Abstract
Purpose of Review
This review provides an update on the developments of adjuvant and neoadjuvant liver-directed and systemic therapy options for patients with resectable hepatocellular carcinoma.
Recent Findings
Data on liver-directed treatment in the adjuvant and neoadjuvant settings are sparse and results are conflicting; many studies suggest that optimizing patient selection criteria is a key milestone required to improve study design and clinical benefit to patients. Systemic treatment options are primarily focused on investigation of anti-PD-1/L1 immunotherapeutic agents, either alone or in combination with other drugs. Numerous clinical trials in both adjuvant and neoadjuvant settings are in progress.
Summary
Exploration of liver-directed and systemic treatment options for adjuvant and neoadjuvant treatment of patients with resectable hepatocellular carcinoma has the potential to improve clinical outcomes for this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
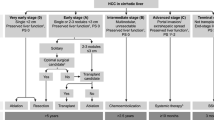
Liver cancer is the sixth-leading cancer diagnosis and the third-leading cause of cancer-related death worldwide; hepatocellular carcinoma (HCC) is the most common type of liver cancer, comprising 75–85% of cases [1]. Advances in imaging and surveillance techniques have increased the number of patients who can be diagnosed at earlier stages, allowing them to potentially receive curative treatment [2, 3]. Surgical resection, transplantation, and ablation comprise current curative treatment options for patients with HCC [2, 4]. However, the risk of recurrence after curative treatment is high, with 5-year recurrence rates ranging from 40 to 70% [5]. Furthermore, there are no FDA-approved treatments to decrease the risk of disease recurrence. Although there have been significant advances in the systemic treatment field for patients with advanced unresectable HCC [2], treatments for patients with resectable HCC are limited to physicians’ discretion or clinical trials. This gap in knowledge has resulted in a significant unmet clinical need for this patient population.
Adjuvant and neoadjuvant treatments have been utilized for many years in a variety of cancers, such as breast [6, 7], colon [8,9,10,11], and pancreas [12,13,14]. These treatments have been shown to reduce risk of disease recurrence and increase length of survival and are recommended treatment options by the National Comprehensive Cancer Network [15,16,17]. Given the promising results demonstrated for adjuvant and neoadjuvant treatment for patients with other types of resectable cancers, the role of adjuvant and neoadjuvant treatment for patients with resectable HCC is a promising field of study.
In this review we report developments in adjuvant and neoadjuvant (including perioperative) treatment for patients with resectable HCC, with a focus on liver-directed treatments and systemic treatments. We conclude with a look at ongoing clinical trials that have the potential to improve outcomes for this patient population.
Adjuvant Treatment
Liver-Directed Treatment
Liver-directed treatment is the preferred treatment modality for patients with intermediate-stage HCC or early-stage HCC for whom curative options are not feasible or unsuccessful [4]. The role of liver-directed treatment in the adjuvant setting has not been well-investigated. Most of the studies on adjuvant liver-directed treatment discussed in this review were performed in China, which may limit generalizability of their results.
The utility of adjuvant transarterial chemoembolization (TACE) was investigated by Wang et al. in a phase 3 single-center randomized controlled trial [18]. Patients with hepatitis B virus (HBV)–related HCC who underwent curative resection and were at intermediate risk (single tumor > 5 cm without microvascular invasion) or high risk (single tumor with microvascular invasion or two or three tumors) of recurrence received adjuvant TACE or no treatment (control). Median recurrence-free survival (RFS) was 49.5 months (95% confidence interval [CI] 37.2–61.8) for TACE compared to 23.8 months (95% CI 15.7–31.9) for control. Subgroup analyses confirmed a clinical benefit with adjuvant TACE across all groups, including patients with alpha fetoprotein > 20 ng/mL and patients at high risk of disease recurrence. Treatment was well-tolerated, with no adverse events (AEs) ≥ grade 3. The benefit of adjuvant TACE was further supported by Wu et al. in a retrospective propensity score matching analysis of patients who underwent hepatectomy and received adjuvant TACE or no treatment (control) [19]. Patients who received adjuvant TACE had improved 3-year disease-free survival (DFS) and overall survival (OS) compared to control patients. However, patients at low risk of disease recurrence (single tumor ≤ 5 cm with no satellite nodules or micro/macrovascular invasion) after curative resection may not benefit from adjuvant TACE [20]. A retrospective propensity score matching analysis by Feng et al. found that low-risk patients who received adjuvant TACE had reduced mean RFS (51.0 ± 4.8 months) compared to patients who did not receive adjuvant TACE (66.0 ± 2.4 months); there were no differences in OS [20]. Identification of patients likely to derive significant clinical benefit from adjuvant TACE is paramount to better refine treatment plans and avoid unnecessary treatment. To address this issue, Liang et al. developed an online calculator to predict survival benefit with or without adjuvant TACE for patients who have undergone surgical resection [21]. The calculator incorporates eight recurrence risk factors (resection margin, tumor size/number, micro/macrovascular invasion, portal hypertension, alpha fetoprotein expression, and Child–Pugh score) and provides estimates for net improvement in survival with or without adjuvant TACE. The authors emphasize that the calculator is meant to provide guidance and serve as a decision aid for treating physicians; it is not meant to provide absolute treatment recommendations. While there are promising data for the use of adjuvant TACE among patients at intermediate or high risk of recurrence, further prospective studies are needed to better investigate its role in this patient population.
Another option for adjuvant liver-directed treatment is stereotactic body radiotherapy (SBRT). Shi et al. performed a single-center randomized controlled trial among patients with BCLC stage 0 or A HCC who underwent hepatectomy with marginal resection with or without adjuvant SBRT [22]. Patients who received SBRT had improved 5-year DFS compared to patients who received surgery alone (56.1% vs 26.3%, p = 0.005). However, there was no significant difference in 5-year OS (75.0% vs 53.7%, p = 0.053). The role of adjuvant SBRT requires further exploration.
Systemic Treatment
The tyrosine kinase inhibitor (TKI) sorafenib was the standard of care first-line systemic treatment option for patients with advanced unresectable HCC from 2007 to 2018 [2]. The phase 3 randomized, double-blind, placebo-controlled STORM trial investigated adjuvant sorafenib compared to placebo in HCC patients who received curative resection or ablation with complete radiologic response at intermediate risk (single tumor > 2 cm, well- or moderately-differentiated, and no microvascular invasion or satellite tumors) or high risk (microvascular invasion, satellite tumors, poorly-differentiated, or two or three tumors (each ≤ 3 cm)) of recurrence [23]. Patients received 400 mg oral sorafenib or placebo twice daily for up to four years or until disease recurrence. Median RFS was 33.3 months (95% CI 27.6–44.0) for patients who received sorafenib and 33.7 months (95% CI 27.6–39.0) for patients who received placebo (hazard ratio [HR] 0.940, 95% CI 0.780–1.134, one-sided p = 0.26). There were no significant differences between sorafenib and placebo for time to recurrence (HR 0.891, 95% CI 0.735–1.081, one-sided p = 0.12) or OS (HR 0.995, 95% CI 0.761–1.300, one-sided p = 0.48). The authors concluded that sorafenib is not a recommended adjuvant treatment. However, Huang et al. performed a meta-analysis comprising 13 studies of 2655 patients and reported that adjuvant sorafenib may improve RFS and OS and reduce recurrence rates [24]. Of note, 11 of the 13 studies were conducted in China; the authors caution that additional studies in a broader patient population are needed to further investigate the role of adjuvant sorafenib.
Lin et al. performed a retrospective propensity score matching analysis comparing patients at high risk (tumor > 5 cm with microvascular invasion, ≥ 3 tumor nodules, ruptured HCC, or tumor thrombus in the portal vein, hepatic vein, or bile duct) of recurrence post-hepatectomy who received adjuvant TACE with or without a TKI (sorafenib, lenvatinib, or rivoceranib (apatinib)) [25]. Patients who received TACE with a TKI had improved 2-year DFS compared to patients who received TACE alone (20.9% vs 12.2%, p = 0.01). Although this study did not compare TKI plus TACE versus TKI alone, it suggests that use of TKIs in the adjuvant setting may be better in combination with other approaches.
Given the inconclusive results for single-agent tyrosine kinase inhibition, others have investigated different mechanisms of action for systemic adjuvant treatment. Sun et al. investigated the VEGFR2 inhibitor rivoceranib (apatinib) in patients who received curative-intent resection and had portal vein tumor thrombosis in an open-label, single-center phase 2 trial [26]. Patients received 500 mg oral rivoceranib daily in 28-day cycles until disease recurrence or intolerable toxicity. Among 30 patients who received rivoceranib, median RFS was 7.6 months (95% CI 5.7–9.5); 1-year OS was 93.3%. Treatment-related grade 3–4 AEs occurred in 46.7% of patients and there were no treatment-related deaths. The authors concluded that rivoceranib is tolerable, with preliminary evidence of tumor control. However, given its relatively low median RFS, current studies are investigating adjuvant rivoceranib in combination with the anti-PD-1 antibody camrelizumab (NCT05367687, NCT04639180). Camrelizumab and rivoceranib have been studied primarily in Asia; the recently published phase 3 CARES-310 study of camrelizumab plus rivoceranib versus sorafenib as first-line treatment in patients with unresectable HCC included only 17% non-Asian patients in each treatment arm [27]. Therefore, the efficacy and safety of camrelizumab and rivoceranib in a broader non-Asian patient population remains unclear.
The phase 3 randomized, open-label IMbrave050 trial compared adjuvant atezolizumab plus bevacizumab versus active surveillance in HCC patients who underwent curative resection or ablation and were at high risk (tumor > 5 cm, > 3 tumors, micro/macrovascular portal vein invasion (Vp1/Vp2), or Grade 3 or 4 tumor differentiation (poorly-differentiated)) of disease recurrence [28, 29••, 30••]. Patients received 1200 mg of intravenous atezolizumab plus 15 mg/kg body weight of bevacizumab every 21 days for up to 17 cycles/12 months of treatment or until disease recurrence or unacceptable toxicity. Independent review facility–assessed RFS was significantly longer in the atezolizumab/bevacizumab arm (not estimable, 95% CI 22.1 months–not estimable) compared to active surveillance (not estimable, 95% CI 21.4 months–not estimable) (HR 0.72, 95% CI 0.56–0.93, p = 0.012). The risk of disease recurrence was lower in the atezolizumab/bevacizumab arm compared to active surveillance (HR 0.67, 95% CI 0.52–0.88, p = 0.003), and there were no significant differences in patient-reported outcomes between treatment arms. Based on these results, atezolizumab plus bevacizumab is a promising adjuvant treatment for HCC patients at high risk of recurrence. Given the positive results with atezolizumab plus bevacizumab for patients with unresectable HCC [31, 32], it will be interesting to see if combination anti-PD-1 immunotherapy plus angiogenesis inhibition can also improve outcomes for HCC patients with resectable disease.
A novel target for HCC treatment is the transmembrane protein CD147, which may promote tumor growth and metastasis [33, 34]. Li et al. performed a phase 2 randomized controlled trial in patients with resected CD147-positive HCC [35•]. Patients received either adjuvant transarterial 131I-metuximab, a radiolabeled anti-CD147 antibody, or no adjuvant treatment (control). Recurrence-free survival at 5 years was higher for patients who received 131I-metuximab compared to control (43.4% vs 21.7%, HR 0.49, 95% CI 0.34–0.72, p < 0.0001). Treatment was well-tolerated, with just 9% of patients reporting grade 3–4 AEs. These results suggest that adjuvant 131I-metuximab for patients with CD147-positive HCC may reduce risk of recurrence. However, metuximab for treatment of unresectable HCC is only approved in China. The roles of CD147 and metuximab for HCC patients in other regions require further research.
Ongoing Trials
Table 1 shows selected clinical trials investigating adjuvant treatment options for patients with resectable HCC. A variety of adjuvant treatment options are being explored, including immunotherapy, tyrosine kinase inhibitors, and combination treatments of systemic and/or liver-directed treatments. In particular, results from the phase 3 studies CheckMate 9DX (nivolumab versus placebo, NCT03383458), KEYNOTE-937 (pembrolizumab versus placebo, NCT03867084), and EMERALD-2 (durvalumab with or without bevacizumab versus placebo, NCT03847428) are highly anticipated. These studies together will better define whether single agent anti-PD-1/L1 immunotherapy is sufficient to result in positive outcomes in resected HCC patients or if the addition of VEGF inhibition is required for demonstrable clinical benefit.
Neoadjuvant/Perioperative Treatment
Liver-Directed Treatment
The role of neoadjuvant TACE for HCC patients planned for resection or transplantation is highly controversial. A retrospective study by Lee et al. looked at patients who received neoadjuvant TACE or no treatment followed by resection; patients who received neoadjuvant TACE had similar recurrence rates (35.90% vs 29.36%, p = 0.955), reduced OS (47.05% vs 52.46%, p = 0.025), and higher rates of re-hospitalization within 6 months of surgery (33.3% vs 20.8%, p = 0.011) compared to resection-only patients [36]. Amisaki et al. reported similar results from a retrospective case–control study; patients who received neoadjuvant TACE had significantly worse RFS (p = 0.043) and OS (p = 0.014) compared to resection-only patients up to 5 years post-resection [37]. Yeh et al. found no significant difference in OS (p = 0.059) or DFS (p = 0.141) between patients who received neoadjuvant TACE followed by curative-intent resection or ablation and those who received curative-intent treatment alone [38]. Dorcaratto et al. showed that neoadjuvant TACE followed by liver transplant provided no clinical benefit in terms of 5-year DFS (70% vs 63%, p = 0.454) or OS (70% vs 65%, p = 0.532) compared to transplant alone, particularly among patients who waited less than 6 months for transplant [39]. Similar results were reported by Li et al., who found that neoadjuvant TACE prior to transplant was associated with lower median OS (51.857 vs 80.930 months) and DFS (50.386 vs 80.281 months) compared to transplant-only patients [40]. While all of these studies are retrospective and have relatively small sample sizes, the consistency between results provides support for each of the authors’ conclusions that neoadjuvant TACE does not provide clinical benefit to patients with resectable HCC and in some cases may be detrimental.
On the other hand, some studies suggest that neoadjuvant TACE may improve outcomes for patients. Notably, a phase 3 randomized trial by Fang et al. reported improvements in OS and progression-free survival for patients who received neoadjuvant TACE followed by hepatectomy compared to patients who did not receive preoperative TACE [41]. Patients received TACE at least twice; patients who recorded stable disease, partial response, or complete response to TACE per modified Response Evaluation Criteria in Solid Tumors (RECIST) proceeded to resection. Patients who received neoadjuvant TACE had significantly greater 3-year OS (HR 0.3602, 95% CI 0.1914–0.6779, p = 0.0011) and progression-free survival (HR 0.4525, 95% CI 0.2891–0.7082, p = 0.0003) compared to resection-only patients. These improvements were consistent between BCLC A and BCLC B patients. For patients listed for liver transplant, a meta-analysis by Butcher et al. of 21 retrospective studies reported no significant differences in 5-year OS (HR 1.02, 95% CI 0.80–1.31, p = 0.88) or DFS (HR 1.18, 95% CI 0.73–1.88, p = 0.50) between patients who received pre-transplant TACE and patients who received transplant only [42]. However, the authors noted that patients who received neoadjuvant TACE were more likely to have larger tumor diameters at baseline and wait longer for transplant, both of which could have negatively impacted clinical outcomes. The authors conclude that neoadjuvant TACE may be used in patients with poor prognostic features, emphasizing the importance of proper patient selection.
Others have identified potential factors that could be used to select patients who may derive benefit from neoadjuvant TACE. Mo et al. performed a multicenter retrospective study of patients who received surgical resection with or without preoperative TACE [43]. Upon multivariate analysis, they found that patients with large tumor diameters (≥ 10 cm) who received preoperative TACE had better RFS (HR 0.419, 95% CI 0.269–0.652, p < 0.005) and OS (HR 0.448, 95% CI 0.260–0.773, p = 0.004) compared to patients who did not receive preoperative TACE; among patients with smaller tumor diameters (5.0–9.9 cm) there were no differences in RFS (HR = 1.045, 95% CI 0.71–1.538, p = 0.823) or OS (HR 0.961, 95% CI 0.601–1.537, p = 0.869). Li et al. reported similar findings in a multicenter retrospective propensity score matching analysis of patients with maximum HCC tumor size ≥ 10 cm who underwent curative liver resection with or without preoperative TACE [44]. Patients who received preoperative TACE had longer median OS (32.8 vs 18.1 months, p = 0.023) and RFS (12.9 vs 4.1 months, p = 0.009) compared to resection-only patients. On multivariate analysis, preoperative TACE was a significant contributor to OS (HR 1.565, 95% CI 1.083–2.262, p = 0.017) and RFS (HR 1.550, 95% CI 1.101–2.180, p = 0.012). Optimization of patient selection for neoadjuvant liver-directed therapy is needed.
Few studies have investigated neoadjuvant TACE in combination with systemic treatment. A phase 3 randomized controlled double-blind study by Hoffmann et al. compared neoadjuvant TACE plus sorafenib or placebo in patients listed for transplantation [45]. Patients who received TACE plus sorafenib had no significant differences in time to progression (71 days vs 85 days, HR 1.106, 95% CI 0.387–3.162), progression-free survival (HR 1.259, 95% CI 0.485–3.270), objective response rate (20.8% vs 26.9%), or disease control rate (66.7% vs 73.1%) compared to patients who received TACE plus placebo. A retrospective multicenter propensity score matching analysis by Wu et al. investigated neoadjuvant TACE plus lenvatinib plus anti-PD-1 immunotherapy (sintilimab, camrelizumab, tislelizumab, pembrolizumab, or toripalimab) in patients planned for resection at high risk of disease recurrence (portal vein tumor thrombus, tumor > 10 cm with tumors close to vasculature that cause resection margin < 1 cm, and > 3 tumors with one tumor > 5 cm) [46]. They concluded that combination neoadjuvant treatment improved OS and DFS; however, comparisons between TACE plus systemic treatment versus TACE alone were not performed. It remains unclear whether TACE combined with systemic therapy provides greater benefit than either treatment modality given alone; prospective studies are needed.
Hepatic arterial infusion chemotherapy (HAIC) is another option for neoadjuvant liver-directed treatment. Oyama et al. performed a single-blind randomized controlled trial comparing patients treated with or without neoadjuvant HAIC followed by radiofrequency ablation [47]. There was no significant difference in overall RFS with neoadjuvant HAIC compared to ablation only (HR 0.597, 95% CI 0.320–1.091, p = 0.094). However, there was a significant improvement in distant RFS with neoadjuvant HAIC (HR 0.468, 95% CI 0.235–0.896, p = 0.022). A retrospective study by Pan et al. investigated the effect of neoadjuvant HAIC versus adjuvant portal vein perfusion chemotherapy on survival in patients with resectable HCC [48]. Neoadjuvant HAIC was associated with improved OS (not reached vs 19.47 months, p = 0.043), intrahepatic progression-free survival (37.57 vs 6.73 months, p = 0.049), and extrahepatic metastasis-free survival (not reached vs 7.03 months, p = 0.040) compared to adjuvant portal vein perfusion chemotherapy. Of note, HAIC is currently used and investigated only in Asia; its utility in other global regions is unknown.
Systemic Treatment
Based on promising results for immunotherapy in the treatment of patients with unresectable advanced HCC [31, 49,50,51], the use of neoadjuvant/perioperative immunotherapy for patients with resectable HCC is of great interest. Ho et al. performed a single-arm phase 1b study investigating neoadjuvant nivolumab plus cabozantinib in patients with locally advanced unresectable HCC [52•]. Patients received 40 mg oral cabozantinib daily for 8 weeks and 240 mg nivolumab intravenously once every 2 weeks (starting 2 weeks after initiation of cabozantinib) for 4 doses; they were restaged 2 weeks after the end of therapy and those patients deemed eligible for surgery were scheduled for surgery at least 28 days after the last dose of cabozantinib. Of 15 patients enrolled in the study, 14 completed therapy and were evaluated for surgical resection; 12 patients underwent successful surgical resection. At the pre-surgical evaluation, 13 of 14 patients had stable disease per RECIST 1.1 and 1 patient had partial response. Upon surgery, 5 of 12 patients had a complete or major (≥ 90% tumor necrosis) pathological response. The study met its primary endpoint of feasibility and supported further research of neoadjuvant systemic treatment for patients with resectable HCC.
The role of perioperative systemic treatment for patients with resectable disease was explored by Kaseb et al. in a single-center, randomized, open-label phase 2 trial [53•]. Patients received 3 doses of 240 mg intravenous nivolumab with or without 1 dose of 1 mg/kg intravenous ipilimumab before partial hepatectomy, followed by 480 mg nivolumab every 4 weeks with or without up to 4 doses of 1 mg/kg ipilimumab every 6 weeks for up to 2 years after surgery or until disease progression or intolerable toxicity. At completion of neoadjuvant treatment, 3 of 13 patients who received single-agent nivolumab had a partial response per RECIST 1.1; there were no objective responses among the 14 patients who received nivolumab plus ipilimumab. After surgical resection, median time to progression was 9.40 months for patients on the nivolumab arm and 19.53 months for patients on the nivolumab/ipilimumab arm (HR 0.89, 95% CI 0.31–2.54, p = 0.83). The incidence of grade 3–4 treatment-related AEs was higher in the combination treatment arm (6 of 14 patients vs 3 of 13 patients); however, AEs did not contribute to any delays in surgery. Three patients in each arm had a major pathological response to neoadjuvant treatment; none of these patients had developed recurrence after a median follow-up of 26.8 months. The authors concluded that perioperative immunotherapy is safe and tolerable among patients with resectable HCC and propose that future studies investigate the efficacy of perioperative nivolumab with or without ipilimumab.
Marron et al. further investigated perioperative single-agent immunotherapy in a single-arm, open-label phase 2 study in patients with resectable HCC using the anti-PD-1 antibody cemiplimab [54•]. Patients received 350 mg intravenous cemiplimab every 3 weeks for 2 cycles, followed by resection and 8 cycles of 350 mg cemiplimab every 3 weeks; the adjuvant section of the trial is not yet complete. Of 21 patients who received neoadjuvant cemiplimab, 20 proceeded to surgical resection; 15% of resected patients had a partial response per RECIST 1.1, with the rest reporting stable disease. Analysis of tumor necrosis at surgery found that 7 patients had tumor necrosis ≥ 50%, including 3 patients with 100% tumor necrosis. There were two grade 3 treatment-related AEs and no grade 4 or 5 AEs. The authors note that their duration of neoadjuvant treatment was shorter than that of Kaseb et al. [53•], which may have contributed to the lower AE incidence and higher number of patients who proceeded to surgery. They conclude that further investigation is needed to identify optimal treatment regimens and trial designs for this new treatment arena.
Combination camrelizumab plus rivoceranib (apatinib) was shown to be superior to sorafenib in terms of improving progression-free survival and OS in the first-line setting for patients with unresectable HCC [27]. This combination was explored by Xia et al. in the perioperative setting [55•]. In this single-arm, open-label phase 2 study patients with resectable HCC received 200 mg intravenous camrelizumab every 2 weeks for 3 cycles plus 250 mg oral rivoceranib (apatinib) for 21 days (starting with the first dose of camrelizumab). Surgical resection occurred 46 days after initiation of camrelizumab. Within 4 weeks post-surgery, patients received 200 mg camrelizumab every 3 weeks plus 250 mg rivoceranib daily for up to 8 cycles or until disease progression or intolerable toxicity. Of 18 patients who received neoadjuvant treatment, 17 received surgery and 13 received adjuvant treatment. Three of 18 patients recorded partial response to neoadjuvant treatment per RECIST 1.1 (16.7% objective response rate) and 14 patients had stable disease (94.4% disease control rate). After surgery, 3 of 17 patients had a major pathologic response to neoadjuvant treatment (17.6%); 1 patient had a complete pathological response (5.9%). Grade ≥ 3 treatment-related AEs occurred in 3 of 18 patients in the neoadjuvant setting and in 5 of 13 patients in the adjuvant setting; no grade 5 events occurred in the perioperative period. At 1 year post-surgery, RFS was 53.85% (95% CI 24.77–75.99). The authors encourage future studies to consider the selection of patients for neoadjuvant treatment based on the extent of disease at baseline and the selection of patients for adjuvant treatment based on pathological response to neoadjuvant treatment. An additional issue to consider is the length and timing of neoadjuvant treatment and surgery; the authors waited 3 weeks after completion of neoadjuvant rivoceranib before surgery but found that surgical resection was challenging and the rate of biliary leakage post-resection was high (3 of 17 patients). The role of tyrosine kinase inhibition in the neoadjuvant setting may require adjustments to improve the surgical experience and outcomes.
Ongoing Trials
Table 2 shows selected clinical trials investigating neoadjuvant and perioperative treatment options for patients with resectable HCC. Of note, some studies are specifically focusing on neoadjuvant treatment for patients listed for liver transplant. A phase 2 study is investigating neoadjuvant durvalumab plus tremelimumab (NCT05027425) and a phase 3 study is investigating neoadjuvant donafenib plus TACE followed by adjuvant donafenib (NCT05576909). Other studies of interest include the recently-launched phase 1b/2 Morpheus-NEO HCC trial (neoadjuvant atezolizumab plus bevacizumab versus atezolizumab plus bevacizumab plus tiragolumab versus bevacizumab plus tobemstomig, NCT05908786), the phase 2 PRIME-HCC study (neoadjuvant nivolumab plus ipilimumab, NCT03682276), and numerous studies investigating neoadjuvant/perioperative camrelizumab in combination with rivoceranib and/or TACE (NCT04930315, NCT04701060, NCT05613478, NCT04521153).
Conclusions
The exploration of adjuvant and neoadjuvant treatment for patients with resectable HCC is a rapidly growing field of study. Expansion of liver-directed and systemic treatment options has the potential to provide significant clinical benefit and improve outcomes for this patient population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. https://doi.org/10.1016/s0140-6736(22)01200-4.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y.
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681–93. https://doi.org/10.1016/j.jhep.2021.11.018.
Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14(3):699–713. https://doi.org/10.1007/s12328-021-01394-7.
Haussmann J, Corradini S, Nestle-Kraemling C, Bölke E, Njanang FJD, Tamaskovics B, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol. 2020;15(1):71. https://doi.org/10.1186/s13014-020-01501-x.
John P, Osani MC, Kodali A, Buchsbaum R, Bannuru RR, Erban JK. Comparative Effectiveness of Adjuvant Chemotherapy in Early-Stage Breast Cancer: A Network Meta-analysis. Clin Breast Cancer. 2021;21(1):e22–37. https://doi.org/10.1016/j.clbc.2020.07.005.
Gelibter AJ, Caponnetto S, Urbano F, Emiliani A, Scagnoli S, Sirgiovanni G, et al. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg Oncol. 2019;30:100–7. https://doi.org/10.1016/j.suronc.2019.06.003.
Fabregas JC, Ramnaraign B, George TJ. Clinical Updates for Colon Cancer Care in 2022. Clin Colorectal Cancer. 2022;21(3):198–203. https://doi.org/10.1016/j.clcc.2022.05.006.
Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36(10):2063–70. https://doi.org/10.1007/s00384-021-03945-3.
Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41(8):1541–52. https://doi.org/10.1200/jco.22.00046.
Tempero MA. NCCN Guidelines Updates: Pancreatic Cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–5. https://doi.org/10.6004/jnccn.2019.5007
Conroy T, Lambert A, Ducreux M. Adjuvant and neoadjuvant approaches in pancreatic cancer. Curr Opin Oncol. 2023;35(4):326–33. https://doi.org/10.1097/cco.0000000000000962.
Park W, Chawla A, O’Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326(9):851–62. https://doi.org/10.1001/jama.2021.13027.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. https://doi.org/10.6004/jnccn.2022.0030
Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen Y-J, Ciombor KK, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2021;19(3):329–59. https://doi.org/10.6004/jnccn.2021.0012
Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19(4):439–57. https://doi.org/10.6004/jnccn.2021.0017
Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018;24(9):2074–81. https://doi.org/10.1158/1078-0432.Ccr-17-2899.
Wu Z, Cui L, Qian J, Luo L, Tu S, Cheng F, et al. Efficacy of adjuvant TACE on the prognosis of patients with HCC after hepatectomy: a multicenter propensity score matching from China. BMC Cancer. 2023;23(1):325. https://doi.org/10.1186/s12885-023-10802-9.
Feng LH, Zhu YY, Zhou JM, Wang M, Xu WQ, Zhang T, et al. Adjuvant TACE may not improve recurrence-free or overall survival in HCC patients with low risk of recurrence after hepatectomy. Front Oncol. 2023;13:1104492. https://doi.org/10.3389/fonc.2023.1104492.
Liang L, Li C, Wang MD, Wang H, Zhou YH, Zeng YY, et al. Development and validation of a novel online calculator for estimating survival benefit of adjuvant transcatheter arterial chemoembolization in patients undergoing surgery for hepatocellular carcinoma. J Hematol Oncol. 2021;14(1):165. https://doi.org/10.1186/s13045-021-01180-5.
Shi C, Li Y, Geng L, Shen W, Sui C, Dai B, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: A randomised controlled trial. Eur J Cancer. 2022;166:176–84. https://doi.org/10.1016/j.ejca.2022.02.012.
Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–54. https://doi.org/10.1016/S1470-2045(15)00198-9.
Huang S, Li D, Zhuang L, Sun L, Wu J. A meta-analysis of the efficacy and safety of adjuvant sorafenib for hepatocellular carcinoma after resection. World J Surg Oncol. 2021;19(1):168. https://doi.org/10.1186/s12957-021-02280-9.
Lin K, Wei F, Huang Q, Lai Z, Zhang J, Chen Q, et al. Postoperative Adjuvant Transarterial Chemoembolization Plus Tyrosine Kinase Inhibitor for Hepatocellular Carcinoma: a Multicentre Retrospective Study. J Hepatocell Carcinoma. 2022;9:127–40. https://doi.org/10.2147/jhc.S352480.
Sun HC, Zhu XD, Zhou J, Gao Q, Shi YH, Ding ZB, et al. Adjuvant apatinib treatment after resection of hepatocellular carcinoma with portal vein tumor thrombosis: a phase II trial. Ann Transl Med. 2020;8(20):1301. https://doi.org/10.21037/atm-20-6181.
Qin S, Chan SL, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023. https://doi.org/10.1016/S0140-6736(23)00961-3.
Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16(15):975–89. https://doi.org/10.2217/fon-2020-0162.
••Chow P, Chen M, Cheng A-L, Kaseb AO, Kudo M, Lee HC, et al. Abstract CT003: IMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Cancer Res. 2023;83(8_Supplement):CT003-CT. https://doi.org/10.1158/1538-7445.AM2023-CT003. (This phase 3 randomized study demonstrated that adjuvant atezolizumab plus bevacizumab significantly improved recurrence-free survival compared to active surveillance in patients at high risk of HCC recurrence after curative-intent resection or ablation.)
••Kudo M, Chen M, Chow PKH, Kaseb AO, Lee HC, Yopp AC, et al. Efficacy, safety and patient reported outcomes (PROs) from the phase III IMbrave050 trial of adjuvant atezolizumab (atezo) + bevacizumab (bev) vs active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. J Clin Oncol. 2023;41(16_suppl):4002-. https://doi.org/10.1200/JCO.2023.41.16_suppl.4002. (Additional results from IMbrave050 showed no significant differences in patient-reported outcomes between patients who received adjuvant atezolizumab plus bevacizumab and those who received active surveillance.)
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–905. https://doi.org/10.1056/NEJMoa1915745.
Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. https://doi.org/10.1016/j.jhep.2021.11.030.
Huang D, Rao D, Jin Q, Lai M, Zhang J, Lai Z, et al. Role of CD147 in the development and diagnosis of hepatocellular carcinoma. Front Immunol. 2023;14. https://doi.org/10.3389/fimmu.2023.1149931
Tseng H-c, Xiong W, Badeti S, Yang Y, Ma M, Liu T, et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat Commun. 2020;11(1):4810. https://doi.org/10.1038/s41467-020-18444-2
•Li J, Xing J, Yang Y, Liu J, Wang W, Xia Y, et al. Adjuvant (131)I-metuximab for hepatocellular carcinoma after liver resection: a randomised, controlled, multicentre, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(6):548–60. https://doi.org/10.1016/S2468-1253(19)30422-4. (This phase 2 study reported promising results for CD147 inhibition as an adjuvant treatment of resectable HCC; this novel target requires further investigation in a broader patient population.)
Lee KT, Lu YW, Wang SN, Chen HY, Chuang SC, Chang WT, et al. The effect of preoperative transarterial chemoembolization of resectable hepatocellular carcinoma on clinical and economic outcomes. J Surg Oncol. 2009;99(6):343–50. https://doi.org/10.1002/jso.21248.
Amisaki M, Honjo S, Morimoto M, Hanaki T, Arai Y, Tokuyasu N, et al. The Negative Effect of Preoperative Transcatheter Arterial Chemoembolization on Long-Term Outcomes for Resectable Hepatocellular Carcinoma: A Propensity Score Matching Analysis. Yonago Acta Med. 2016;59(4):270–8.
Yeh ML, Huang CI, Huang CF, Hsieh MY, Huang JF, Dai CY, et al. Neoadjuvant transcatheter arterial chemoembolization does not provide survival benefit compared to curative therapy alone in single hepatocellular carcinoma. Kaohsiung J Med Sci. 2015;31(2):77–82. https://doi.org/10.1016/j.kjms.2014.11.003.
Dorcaratto D, Udupa V, Hogan NM, Brophy DP, McCann JW, Maguire D, et al. Does neoadjuvant doxorubicin drug-eluting bead transarterial chemoembolization improve survival in patients undergoing liver transplant for hepatocellular carcinoma? Diagn Interv Radiol. 2017;23(6):441–7. https://doi.org/10.5152/dir.2017.17106.
Li HL, Ji WB, Zhao R, Duan WD, Chen YW, Wang XQ, et al. Poor prognosis for hepatocellular carcinoma with transarterial chemoembolization pre-transplantation: retrospective analysis. World J Gastroenterol. 2015;21(12):3599–606. https://doi.org/10.3748/wjg.v21.i12.3599.
Fang C, Luo R, Zhang Y, Wang J, Feng K, Liu S, et al. Hepatectomy versus transcatheter arterial chemoembolization for resectable BCLC stage A/B hepatocellular carcinoma beyond Milan criteria: A randomized clinical trial. Front Oncol. 2023;13:1101162. https://doi.org/10.3389/fonc.2023.1101162.
Butcher DA, Brandis KJ, Wang H, Spannenburg L, Bridle KR, Crawford DH, et al. Long-term survival and postoperative complications of pre-liver transplantation transarterial chemoembolisation in hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Surg Oncol. 2022;48(3):621–31. https://doi.org/10.1016/j.ejso.2021.09.017.
Mo A, Zhang Q, Xia F, Huang Z, Peng S, Cao W, et al. Preoperative transcatheter arterial chemoembolization and prognosis of patients with solitary large hepatocellular carcinomas (≥5 cm): Multicenter retrospective study. Cancer Med. 2023;12(7):7734–47. https://doi.org/10.1002/cam4.5529.
Li C, Wang MD, Lu L, Wu H, Yu JJ, Zhang WG, et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥ 10 cm): a multicenter propensity matching analysis. Hepatol Int. 2019;13(6):736–47. https://doi.org/10.1007/s12072-019-09981-0.
Hoffmann K, Ganten T, Gotthardtp D, Radeleff B, Settmacher U, Kollmar O, et al. Impact of neo-adjuvant Sorafenib treatment on liver transplantation in HCC patients - a prospective, randomized, double-blind, phase III trial. BMC Cancer. 2015;15:392. https://doi.org/10.1186/s12885-015-1373-z.
Wu JY, Wu JY, Li YN, Qiu FN, Zhou SQ, Yin ZY, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for neoadjuvant treatment of resectable hepatocellular carcinoma with high risk of recurrence: A multicenter retrospective study. Front Oncol. 2022;12:985380. https://doi.org/10.3389/fonc.2022.985380
Oyama A, Nouso K, Yoshimura K, Morimoto Y, Nakamura S, Onishi H, et al. Randomized controlled study to examine the efficacy of hepatic arterial infusion chemotherapy with cisplatin before radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2021;51(6):694–701. https://doi.org/10.1111/hepr.13633.
Pan Y, Mei J, Chen J, Zhang D, Wang J, Wang X, et al. Comparison Between Portal Vein Perfusion Chemotherapy and Neoadjuvant Hepatic Arterial Infusion Chemotherapy for Resectable Intermediate to Advanced Stage Hepatocellular Carcinoma. Ann Surg Oncol. 2022;29(3):2016–29. https://doi.org/10.1245/s10434-021-10903-4.
Abou-Alfa Ghassan K, Lau G, Kudo M, Chan Stephen L, Kelley Robin K, Furuse J, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. https://doi.org/10.1056/EVIDoa2100070
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38(3):193–202. https://doi.org/10.1200/JCO.19.01307.
Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6(11):e204564. https://doi.org/10.1001/jamaoncol.2020.4564
•Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat Cancer. 2021;2(9):891–903. https://doi.org/10.1038/s43018-021-00234-4. (This phase 1b study was the first to report positive results for neoadjuvant combination anti-PD-1 immunotherapy plus tyrosine kinase inhibition for resectable HCC.)
•Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey J-N, Lee SS, et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(3):208–18. https://doi.org/10.1016/S2468-1253(21)00427-1 (This phase 2 study reported that perioperative immunotherapy is safe and tolerable among patients with resectable HCC and proposed that further studies are needed to investigate the efficacy of single-agent versus combination immunotherapy in this patient population.)
•Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(3):219–29. https://doi.org/10.1016/S2468-1253(21)00385-X. (This single-arm phase 2 trial suggests that single-agent anti-PD-1 immunotherapy in the neoadjuvant setting may be sufficient to promote significant tumor necrosis; this trial includes an adjuvant phase of cemiplimab treatment which is not yet complete.)
•Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022;10(4). https://doi.org/10.1136/jitc-2022-004656. (This phase 2 study demonstrated preliminary safety and efficacy for perioperative combination anti-PD-1 immunotherapy plus VEGF inhibition; however, it raises questions about the role of neoadjuvant tyrosine kinase inhibition and its impact on surgical resection.)
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Contributions
Christiana Crook and Daneng Li conceptualized the article. Christiana Crook performed the literature search. Christiana Crook and Daneng Li drafted and critically revised the article.
Corresponding author
Ethics declarations
Competing Interests
Daneng Li reports research funding to his institution from AstraZeneca and Brooklyn ImmunoTherapeutics. He serves as a consultant and has received honoraria from Adagene, AstraZeneca, Coherus, Delcath, Eisai, Exelixis, Genentech, Ipsen Biopharmaceuticals, Merck, Servier, Sumitomo, and TerSera Therapeutics, all outside the submitted work. Christiana Crook has no competing interests to declare.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crook, C.J., Li, D. Adjuvant and Neoadjuvant Treatments for Resectable Hepatocellular Carcinoma. Curr Oncol Rep 25, 1191–1201 (2023). https://doi.org/10.1007/s11912-023-01455-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01455-9