Abstract
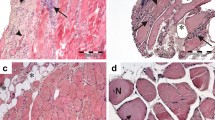
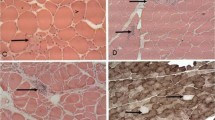
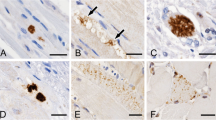
In 11 patients with distal myopathy with rimmed vacuole formation (DMRV), a well-known autosomal recessively inherited disorder, the rimmed vacuole formation appears to be the main pathological change accounting for the progressive muscle fiber degeneration. To gain a better understanding of the pathophysiology of the vacuole formation, we applied Congo red and immunohistochemical stains to muscle biopsies from these patients and the results were compared with those of patients with inclusion body myositis (IBM). The vacuoles in DMRV contained Congophilic amyloid material and deposits immunoreactive for β-amyloid protein, both the NH2 and COOH termini of β-amyloid protein precursor, ubiquitin, and tau protein. These results were similar to those seen in our present cases of IBM as well as in previously reported cases. Therefore, there may be no pathogenetic differences in the formation of rimmed vacuoles in DMRV and IBM. Nevertheless, the degenerative process involved in rimmed vacuole formation in various diseases may share a common pathogenetic mechanism with that in amyloid-plaque formation in Alzheimer's disease brain as has been proposed previously.
Similar content being viewed by others
References
Askanas V, Engel WK (1993) New advances in inclusion-body myositis. Curr Opin Rheumatol 5: 732–741
Askanas V, Serdaroglu P, Engel WK, Alvarez RB (1991) Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci Lett 130: 73–76
Askanas V, Engel WK, Alvarez RB (1992) Light and electron microscopic localization of β-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol 141: 31–36
Askanas V, Alvarez RB, Engel WK (1993) β-amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol 34: 551–560
Askanas V, Bilak M, Engel WK, Alvarez RB, Tomé F, Leclerc A (1993) Prion protein is abnormally accumulated in inclusionbody myositis. Neuroreport 5: 25–28
Askanas V, Engel WK, Alvarez RB (1993) Enhanced detection of Congo-red-positive amyloid deposits in muscle fibers of inclusion body myositis and brain of Alzheimer's disease using fluorescence technique. Neurology 43: 1265–1267
Askanas V, Mirabella M, Engel WK, Alvarez RB, Weisgraber KH (1994) Apolipoprotein E immunoreactive deposits in inclusion-body muscle diseases. Lancet 343: 364–365
Askanas V, Engel WK, Bilak M, alvarez RB, Selkoe DJ (1994) Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol 144: 177–187
Borg K, Tomé FMS, Edström L (1991) Intranuclear and cytoplasmic filamentous inclusions in distal myopathy (Welander). Acta Neuropathol 82: 102–106
Carpenter S, Karpati G, Heller I, Eisen A (1978) Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology 28: 8–17
Dieler R, Schröder JM (1990) Lacunar dilatations of intrafusal and extrafusal terminal cisternae, annulate lamellae, confronting cisternae and tubulofilamentous inclusions within the spectrum of muscle and nerve fiber changes in myotonic dystrophy. Pathol Res Pract 186: 371–382
Eisen A, Berry K, Gibson G (1983) Inclusion body myositis (IBM): myopathy or neuropathy? Neurology 33: 1109–1114
Golde TE, Estus S, Younkin HY, Selkoe DJ, Younkin SG (1992) Processing of the amyloid protein precursor to potentially amyloidgenic derivatives. Science 255: 728–730
Haass C, Selkoe DJ (1993) Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 75: 1039–1042
Haass C, Koo E, Mellon A, Hung AY, Selkoe DJ (1992) Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357: 500–503
Ihara Y (1988) Massive somatodendritic sprouting of the cortical neurons in Alzheimer's disease. Brain Res 459: 138–144
Ii K, Hizawa K, Nonaka I, Sugita H, Kominami E, Katunuma N (1986) Abnormal increases of lysosomal cysteinine proteinases in rimmed vacuoles in the skeletal muscle. Am J Pathol 122: 193–198
Leclerc A, Tomé FMS, Fardeau M (1993) Ubiquitin and β-amyloid-protein in inclusion body myositis (IBM), familial IBM-like disorder and oculopharyngeal muscular dystrophy: an immunocytochemical study. Neuromusc Disord 3: 283–291
Lindberg C, Borg K, Edström L, Hedström A, Oldfors A (1991) Inclusion body myositis and Welander distal myopathy: a clinical, neurophysiological and morphological comparison. J Neurol Sci 1991: 76–81
Lotz BP, Engel AG, Nishino H, Stevens JC, Lichy WJ (1989) Inclusion body myositis. Brain 112: 724–747
Massa R, Weller B, Karpati G, Shoubridge E, Carpenter S (1991) Familial inclusion body myositis among Kurdish-Iranian Jews. Arch Neurol 48: 519–522
Matsubara S, Tanabe H (1982) Hereditary distal myopathy with filamentous inclusion. Acta Neurol Scand 65: 363–368
Mendell JR, Sahenk Z, Gales T, Paul L (1991) Amyloid filaments in inclusion body myositis: novel findings provide insight into nature of filaments. Arch Neurol 48: 1229–1234
Neville HE, Baumbach LL, Ringel SP, Russo LS Jr, Sujansky E, Garcia CA (1992) Familial inclusion body myositis: evidence for autosomal dominant inheritance. Neurology 42: 897–902
Nonaka I, Sunohara N, Ishiura S, Satoyoshi E (1981) Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci 51: 141–155
Nonaka I, Sunohara N, Satoyoshi E, Terasawa K, Yonemoto K (1985) Autosomal recessive distal muscular dystrophy: a comparative study with distal myopathy with rimmed vacuole formation. Ann Neurol 17: 51–59
Satoyoshi E, Murakami N, Takemitsu M, Nonaka I (1993) Significance of “rimmed” vacuoles in various neuromuscular disorders. In: Serratrice G (ed) Nervous system muscles and systemic diseases. Expansion Scientifique Française, Paris, pp 83–92
Sunohara N, Nonaka I, Kamei N, Satoyoshi E (1989) Distal myopathy with rimmed vacuole formation: a follow-up study. Brain 112: 65–83
Takio K, Hasegawa M, Titani K, Ihara Y, Ihara Y (1989) Identification of β protein precursor in newborn rat brain. Biochem Biophys Res Commun 160: 1296–1301
Yunis EJ, Samaha FJ (1971) Inclusion body myositis. Lab Invest 25: 240–248
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murakami, N., Ihara, Y. & Nonaka, I. Muscle fiber degeneration in distal myopathy with rimmed vacuole formation. Acta Neuropathol 89, 29–34 (1995). https://doi.org/10.1007/BF00294256
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294256