Abstract
Purpose of the review
The human gut harbors a complex community of microbes that influence many processes regulating musculoskeletal development and homeostasis. This review gives an update on the current knowledge surrounding the impact of the gut microbiota on musculoskeletal health, with an emphasis on research conducted over the last three years.
Recent findings
The gut microbiota and their metabolites are associated with sarcopenia, osteoporosis, osteoarthritis, and rheumatoid arthritis. The field is moving fast from describing simple correlations to pursue establishing causation through clinical trials.
Summary
The gut microbiota and their microbial-synthesized metabolites hold promise for offering new potential alternatives for the prevention and treatment of musculoskeletal diseases given its malleability and response to environmental stimuli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The musculoskeletal system is an important determinant of overall human health. Besides serving as a scaffold for the body and its locomotive function, it is in constant communication with other organs in the body through biochemical signaling, increasingly recognized to harbor fundamental endocrine functions. For instance, it has been postulated that bone can exert critical functions regulating male fertility and whole-body glucose metabolism [1] and that myokines (i.e., cytokines and other peptides released by muscle fibers) can influence cognition, lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, muscle hypertrophy, skin structure, and tumor growth [2].
Musculoskeletal disorders constitute a major cause of disability and morbidity [3]. The economic burden of these diseases on health systems worldwide is predicted to continue increasing together with life expectancy. This global challenge requires urgent and feasible solutions. The pharmacological treatment of most skeletal conditions is broad, ranging from anti-inflammatories and analgesics to topical preparations and nutraceuticals [4], whereas no pharmacological treatment exists for instance for sarcopenia. There is also a lot of emphasis on lifestyle-modification approaches, including physical activity and diet changes to improve the prevention and treatment of musculoskeletal diseases.
Among the novel approximations to preserve musculoskeletal health, the study of the gut microbiota (GM) and their microbial-synthesized metabolites holds promise offering new potential alternatives for the prevention and treatment of musculoskeletal diseases, as diet and lifestyle modifications can impact the composition, richness, and predicted functional profiles of the gut microbiota [5]. The GM encompasses a set of over 2000 different kinds of microorganisms residing in our gastrointestinal tract, encoding 150-fold more genes than the human genome [6]. These microbial communities are assembled during the first 2 years of life after which they stabilize; as such, disruption of this colonization at early ages could affect maturation and developmental pathways [7]. Unsurprisingly, microbiome metabolites are now believed to influence numerous diseases, such as cancer, diabetes, cardiovascular disease, multiple sclerosis and autism spectrum disorder, amid many others [8]. Besides this, the GM strongly interacts with certain drugs and influences their action [9]. The GM is now considered to be the leading edge of scientific research accounting for more than 9500 research publications in the last year [9], and the musculoskeletal field is no exception. The last year has witnessed an upsurge in research on the viral component of the microbiome (i.e., the virome), which is dominated by bacteriophages, determinant in shaping bacterial communities [10]. Their study is essential to bridge gaps of knowledge on the ecology and functionality of the GM.
There is an increasing body of evidence showing that the GM can exert effects in the musculoskeletal system as it modulates gut permeability, hormonal secretion, and immune response, and stimulates calcium and vitamin D absorption [11]. Therefore, the modulation of the GM could be seen as a next-generation treatment for musculoskeletal disorders. The influence of the gut microbiome on musculoskeletal health and disease processes can be direct or indirect. For example, the GM can play key roles in the success of lifestyle interventions aimed at mitigating the impact of aging in the musculoskeletal system or the development of disease. In this review, we will provide an overview of studies that examined the impact of the GM or their products on the musculoskeletal system, with special emphasis on the effects targeting bone homeostasis through the life course.
Gut Microbiome as Determinant of Musculoskeletal Health and Disease
In this review, we prioritized work published during the last three years, strategy that may have resulted in underrepresentation of previously published high-quality work and reviews on this topic [11,12,13,14,15,16,17,18,19]. We cover in detail the relationship with skeletal outcomes i.e., bone metabolism/osteoporosis, but also provide an overview of its significance to muscle function/sarcopenia, cartilage integrity/osteoarthritis and its role on the immune response/specifically in rheumatoid arthritis.
Gut Microbiome Effects on Bone Metabolism
Bone metabolism depends on the balance between bone formation and resorption orchestrated by the action of osteoblasts, osteocytes, and osteoclasts. Bone mass starts to be accrued after birth and peaks in young adulthood, decreasing thereafter [20]. A high peak bone mass is associated with reduced osteoporosis risk in later life, with simulation studies showing that a 10% increase in peak bone mass delays the onset of osteoporosis by 13 years [21]. Therefore, measures intending to maximize peak bone mass and strength are important when designing strategies aimed at reducing the risk of osteoporosis or low bone mass later in life. Osteoporosis is a disease affecting > 200 million elderly worldwide, characterized by increased microstructural deterioration of bone tissue and low bone mass which ultimately leads to fragility fractures. Despite a range of effective compounds to reduce fracture risk, treatment rates are low among high-risk individuals [22].
With over 435 publications appearing in PubMed between the years 2013 and 2019, microbiome research applied to bone health is clearly booming. This is not unexpected, considering that host metabolic pathways, the immune system, and the hormonal environment constitute important determinants of bone metabolism. Consequently, it is rational to think that the GM also plays an important role in bone homeostasis (Fig. 1). Indeed, the risk of osteoporosis has been associated with inter-individual variation of the gut microbiome [23]. Understanding relevant host-microbe interactions would in principle open the door to better diagnostic and therapeutic options in osteoporosis management. Here, we summarize research illustrating different routes by which the GM affects bone, derived mainly from work in animal models and evidence emerging from human studies. This work has evolved from describing simple correlations to pursue establishing causation through clinical trials.
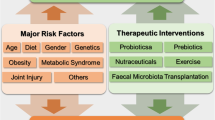
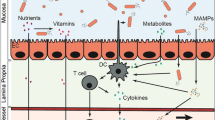
a Influence of gut microbiota on bone. The gut microbiota (GM) contributes to preserve gut barrier integrity. The GM affects absorption of calcium and vitamin D, maturation of the immune system, and production and activation of hormones as estrogens and androgens. Moreover, GM dysbiosis can result in production of inflammatory cytokines that translocate intestinal barrier and exert detrimental effects on bone. Probiotics and prebiotics have shown potential to mitigate or restore bone health. b Influence of gut microbiota on joints and muscles. The GM can influence joint health through some mechanisms including host immunity, and inflammation. Whereas influence of muscle health involves also glucose intake, energy metabolism, and fiber protein synthesis
Nutrition and Bone Development
Calcium and vitamin D are key bone health nutrients, whose depletion or deficit results in adverse skeletal complications [24]. These nutrients have been considered critical, to the point that clinical trials aimed at showing the effectiveness of osteoporosis medications systematically include vitamin D and calcium as part of the treatment regimen [25,26,27]. Yet, over the last years, a growing number of studies have questioned the use of supplementing these nutrients in the general population [28,29,30], in contrast to their established benefit in deficient individuals. During skeletal development, calcium and vitamin D exert critical roles [31] and the involvement of the GM in the absorption and activation of these nutrients might then be of great significance during early ages. Another molecule to consider is vitamin K, which albeit some contradictory findings has shown a positive effect on bone health [32].
Vitamin D
Childhood vitamin D deficiency is considered a significant public health issue around the world [33]. Randomized controlled trials of vitamin D supplementation in children with deficiency have shown improvement on bone mineral density (BMD) [34]. Besides its direct effect on calcium absorption, vitamin D regulates the homeostasis of the gut mucosa by maintaining the integrity of the epithelial barrier and thus the translocation of microbial metabolites to the host. This regulation also influences the maturation of the immune system and inflammation responses [35]. With respect to its relation with the GM, a recent small intervention study showed that high vitamin D supplementation in adolescent girls (i.e., 9 weekly doses of 50,000 IU) resulted in an increase of Firmicutes, Bifidobacterium, and Enterococcus and a decrease of Bacteroidetes and Lactobacillus [36]. In line with these findings, vitamin D receptor (VDR) knock-out (KO) mice have a microbiome enriched for Bacteroides and Clostridium but depleted in Lactobacillus [37]. In addition, these mice had alterations in metabolites specifically produced from carbohydrate, protein, lipid, and bile acid metabolism [38]. These studies support the contention that vitamin D regulates the GM, also supported by the identification of variants in the VDR gene in a GM genome-wide association study (GWAS) [39]. Conversely, there is also evidence of the GM influencing the levels of circulating vitamin D. For instance, a clinical trial in 127 individuals showed that supplementation of Lactobacillus reuteri NCIMB 30242 increased mean circulating 25-hydroxyvitamin D by 25.5% after a 9-weeks intervention [40]. On top of this, bacteria as Streptomyces griseolus can hydroxilize vitamin D3, essential step to its activation, as human metabolic enzymes do [41].
Calcium Absorption
Calcium is the most common mineral in the human body. For a high peak bone mass to be achieved, the intake of calcium needs to be adequate, particularly during periods of rapid growth when absorbed calcium is retained, rather than excreted in the urine [42]. Several studies have demonstrated that short-chain fatty acids (SCFAs) produced by the GM help improving calcium absorption in humans and increase bone density and strength in animal models [43,44,45]. These studies focused on the advantage of consumption of dietary fibers and their effect in the increment of SCFAs as well as Parabacteroides, Bifidobacterium, and Bacteroides relative abundances (a nice review on the topic can be found [46]). The importance of these studies lies in the use of prebiotics (i.e., compounds that induce the growth or activity of beneficial microorganisms) or postbiotics (i.e., factors secreted by live microorganisms) to correct calcium deficiency without the need for an increase in calcium-rich foods or supplements. However, there are studies suggesting that the relationship between the GM and calcium levels is not unidirectional. Calcium supplementation has been shown to increase the microbial diversity and the number of Bifidobacterium sp [47], Ruminococcaceae, and Akkermansia in mice [48]. Also, in healthy men, intake of 1000 mg of calcium and 1000 mg of phosphorus per day, for 8 weeks, increased the fraction of Clostridium XVIII in the fecal samples [49].
Vitamin K
It has been shown that vitamin K is implicated in bone health by promoting the osteoblast-to-osteocyte transition, limiting osteoclastogenesis and intermediating the process of osteocalcin carboxylation [32]. However, a recent study has also implicated this vitamin in changes in the composition and structure of the organic or mineral material [50]. By using metagenomic analysis of the fecal microbiota from mice as well as nanoscale chemical analysis of bone tissue, the authors were able to identify reductions in the concentrations of forms of vitamin K generated by microbes in mice with impaired bone strength [50].
Dietary Fibers
Despite the known caveats of nutrition epidemiology such as compositionality of the data and correlation with varying social and behavioral factors [51], two large epidemiological studies have shown a positive effect of fiber intake on bone outcomes [52, 53]. It is hypothesized that the effect of fibers in health is at least partially explained by their fermentation to SCFAs (acetate, propionate, butyrate) by the GM. Besides the positive effects of SCFAs already described, administration of propionate (C3) or butyrate (C4) prevents ovariectomy-induced, as well as, inflammation-dependent bone loss in mice [44]. Butyrate can also inhibit histone deacetylase and stimulate osteoblast differentiation [54] and increase bone formation with increased bone sialoprotein and osteoprotegerin production [55]. Moreover, butyrate could stimulate bone formation via T regulatory cell-mediated regulation of WNT10B expression [56]. Mechanistically, C3 and C4 induce metabolic reprogramming of osteoclasts, resulting in enhanced glycolysis at the expense of oxidative phosphorylation, thereby downregulating essential osteoclast genes such as TRAF6 and NFATc1 [44]. Reduction of osteoclast differentiation was also recently observed in the alveolar bone in mice in response to SCFAs administration [57]. In line with these results, mice fed with a diet rich in short-chain Galacto-Oligosaccharides and long-chain Fructo-Oligosaccharides (scGOS/lcFOS), prebiotics used by GM as substrate for the production of SCFAs, showed an improved BMD [58].
The Role of Hormones on Bone Metabolism
Insulin-Like Growth Factor 1 (IGF-1)
IGF-1 plays an essential role in regulating skeletal development and postnatal growth. Igf1 KO mice exhibit decreased post-natal growth rate and delayed skeletal ossification, whereas overexpression of Igf1 significantly increases radial bone growth in male and female mice [59, 60]. IGF-1 serum levels have been shown to be higher in mice with an intact GM as compared with germ-free (GF) mice. As expected, GF mice showed decreased linear growth, femur length, cortical thickness, and trabecular bone [59]. Administration of SCFAs was sufficient to increase circulating IGF-1 [59]. However, a study performed in a different mice strain showed the opposite results [61]. These seemingly contradictory findings might be explained by the specific genetic background of the experimental subjects and/or age-dependent effects. Viruses of the Irdoviradae family in the human gut virome have been shown to produce viral insulin/IGF-1-like peptides (VILPs). These peptides are able to bind to murine and human IGF-1 receptors and stimulate cell growth in vitro [62].
Sex Hormones
The estrogen depletion observed in post-menopausal women adversely impacts bone homeostasis, and one of the principal regulators of circulating estrogens is the GM [63]. The GM regulates estrogens through the secretion of β-glucuronidase, an enzyme that deconjugates estrogens into their active forms. When this process is impaired through, for example, lower diversity of the GM, the decrease in deconjugation results in a reduction of circulating estrogens [63]. Excessive osteoclast formation and resorption are considered as the key pathological changes in estrogen-deficiency-induced osteoporosis [64]. Moreover, estrogen deprivation increases intestinal permeability allowing the translocation of bacteria and increasing the number of antigens entering the epithelial mucosa what could lead to systemic inflammation. Compared with normal mice, GF mice showed less bone loss, following estrogen deficiency, due to the reduction of osteoclastogenic cytokines [59]. In addition, probiotic treatment based on different Lactobacillus species reduced the expression of osteoclastogenic cytokines and increased the expression of OPG in bone, protecting mice from ovariectomy (OVX)-induced bone loss [65]. Bifidobacterium longum has also been reported to alleviate bone loss in OVX rats [66]. Further, treatments to prevent gut leakage either by antibiotic depletion of the gut microbiota or administration of Lactobacillus reuteri were shown to be effective in the treatment of glucocorticoid-induced osteoporosis in mice [67]. Androgens, other type of sex hormones, are also essential for bone development and maintenance [68]. Recently, it has been shown that the GM modulates levels of free hydrotestosterone (DHT), a potent androgen, in the distal intestine. However, further studies are necessary to clarify if the GM has the capacity to regulate androgen metabolism and action, also at extra intestinal locations [68].
Role of the Microbiota in Immunity and inflammation
The GM plays a central role in the maturation of the immune system. It is involved in the production of circulating cytokines and the development of lymphoid cells, particularly of T-helper lymphocytes. With age and particularly in response to estrogen deficiency, T cells increase their production of pro-inflammatory and pro-osteoclastogenic cytokines, such as TNF-α and RANKL [69]. The ability of the GM to increase these cytokines and reduce cortical bone in mice is actually dependent on NOD1 and NOD2 signaling which elicits an inflammatory response [70]. Studies have also shown that activation of the toll-like receptor 5 (TLR5), another pattern recognition receptor used by the innate immune system, prompts osteoclast formation and bone loss in mice. Besides, TLR5-KO mice present with increasing periosteal expansion [71], which is normalized when there is a disruption of the GM, in line with a mediation role of the GM.
Exercise is another integral component of osteoporosis management, as physical activity increases BMD and reduces inflammatory markers [72]. Recently, it was proposed that exercise may prevent bone loss through changes in GM. This was based on the results of an activity study in mice, where members of the Bifidobacteriaceae family, known to reduce intestinal inflammation, positively correlated with BMD [73].
Clinical Studies Assessing the Effect of GM in Osteoporosis
Observational Studies
An association study by Das et al. found a higher abundance of Actinomyces, Eggerthella, Clostridium Cluster XlVa, and Lactobacillus genera in individuals with osteoporosis (N = 60) compared with individuals with normal BMD (N = 60) and a lower abundance of Escherichia/Shigella and Veillonella species in the osteoporotic individuals compared with an osteopenic group (N = 61) [74]. No statistical differences were found in diversity metrics among the groups [74]. In contrast, another study found higher diversity and a higher abundance of Dialister and Faecalibacterium in individuals with osteoporosis (N = 48) as compared with individuals with normal levels of BMD (N = 48) [75], whereas two Chinese studies, each in about hundred individuals, reported correlations between the abundance of Bifidobacterium, Roseburia, and Lactobacillus [76] and Allisonella, Klebsiella, and Megasphaera [77] and BMD, respectively. These inconsistent results show the importance of using adequate sample sizes and controlling for multiple testing when investigating possible new associations.
Microbiome-Based Clinical Trials
Two different clinical trials carried out in Sweden have shown a substantial decrease in bone loss in postmenopausal women after probiotic use. The first one, enrolled 90 postmenopausal women and showed that after 1 year of daily supplementation with Lactobacillus reuteri 6475, the treatment group presented reduced volumetric BMD loss at the tibia (mean difference between groups =1.02%; 95% CI: 0.02–2.03%) [78]. The second one focused on bone loss at the lumbar spine (LS). Two hundred thirty-two early postmenopausal women completed the trial in which half of them (116) received probiotic treatment consisting of a daily dose of three Lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and Lactobacillus plantarum DSM 15313; 1×10-10 colony-forming units per capsule) per 12 months or placebo. LS-DXA scans were taken the day of intake and 1 year later when the treatment ended. Lactobacillus treatment reduced the LS-BMD loss compared with placebo (mean difference 0.71%, 95% CI 0.06 to 1.35) [79]. The LS-BMD loss was significant in the placebo group (–0.72%, −1.22 to −0.22), whereas no bone loss was observed in the Lactobacillus-treated group (–0.01%, −0.50 to 0.48) [79]. The authors concluded that the Lactobacillus strains seem to target mechanisms with differential action on trabecular and cortical bone. Conversely, a clinical trial in 76 healthy postmenopausal Japanese women observed a positive effect of administration of Bacillus subtilis C-3102 for 24 weeks in total hip BMD (placebo = 0.83 ± 0.63%, C-3102 = 2.53 ± 0.52%, p =0.043), whilst no significant effect was observed in the LS-BMD of the participants taking the probiotic as compared with the placebo group. Based on microbiome profiles, urinary type I collagen cross-linked N-telopeptide and tartrate-resistant acid phosphatase isoform 5b measurements the authors presume that C-3102 improves BMD by inhibiting bone resorption and modulating the GM [80]. In a study comprising 50 healthy post-menopausal Iranian women [81], a lower serum collagen type 1 cross-linked C-telopeptide (CTX) was also detected in the intervention group as compared with the placebo group. The intervention group, comprising 25 women, took a multispecies probiotic capsule (GeriLact) daily for 6 months. GeriLact contains Lactobacillus casei, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, and Streptococcus thermophilus [81]. The presence of reduced bone turnover was also supported by lower levels of bone-specific alkaline phosphatase (BALP) in the intervention group after treatment [81].
Gut Microbiome Effects on Skeletal Muscle Mass and Function
Even if the gut-muscle axis has not been studied to the extent of the gut-bone axis, this field is gaining momentum [82,83,84,85,86,87,88]. This axis may be involved in the pathogenesis of muscle wasting disorders through the transduction of pro-anabolic stimuli from dietary nutrients, modulation of inflammation and insulin sensitivity among others (Fig. 1) [19]. It has been shown that skeletal muscle mass and physical function are reduced in GF and in antibiotic-treated mice [83, 85, 89]. Transplanting the GM from conventionally raised mice to GF mice resulted in an increase in skeletal muscle mass and a reduction in muscle atrophy markers [85]. Moreover, merely treatment with SCFAs partly reversed skeletal muscle impairments in these mice [85]. Another study demonstrated that the reduced physical fitness, exercise performance, and energy metabolism in young GF mice could be improved by inoculation of either Eubacterium rectale, Lactobacillus plantarum TWK10, or Clostridium coccoides [87]. Similarly, reduced running endurance in conjunction with increased ex vivo muscle fatigability was found in antibiotic-treated mice [83], which could be entirely normalized by natural reseeding of the gut microbiota [83]. In humans, genus Prevotella and Barnesiella have been shown to be more abundant in the fecal samples of elderly with higher lean mass and better physical performance as compared with low-functioning elderly in a small study (high-functioning, N = 18; low-functioning, N = 11) [88]. Colonization of GF mice with the human microbiota of the highly functional individuals resulted in higher grip-strength in these mice. However, no differences were observed in total lean mass or endurance between mice colonization with the high-functioning human microbiota or low-functioning microbiota [88]. Currently, there is an ongoing clinical trial in Ireland aiming to assess the effect of Bacillus coagulans as probiotic on the rates of muscle protein synthesis [90]. If Bacillus coagulans supplementation can improve muscle protein synthesis rates following plant protein consumption, then that could embody an effective and environmentally sensitive strategy to attenuate adverse age-related loss of muscle mass and physical function in the elderly.
Gut Microbiome Effects on the Joints
Osteoarthritis (OA) is the most common chronic degenerative joint disease and a leading cause for joint disability worldwide [91]. Currently, no curative treatment exists for OA. Well-established risk factors for OA are obesity and macrophage-mediated inflammation, both linked to the GM (Fig. 1) [92, 93]. In principle, different mechanisms by which the GM can reduce obesity would have a high impact on modifying OA risk. Accordingly, mice following a long-term high-fat diet are prone to develop obesity-mediated OA. However, this risk is reduced by intervention with Lactobacillus paracasei subsp. paracasei M5 or the prebiotic oligofructose [94]. In addition, a small-scale study recently demonstrated worsening of OA pathology in the presence of serum and synovial fluid containing high bacterial LPS (lipopolysaccharides) levels with activated macrophages in the knee joint capsule and synovium [95]. Moreover, the abundance of Streptococcus species was recently associated with increased knee pain and knee joint inflammation in a large population study of older adults [96]. Clinical trials in humans have already shown a positive effect of Lactobacillus casei Shirota [97] and Streptococcus thermophilus [98] in the progression of knee OA.
Gut Microbiome Effects on the Immune Response
Rheumatoid arthritis (RA) is an autoimmune disease in which systemic chronic inflammation leads to joint destruction. Altered composition of the oral and gut microbiota has been observed in RA both in mice and human studies (reviewed recently in [17]). Several GM bacteria species have been found to be enriched in RA cases, among which Prevotella species [99] and different species of Lactobacillus. Also, the oral microbiome species Cryptobacterium curtum has been found to be enriched in RA cases. This bacterium is capable of producing large amounts of citrulline which is known for acting as an autoantigen in RA [100]. Also in relation to RA, Lactobacillus casei was able to suppress the induction of RA and protect bones from destruction in a study using rats [101].
Future Perspectives
It is clear that the GM presents an exciting new frontier in musculoskeletal research. Although the potential benefit of GM research is high, several hurdles still need to be overcome before GM research can be translated to the clinic. The majority of the GM research related to (musculoskeletal) health has been done using 16S ribosomal RNA (rRNA) sequencing. The 16S rRNA technique, while technically robust, has limited resolution to identify specific bacteria related to disease (i.e., taxonomical orders bellow genus level) or to assess functional potential, as opposed to for example shotgun metagenomics sequencing. This limitation hampers the possibility of translating microbiome research to the development of advanced therapeutics. Moreover, most microbiome studies have been carried out in small sample sizes that are not representative of the population and can be hindered by the high inter-individual variability of the GM and high dimensionality of the data. On top of this, methods and procedures for collection, extraction, and analysis of microbiome data are not standardized across studies, which has led to a lack of reproducibility in the field. Therefore, harmonization of large-scale studies will allow guarantying reproducible science. As large GWAS of GM variability continue to emerge, leveraging genetic information to construct instrumental variables allowing systematic exploration of unconfounded relationships between GM and musculoskeletal traits starts to materialize using the Mendelian randomization approach [102]. Recently, the relationship of a microbiome-based polygenic risk score and BMD was assessed in the UK Biobank and found only one nominal significant association with pelvic BMD [103]. However, the GM-GWAS selected by the authors as the base for their analyses were rather small [103]. The recent publication of the MiBioGen consortium meta-analysis including more than 18,000 individuals [104] opens the opportunity to evaluate the association of multiple microbes and musculoskeletal health outcomes in well-powered settings. This strategy, will also allow the efficient designs of clinical trials, with increased likelihood of success. Altogether, the efficacious clinical trials summarized here are likely to fuel further studies and the development of new therapies based on our ever-growing knowledge of the relation of GM and bone metabolism. Moreover, patient surveys in the USA have shown the great acceptance of prebiotics and probiotics suggesting that microbiome-based therapies could increase treatment compliance in patients [105, 106].
Last but not least, the characterization of the gut virome is still in its infancy. Yet, viruses can alter microbiota structure by infecting specific populations of bacteria as well as trigger apoptosis or induce alterations in host cells [107]. Therefore, they are intrinsically involved in the maturation of the immune system and the inflammation process. Given these findings, it is timely to evaluate the potential role of the gut virome in musculoskeletal health and disease.
Concluding Remarks
This review highlights studies in which the GM is shown to have not just an association but also a key modulatory role in the musculoskeletal system. There is substantial space for improving the current management of musculoskeletal diseases, and the GM-derived treatments are an exciting point of inflection with growing number of opportunities. This increasing body of evidence, together with the GM response to environmental stimuli and malleability, positions it as a potential crucial driver of the personalized healthcare revolution, including musculoskeletal diseases.
References
Oldknow KJ, MacRae VE, Farquharson C. Endocrine role of bone: recent and emerging perspectives beyond osteocalcin. J Endocrinol. 2015;225(1):R1–19.
Severinsen MCK, Pedersen BK. Muscle–organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41(4):594–609.
Lewis R, Gómez Álvarez CB, Rayman M, Lanham-New S, Woolf A, Mobasheri A. Strategies for optimising musculoskeletal health in the 21st century. BMC Musculoskelet Disord. 2019;20(1):164.
Loveless MS, Fry AL. Pharmacologic Therapies in Musculoskeletal Conditions. Med Clin N Am. 2016;100(4):869–90.
Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 2020;11(1):5206.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Robertson R, Manges A, Finlay B, Prendergast A. The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol. 2019;27(2):131–47.
Steves CJ, Bird S, Williams FM, Spector TD. The microbiome and musculoskeletal conditions of aging: a review of evidence for impact and potential therapeutics. J Bone Miner Res. 2016;31(2):261–9.
Vila AV, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11(1):362.
Andrey N, Shkoporov AGC, Sutton TDS, Velayudhan V, Ross RP, Hill C. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. 2019;26:527–41.
Locantore P, Del Gatto V, Gelli S, Paragliola RM, Pontecorvi A. The interplay between immune system and microbiota in osteoporosis. Mediat Inflamm. 2020;2020:3686749.
Medina-Gomez C. Bone and the gut microbiome: a new dimension. J Lab Precis Med. 2018;3:96.
Yan J, Charles JF. Gut microbiome and bone: to build, destroy, or both? Curr Osteoporos Rep. 2017;15(4):376–84.
Xu X, Jia X, Mo L, Liu C, Zheng L, Yuan Q, et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017;5:17046.
Pacifici R. Bone Remodeling and the Microbiome. Cold Spring Harb Perspect Med. 2018;8(4):a031203.
Ibanez L, Rouleau M, Wakkach A, Blin-Wakkach C. Gut microbiome and bone. Joint Bone Spine. 2019;86(1):43–7.
Maeda Y, Takeda K. Host–microbiota interactions in rheumatoid arthritis. Exp Mol Med. 2019;51(12):1–6.
Lustgarten MS. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Front Physiol. 2019;10:1435.
Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev. 2019;25:84–95.
Lu J, Shin Y, Yen M-S, Sun SS. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom. 2016;19(2):180–91.
Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14(10):843–7.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–76.
Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–9.
Uday S, Hogler W. Nutritional rickets and osteomalacia in the twenty-first century: revised concepts, public health, and prevention strategies. Curr Osteoporos Rep. 2017;15(4):293–302.
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, et al. Annual high-dose oral vitamin d and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–22.
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin d supplementationa meta-analysis of randomized controlled trials. JAMA. 2005;293(18):2257–64.
Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J Bone Miner Res. 2006;21(3):397–405.
Cerani A, Zhou S, Forgetta V, Morris JA, Trajanoska K, Rivadeneira F, et al. Genetic predisposition to increased serum calcium, bone mineral density, and fracture risk in individuals with normal calcium levels: mendelian randomisation study. BMJ. 2019;366:l4410.
Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, et al. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and mendelian randomisation study. BMJ. 2018;362:k3225.
Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–58.
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–386.
Palermo A, Tuccinardi D, D'Onofrio L, Watanabe M, Maggi D, Maurizi AR, et al. Vitamin K and osteoporosis: Myth or reality? Metabolism. 2017;70:57–71.
Antonucci R, Locci C, Clemente MG, Chicconi E, Antonucci L. Vitamin D deficiency in childhood: old lessons and current challenges. J Pediatr Endocrinol Metab. 2018;31(3):247–60.
Winzenberg T, Powell S, Shaw KA, Jones G. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
Farre R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients. 2020;12(4):1185.
Tabatabaeizadeh SA, Fazeli M, Meshkat Z, Khodashenas E, Esmaeili H, Mazloum S, et al. The effects of high doses of vitamin D on the composition of the gut microbiome of adolescent girls. Clin Nutr ESPEN. 2020;35:103–8.
Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, et al. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin Ther. 2015;37(5):996–1009 e7.
Chatterjee I, Lu R, Zhang Y, Zhang J, Dai Y, Xia Y, et al. Vitamin D receptor promotes healthy microbial metabolites and microbiome. Sci Rep. 2020;10(1):7340.
Wang J, Thingholm LB, Skieceviciene J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396–406.
Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(7):2944–51.
Yamamoto EA, Jorgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. 2019;10:3141.
Matkovic V. Calcium intake and peak bone mass. N Engl J Med. 1992;327(2):119–20.
Jakeman SA, Henry CN, Martin BR, McCabe GP, McCabe LD, Jackson GS, et al. Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: a randomized crossover trial. Am J Clin Nutr. 2016;104(3):837–43.
Lucas S, Omata Y, Hofmann J, Bottcher M, Iljazovic A, Sarter K, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55.
Weaver CM, Martin BR, Story JA, Hutchinson I, Sanders L. Novel fibers increase bone calcium content and strength beyond efficiency of large intestine fermentation. J Agric Food Chem. 2010;58(16):8952–7.
Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017;36(3):218–22.
Chaplin A, Parra P, Laraichi S, Serra F, Palou A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol Nutr Food Res. 2016;60(2):468–80.
Nadeem Aslam M, Bassis CM, Zhang L, Zaidi S, Varani J, Bergin IL. Calcium Reduces Liver Injury in Mice on a High-Fat Diet: Alterations in Microbial and Bile Acid Profiles. PLoS One. 2016;11(11):e0166178.
Trautvetter U, Camarinha-Silva A, Jahreis G, Lorkowski S, Glei M. High phosphorus intake and gut-related parameters - results of a randomized placebo-controlled human intervention study. Nutr J. 2018;17(1):23.
Guss JD, Taylor E, Rouse Z, Roubert S, Higgins CH, Thomas CJ, et al. The microbial metagenome and bone tissue composition in mice with microbiome-induced reductions in bone strength. Bone. 2019;127:146–54.
Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320(10):969–70.
Dai Z, Zhang Y, Lu N, Felson DT, Kiel DP, Sahni S. Association between dietary fiber intake and bone loss in the Framingham offspring study. J Bone Miner Res. 2018;33(2):241–9.
Lee T, Suh HS. Associations between dietary fiber intake and bone mineral density in adult Korean population: analysis of national health and nutrition examination survey in 2011. J Bone Metab. 2019;26(3):151–60.
Lee HW, Suh JH, Kim AY, Lee YS, Park SY, Kim JB. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol Endocrinol. 2006;20(10):2432–43.
Chang M-C, Chen Y-J, Lian Y-C, Chang B-E, Huang C-C, Huang W-L, et al. Butyrate stimulates histone H3 acetylation, 8-isoprostane production, RANKL expression, and regulated osteoprotegerin expression/secretion in MG-63 osteoblastic cells. Int J Mol Sci. 2018;19(12):4071.
Tyagi AM, Yu M, Darby TM, Vaccaro C, Li J-Y, Owens JA, et al. The microbial metabolite butyrate stimulates bone formation via t regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49(6):1116–31.e7.
Montalvany-Antonucci CC, Duffles LF, de Arruda JAA, Zicker MC, de Oliveira S, Macari S, et al. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone. 2019;125:112–21.
Rogier R, Ederveen THA, Wopereis H, Hartog A, Boekhorst J, van Hijum SAFT, et al. Supplementation of diet with non-digestible oligosaccharides alters the intestinal microbiota, but not arthritis development, in IL-1 receptor antagonist deficient mice. PLoS One. 2019;14(7):e0219366.
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci. 2016;113(47):E7554–E63.
Schwarzer M, Makki K, Storelli G, Machuca-Gayet I, Srutkova D, Hermanova P, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–7.
Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, et al. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Sci Rep. 2017;7(1):5747.
Altindis E, Cai W, Sakaguchi M, Zhang F, GuoXiao W, Liu F, et al. Viral insulin-like peptides activate human insulin and IGF-1 receptor signaling: A paradigm shift for host-microbe interactions. Proc Natl Acad Sci U S A. 2018;115(10):2461–6.
Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53.
Horowitz M. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993;260(5108):626–7.
Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, et al. Probiotics Protect Mice from Ovariectomy-Induced Cortical Bone Loss. PLoS One. 2014;9(3):e92368.
Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, et al. Probiotics Bifidobacterium longum Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed Res Int. 2015;2015:897639.
Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35(4):801–20.
Colldén H, Landin A, Wallenius V, Elebring E, Fändriks L, Nilsson ME, et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab. 2019;317(6):E1182–E92.
D'Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone. 2008;43(1):92–100.
Ohlsson C, Nigro G, Boneca IG, Bäckhed F, Sansonetti P, Sjögren K. Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell Immunol. 2017;317:55–8.
Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32(6):1343–53.
Lombardi G, Ziemann E, Banfi G. Physical Activity and Bone Health: What Is the Role of Immune System? A Narrative Review of the Third Way. Front Endocrinol (Lausanne). 2019;10:60.
McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N, et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20–31.
Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford). 2019;58(12):2295–304.
Xu Z, Xie Z, Sun J, Huang S, Chen Y, Li C, et al. Gut microbiome reveals specific dysbiosis in primary osteoporosis. Front Cell Infect Microbiol. 2020;10:160.
Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30(5):1003–13.
He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY). 2020;12(9):8583–604.
Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–17.
Per-Anders J, Curiac D, Ahren IL, Hansson F, Niskanen TM, Sjogren K, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1(3):E154–E62.
Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, et al. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Biosci Microbiota Food Health. 2018;37(4):87–96.
Jafarnejad S, Djafarian K, Fazeli MR, Yekaninejad MS, Rostamian A, Keshavarz SA. Effects of a multispecies probiotic supplement on bone health in osteopenic postmenopausal women: a randomized, double-blind, controlled trial. J Am Coll Nutr. 2017;36(7):497–506.
Manickam R, Oh HYP, Tan CK, Paramalingam E, Wahli W. Metronidazole causes skeletal muscle atrophy and modulates muscle chronometabolism. Int J Mol Sci. 2018;19(8):2418.
Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019;317(1):E158–E71.
Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, et al. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab. 2019;316(5):E956–E66.
Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11(502):eaan5662.
Hsu YJ, Chiu CC, Li YP, Huang WC, Huang YT, Huang CC, et al. Effect of intestinal microbiota on exercise performance in mice. J Strength Cond Res. 2015;29(2):552–8.
Huang WC, Chen YH, Chuang HL, Chiu CC, Huang CC. Investigation of the effects of microbiota on exercise physiological adaption, performance, and energy utilization using a gnotobiotic animal model. Front Microbiol. 2019;10:1906.
Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. 2019;127:110722.
Jäger R, Shields K, Sharp M, Partl J, Wilson JM, Lowery RP, et al. Effects of probiotic supplementation on markers of skeletal mus-cle damage, perceived recovery and athletic performance after an intense single leg training bout. J Int Soc Sports Nutr. 2015;12(S1):P36.
Roche HM. University College Dublin (November 25, 2019- December 1, 2021) Effect of Bacillus Coagulans on skeletal muscle protein synthesis in response to vegetable protein ingestion. Identifier NCT04297111. https://clinicaltrials.gov/ct2/show/NCT04297111. Accessed on 22 Nov 2020.
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Favazzo LJ, Hendesi H, Villani DA, Soniwala S, Dar QA, Schott EM, et al. The gut microbiome-joint connection: implications in osteoarthritis. Curr Opin Rheumatol. 2020;32(1):92–101.
Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Song W, Liu Y, Dong X, Song C, Bai Y, Hu P, et al. Lactobacillus M5 prevents osteoarthritis induced by a high-fat diet in mice. J Funct Foods. 2020;72:104039.
Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr Cartil. 2016;24(10):1769–75.
Boer CG, Radjabzadeh D, Medina-Gomez C, Garmaeva S, Schiphof D, Arp P, et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10(1):4881.
Lei M, Guo C, Wang D, Zhang C, Hua L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benefic Microbes. 2017;8(5):697–703.
Lyu J-L, Wang T-M, Chen Y-H, Chang S-T, Wu M-S, Lin Y-H, et al. Oral intake of Streptococcus thermophilus improves knee osteoarthritis degeneration: A randomized, double-blind, placebo-controlled clinical study. Heliyon. 2020;6(4):e03757.
Wells P, Adebayo A, Bowyer R, Freidin M, Finckh A, Strowig T, et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 2020;2:e418–e27.
Lopez-Oliva I, Paropkari AD, Saraswat S, Serban S, Yonel Z, Sharma P, et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2018;70(7):1008–13.
Pan H, Guo R, Ju Y, Wang Q, Zhu J, Xie Y, et al. A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome. 2019;7(1):107.
Trajanoska K, Rivadeneira F. Using Mendelian Randomization to Decipher Mechanisms of Bone Disease. Curr Osteoporos Rep. 2018;16(5):531–40.
Cheng S, Qi X, Ma M, Zhang L, Cheng B, Liang C, et al. Assessing the Relationship Between Gut Microbiota and Bone Mineral Density. Front Genet. 2020;11(6). https://doi.org/10.3389/fgene.2020.00006
Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Genetics of human gut microbiome composition. 2020;15(8):e1008073.
Chin-Lee B, Curry WJ, Fetterman J, Graybill MA, Karpa K. Patient experience and use of probiotics in community-based health care settings. Patient Prefer Adherence. 2014;8:1513–20.
Nguyen M, Ferge KK, Vaughn AR, Burney W, Teng LH, Pan A, et al. Probiotic Supplementation and Food Intake and Knowledge Among Patients and Consumers. Probiotics Antimicrob Proteins. 2020;12(3):824–33.
Mukhopadhya I, Segal JP, Carding SR, Hart AL, Hold GL. The gut virome: the 'missing link' between gut bacteria and host immunity? Ther Adv Gastroenterol. 2019;12:1756284819836620.
Acknowledgments
We would like to thank Prof. Dr. Fernando Rivadeneira, Dr. Robert Kraaij, and Dr. Joyce Van Meurs for their critical comments on the manuscript.
Funding
R.L is supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860898 [FIDELIO]. L.O. is funded by an Erasmus MC fellowship grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
R. Li, C.G. Boer, and C. Medina-Gomez declare no conflict of interest. L. Oei reports personal fees from Amgen speaker fee at a conference, outside the submitted work. The other authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetics
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, R., Boer, C.G., Oei, L. et al. The Gut Microbiome: a New Frontier in Musculoskeletal Research. Curr Osteoporos Rep 19, 347–357 (2021). https://doi.org/10.1007/s11914-021-00675-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00675-x