Abstract
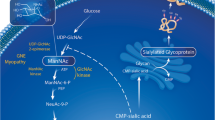
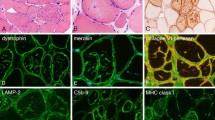
Distal myopathy with rimmed vacuoles (DMRV) and hereditary inclusion body myopathy (hIBM) share similar clinical features, including onset in young adulthood with preferential involvement of the anterior compartment of the lower legs and sparing of the quadriceps femoris muscles. The most significant muscle pathology is the presence of rimmed vacuoles, which appear to play a major role in muscle atrophy and weakness. After the discovery of the gene locus in both DMRV and hIBM on chromosome 9 and mutations in the gene encoding the enzyme UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE), it became clear that they are allelic disorders. From gene analysis, it is evident that these diseases are not restricted to people of Japanese and Jewish ancestry, but that they are widely distributed throughout all ethnic groups. Although defective glycosylation to a muscle fiber has been suggested, the mechanism by which myofibrillar degeneration is followed by rimmed vacuole formation remains to be clarified.
Similar content being viewed by others
References and Recommended Reading
Nonaka I, Sunohara N, Ishiura S, et al.: Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci 1981, 51:141–155.
Kumamoto T, Fukuhara N, Nagashima M, et al.: Distal myopathy. Histochemical and ultrastructural study. Arch Neurol 1982, 39:367–371.
Nonaka I, Murakami N, Suzuki Y, et al.: Distal myopathy with rimmed vacuoles. Neuromusc Disord 1998, 8:333–337.
Nonaka I: Distal myopathies. Curr Opin Neurol 1999, 12:493–499.
Argov Z, Yarom R: ‘Rimmed vacuole myopathyrs sparing quadriceps. A unique disorder in Iranian Jews. J Neurol Sci 1984, 64:33–43.
Askanas V, Engel WK: New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusionbody myopathies. Curr Opin Rheumatol 1995, 7:486–496.
Askanas V, Engel WK: Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol 1998, 10:543–547.
Sunohara N, Nonaka I, Kamei N, et al.: Distal myopathy with rimmed vacuole formation. A follow-up study. Brain 1989, 112:65–83.
Mitrani-Rosenbaum S, Argov Z, Blumenfeld A, et al.: Hereditary inclusion body myopathy maps to chromosome 9p1-q1. Hum Mol Genet 1996, 5:159–163.
Ikeuchi T, Asaka T, Saito M, et al.: Gene locus for autosomal recessive distal myopathy with rimmed vacuoles maps to chromosome 9. Ann Neurol 1997, 41:432–437.
Eisenberg I, Avidan N, Potikha T, et al.: The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive inclusion body myopathy. Nat Genet 2001, 29:83–87.
Seppala R, Lehto VP, Gahl WA: Mutations in the human UDPN-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet 1999, 64:1563–1569.
Leroy JG, Seppala R, Huizing M, et al.: Dominant inheritance of sialuria, an inborn error of feedback inhibition. Am J Hum Genet 2001, 68:1419–1427.
Kayashima T, Matsuo H, Satoh A, et al.: Nonaka myopathy is caused by mutations in the UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase gene. J Hum Genet 2002, 47:77–79.
Tomimitsu H, Ishikawa K, Shimizu J, et al.: Distal myopathy with rimmed vacuoles: novel mutations in the GNE gene. Neurology 2002, 13:451–454.
Arai A, Tanaka K, Ikeuchi T, et al.: A novel mutation in the GNE gene and a linkage disequilibrium in Japanese pedigrees. Ann Neurol 2002, 52:516–519. All six Japanese DMRV pedigrees analyzed had a homozygous V572L mutation with a strong linkage disequilibrium, suggesting a strong founder effect in Japanese DMRV pedigree.
Nishino I, Noguchi S, Murayama K, et al.: Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology 2002, 59:1689–1693. Twenty-seven of 34 patients with the clinical characteristics of DMRV had mutations in the GNE gene. A mutation of V572L accounted for 61%. The UDP-N-acetylglucosamine 2-epimerase activity in leukocytes was significantly decreased, suggesting that the loss-of-function mutation in GNE gene is responsible for inducing the disease state.
Tomimitsu H, Shimizu J, Ishikawa K, et al.: Distal myopathy with rimmed vacuoles (DMRV): new GNE mutations and splice variant. Neurology 2004, 62:1607–1610. In an examination of GNE gene mutation in patients who had no typical DMRV symptoms, one had predominantly proximal muscle involvement. Patients with the common V572L mutations had the typical clinical features of DMRV as compared with those without the common mutation.
Eisenberg I, Grabov-Nardini G, Hochner H, et al.: Mutations spectrum of GNE in hereditary inclusion body myopathy sparing the quadriceps. Hum Mutat 2003, 21:99. Patients with hIBM with mutations in the GNE gene are found throughout the world, even in people of non-Jewish ancestry.
Argov Z, Eisenberg I, Grabov-Nardini G, et al.: Hereditary inclusion body myopathy: the Middle Eastern genetic cluster. Neurology 2003, 60:1519–1523.
Broccolini A, Ricci E, Cassandrini D, et al.: Novel GNE mutations in Italian families with autosomal recessive hereditary inclusion-body myopathy. Hum Mutat 2004, 23:632.
Yabe I, Higashi T, Kikuchi S, et al.: GNE mutations causing distal myopathy with rimmed vacuoles with inflammation. Neurology 2003, 61:384–386.
Krause S, Schlotter-Weigel B, Walter MC, et al.: A novel homozygous missense mutation in the GNE gene of a patient with quadriceps-sparing hereditary inclusion body myopathy associated with muscle inflammation. Neuromusc Disord 2003, 13:830–834.
Schwarzkopf M, Knobeloch KP, Rohde E, et al.: Sialylation is essential for early development of in mice. Proc Natl Acad Sci U S A 2002, 99:5267–5270.
Hinderlich S, Salama I, Eisenberg I, et al.: The homozygous M712T mutation of UDP-N-acetylglucosamine 2-epimerase/ N-acetylmannosamine kinase results in reduced enzyme activities but not in altered overall cellular sialylation in hereditary inclusion body myopathy. FEBS Lett 2004, 566:105–109. Although UDP-N-acetylglucosamine 2-epimerase activity was reduced in B lymphoblastoid cell lines from patients, cellular sialylation was not altered by colorimetric assays and lectin analysis.
Noguchi S, Keira Y, Murayama K, et al.: Reduction of UDP-Nacetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem 2004, 279:11402–11407. Sialic acid levels were reduced to 60% to 75% of control in muscle and cultured cells from DMRV patients. Addition of ManNAc and NeuAc to primary cultured cells normalized sialylation levels, suggesting a possible therapy.
Saito F, Tomimatsu H, Arai K, et al.: A Japanese patient with distal myopathy with rimmed vacuoles: missense mutations in the epimerase domain of the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene accompanied by hyposialylation of skeletal muscle glycoproteins. Neuromusc Disord 2004, 14:158–161.
Huizing M, Rakocevic G, Sparks SE, et al.: Hypoglycosylation of _-dystroglycan in patients with hereditary IBM due to GNE mutations. Mol Genet Metab 2004, 81:196–202.
Ii K, Hizawa K, Nonaka I, et al.: Abnormal increases of lysosomal cysteine proteinases in rimmed vacuoles in the skeletal muscle. Am J Pathol 1986, 122:193–198.
Nishino I: Autophagic vacuolar myopathies. Curr Neurol Neurosci Rep 2003, 3:64–69.
Kumamoto T, Fujimoto S, Nagao S, et al.: Proteasomes in distal myopathy with rimmed vacuoles. Intern Med 1998, 37:746–752.
Fukuhara N, Kumamoto T, Tsubaki T, et al.: Rimmed vacuoles. Acta Neruopathol 1980, 32:229–235.
Murakami N, Ihara Y, Nonaka I, et al.: Muscle fiber degeneration in distal myopathy with rimmed vacuole formation. Acta Neuropathol 1995, 89:29–34.
Yan C, Ikezoe K, Nonaka I: Apoptotic muscle fiber degeneration in distal myopathy with rimmed vacuoles. Acta Neuropathol 2001, 101:9–16.
Watts GD, Wymer J, Kovach MJ, et al.: Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 2004, 36:377–381. An unusual form of autosomal dominantly inherited hIBM with Paget disease and dementia had mutations in valosin-containing protein (VCP). Because dysfunction of the VCP caused cytoplasmic vacuole and inclusion body formation, identification of the VCP as causing hIBM has implications for understanding rimmed vacuole formation and pathologic ubiquitin-based pathway.
Hirabayashi M, Inoue M, Tanaka K, et al.: VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ 2001, 8:977–984.
Mizuno Y, Hori S, Kakizuka A, et al.: Vacuole-creating protein in neurodegenerative diseases in humans. Neurosci Lett 2003, 34:77–80.
Snow DM, Hart GW: Nuclear and cytoplasmic glycosylation. Int Rev Cytol 1998, 181:43–74.
Wells L, Vosseller K, Hart GW: Glycosylation of nucleoplasmic proteins: Signal transduction and O-GlcNAc. Science 2001, 291:2376–2378.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nonaka, I., Noguchi, S. & Nishino, I. Distal myopathy with rimmed vacuoles and hereditary inclusion body myopathy. Curr Neurol Neurosci Rep 5, 61–65 (2005). https://doi.org/10.1007/s11910-005-0025-0
Issue Date:
DOI: https://doi.org/10.1007/s11910-005-0025-0