;36(2):169–209. https://doi.org/10.1097/00005792-195705000-00002.
Fakour F, Fazeli B. Visceral bed involvement in thromboangiitis obliterans: a systematic review. Vasc Health Risk Manag. 2019;15:317–53. https://doi.org/10.2147/VHRM.S182450.
Vodopivec I, Rizzo JF 3rd. Ophthalmic manifestations of giant cell arteritis. Rheumatology (Oxford). 2018;57(suppl_2):ii63–72. https://doi.org/10.1093/rheumatology/kex428.
Noel N, Butel N, Le Hoang P, Koskas F, Costedoat-Chalumeau N, Wechsler B, et al. Small vessel involvement in Takayasu’s arteritis. Autoimmun Rev. 2013;12(3):355–62. https://doi.org/10.1016/j.autrev.2012.05.010.
Karadag O, Jayne DJ. Polyarteritis nodosa revisited: a review of historical approaches, subphenotypes and a research agenda. Clin Exp Rheumatol. 2018;36(Suppl 111(2)):135–42. Points out the problems with the current nomenclature and the many different disease processes that can present with PAN-like vasculitis.
Kint N, De Haes P, Blockmans D. Comparison between classical polyarteritis nodosa and single organ vasculitis of medium-sized vessels: a retrospective study of 25 patients and review of the literature. Acta Clin Belg. 2016;71(1):26–31. https://doi.org/10.1179/2295333714Y.0000000114.
Lefèvre G, Leurs A, Gibier JB, Copin MC, Staumont-Sallé D, Dezoteux F, et al. CEREO—French National Reference Center for Hypereosinophilic Syndromes. “Idiopathic eosinophilic vasculitis”: another side of hypereosinophilic syndrome? A comprehensive analysis of 117 cases in asthma-free patients. J Allergy Clin Immunol Pract. 2020;8(4):1329–1340.e3. https://doi.org/10.1016/j.jaip.2019.12.011.
Harikrishnan G, Jain N, Verma V, Agarwal V, Misra DP. IgA vasculitis with large vessel involvement. Rheumatology (Oxford). 2020;59(7):1790–1. https://doi.org/10.1093/rheumatology/kez603.
Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine (Baltimore). 1980;59(6):426–41.
Jung DH, Nadol JB Jr, Folkerth RD, Merola JF. Histopathology of the inner ear in a case with recent onset of cogan’s syndrome: evidence for vasculitis. Ann Otol Rhinol Laryngol. 2016;125(1):20–4. https://doi.org/10.1177/0003489415595426.
Antonios N, Silliman S. Cogan syndrome: an analysis of reported neurological manifestations. Neurologist. 2012;18(2):55–63. https://doi.org/10.1097/NRL.0b013e31823fa3a0.
Gül A. Pathogenesis of Behçet’s disease: autoinflammatory features and beyond. Semin Immunopathol. 2015;37(4):413–8. https://doi.org/10.1007/s00281-015-0502-8.
Nikiphorou E, Galloway J, Fragoulis GE. Overview of IgG4-related aortitis and periaortitis. A decade since their first description. Autoimmun Rev. 2020;19(12):102694. https://doi.org/10.1016/j.autrev.2020.102694. Conceptually strong review on the topic of IgG4-related aortitis and periaortitis.
Kondo A, Ikeguchi R, Shirai Y, Kobayashi M, Toi S, Shimizu Y, Kitagawa K. Association of IgG4-related arteritis with recurrent stroke. J Stroke Cerebrovasc Dis. 2020;29(2):104514. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104514.
Marlin ES, Dornbos D 3rd, Ikeda DS, Lehman NL, Powers CJ. IgG4-related disease: a new etiology underlying diffuse intracranial dilating vasculopathy. World Neurosurg. 2017;107:1048.e15–20. https://doi.org/10.1016/j.wneu.2017.08.012.
Kimbrough B, Warrington K, Langenfeld H, Crowson C, Carmona E, Virata A, Koster M. Vasculitis in patients with sarcoidosis: a single-institution case series of 17 patients [abstract]. Arthritis Rheumatol. 2021;73(suppl 10):652 . https://acrabstracts.org/abstract/vasculitis-in-patients-with-sarcoidosis-a-single-institution-case-series-of-17-patients. Accessed 12 Jan 2022
Journeau L, Pistorius MA, Michon-Pasturel U, Lambert M, Lapébie FX, Bura-Riviere A, et al. Groupe d’Étude Français des Artérites des gros Vaisseaux. Juvenile temporal arteritis: A clinicopathological multicentric experience. Autoimmun Rev. 2019;18(5):476–83. https://doi.org/10.1016/j.autrev.2019.03.007.
Cinar I, Wang H, Stone JR. Clinically isolated aortitis: pitfalls, progress, and possibilities. Cardiovasc Pathol. 2017;29:23–32. https://doi.org/10.1016/j.carpath.2017.04.003.
Aghayev A, Bay CP, Tedeschi S, Monach PA, Campia U, Gerhard-Herman M, et al. Clinically isolated aortitis: imaging features and clinical outcomes: comparison with giant cell arteritis and giant cell aortitis. Int J Card Imaging. 2021;37(4):1433–43. https://doi.org/10.1007/s10554-020-02087-x.
Maningding E, Kermani TA. Mimics of vasculitis. Rheumatology (Oxford). 2021;60(1):34–47. https://doi.org/10.1093/rheumatology/keaa495. Recent review of vasculitis mimics with practical tables.
Llamas-Velasco M, Alegría V, Santos-Briz Á, Cerroni L, Kutzner H, Requena L. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39(9):637–62. https://doi.org/10.1097/DAD.0000000000000766.
Lecler A, Obadia M, Savatovsky J, Picard H, Charbonneau F, Menjot de Champfleur N, et al. TIPIC syndrome: beyond the myth of carotidynia, a new distinct unclassified entity. AJNR Am J Neuroradiol. 2017;38(7):1391–8. https://doi.org/10.3174/ajnr.A5214.
Upton PD, Smith JG, Charnock DR. Histologic confirmation of carotidynia. Otolaryngol Head Neck Surg. 2003;129(4):443–4. https://doi.org/10.1016/s0194-5998(03)00611-9.
Goyal G, Heaney ML, Collin M, Cohen-Aubart F, Vaglio A, Durham BH, et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929–45. https://doi.org/10.1182/blood.2019003507.
Haroche J, Amoura Z, Dion E, Wechsler B, Costedoat-Chalumeau N, Cacoub P, et al. Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review. Medicine (Baltimore). 2004;83(6):371–92. https://doi.org/10.1097/01.md.0000145368.17934.91.
Harrington CA, Brescia AA, Tieu BH, Fuss C. VIDEO: Rethinking “mural thickening:” vasculitis or aortic dissection? AJR Am J Roentgenol. 2018;210(5):W240–1. https://doi.org/10.2214/AJR.17.19090.
Torre S, Caramaschi P, Faggian G, Luciani GB. Takayasu arteritis mimicking type a intramural hematoma. Ann Thorac Surg. 2017;104(1):e35–7. https://doi.org/10.1016/j.athoracsur.2017.01.121.
Thalhammer C, Husmann M, Glanzmann C, Studer G, Amann-Vesti BR. Carotid artery disease after head and neck radiotherapy. Vasa. 2015;44(1):23–30. https://doi.org/10.1024/0301-1526/a000403.
Seitz L, Lötscher F. The intima-media thickness in suspected giant cell arteritis-sometimes it is worth taking a closer look. Rheumatology (Oxford). 2021;60(7):3039–41. https://doi.org/10.1093/rheumatology/keab316.
Funding
Open access funding provided by University of Bern
Author information
Authors and Affiliations
Contributions
LS, PS, RP, and FL all performed literature searches and wrote parts of the manuscript. LS and FL planned and structured the project, co-wrote, and edited all sections and compiled the tables. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on on Vasculitis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seitz, L., Seitz, P., Pop, R. et al. Spectrum of Large and Medium Vessel Vasculitis in Adults: Primary Vasculitides, Arthritides, Connective Tissue, and Fibroinflammatory Diseases. Curr Rheumatol Rep 24, 352–370 (2022). https://doi.org/10.1007/s11926-022-01086-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01086-2