Abstract
Purpose of Review
In this narrative review encompassing relevant scientific publications regarding critically ill patients in the last 5 years, we discuss key questions regarding the concept, pathophysiology, identification, epidemiology, and implications of augmented renal clearance (ARC) in the treatment of sepsis.
Recent Findings
Mathematical estimates of renal function show low accuracy when evaluating renal function in the intensive care unit, jeopardizing the correct dosing of antimicrobials. The description of ARC in critically ill patients in several, distant geographical areas worldwide reveals that this condition is more frequent than anticipated. Several new risk factors have been recently reported, needing future confirmation. Pathophysiology is still largely unknown; however, intact kidney physiology, inflammatory mediators, and tubular secretion seem to play a role. Several studies have demonstrated the association between ARC and subtherapeutic levels of several β-lactams, vancomycin, and fluconazole. Lately, there have been recommendations of dosage regimen adjustments for patients with ARC, namely, through increases in total daily dose or prolonged infusion for various antimicrobials. Literature is scarce describing the influence of ARC on clinical outcomes of patients receiving antibiotics, and results are contradictory.
Summary
Growing body of evidence supports that measured creatinine clearance based on time-defined urine output is strongly recommended for the identification of ARC and for reliable evaluation of its prevalence and risk factors. Clinicians should be alert for the need to use off-label dosing of antimicrobials in septic patients showing ARC. Concise recommendations for antibiotic dosage regimens, based on clinical data, are still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When a patient is critically ill, kidney function can be significantly altered, leading to profound physiological and clinical alterations. Clinicians used to focus on acute renal injury; however, concentrating solely on one end of the range of renal function can limit our understanding and hinder a comprehensive analysis. Augmented renal clearance (ARC) remains an underappreciated clinical condition, and strategies for managing it are still being developed. ARC can potentially decrease the plasma concentrations of renally eliminated drugs. This has been extensively demonstrated with antibiotics, but also with other drugs, such as enoxaparin [1], metformin [2], and levetiracetam [3,4,5].
Antibiotics are one of the touchstones of the treatment of sepsis, and their early and appropriate administration improves clinical outcome. Clinicians are used to adjusting antibiotics to decreased renal performance; however, the reverse is quite rare. Dosing adaptation in critically ill patients is crucial due to the complex interplay of physiological changes, altered drug pharmacokinetics, and multiple co-existing medical conditions. Consequently, standard dosing regimens may not achieve the desired therapeutic effect or could lead to adverse drug reactions. Particularly, the critically ill frequently shows ARC, and this condition shows a robust association with under-therapeutic serum concentrations of several antibiotics. ARC impact has been increasingly described in intensive care units (ICU) around the world and has become included in recent guidelines and recommendations [6,7,8,9,10,11].
Methodology
A literature search was conducted on PubMed/MEDLINE between January 2018 and July 2023 to focus on publications within the last 5 years. All references that reported information on definition, identification, epidemiology, pathophysiology, and clinical relevance in sepsis of ARC were included. The search was limited to adult humans and articles published in English.
Definition of Augmented Renal Clearance
Although its recognition is not recent [12], the concept of ARC was first proposed in 2010 by Udy et al. and defined as an “increased elimination of circulating solutes compared with an expected baseline, involving changes in glomerular filtration and renal tubular function” [13]. There is still no standard definition for ARC, but there is a broad consensus that a creatinine clearance (CLCR) ≥ 130 ml/min/1.73 m2 seems to be an acceptable and clinically important cut-off value to define ARC: it is clearly supra-physiological, and it is the most used value in investigation and is undoubtedly associated with underexposure to antibiotics. By definition, the critically ill patient is often in unstable condition, and renal function varies quite substantially during the ICU stay. For that reason, augmented renal function should be interpreted more as a continuum and less as a dichotomic factor (presence/absence of ARC), as there is a linear correlation between renal function and elimination of most hydrophilic antibiotics.
Identification of Patients with ARC
Variations of glomerular filtration rate (GFR) are poorly reflected by daily changes in serum creatinine concentrations in critically ill patients. For that reason, creatinine-based equations are flawed in the critically ill and will tend to significantly underestimate renal function in patients with ARC [14, 15]. Despite the overwhelming medical evidence demonstrating the insensitivity of these methods, clinicians and investigators persist in assessing renal function this way. These considerations are strengthened in several recently published studies in ICU settings, including studies where a significant percentage of patients exhibited ARC.
In a study by Troisi et al., adult critically ill patients who underwent therapeutic drug monitoring (TDM) for meropenem and for whom a 24 h urine collection for measuring CLCR (24 h-CLCR) was performed were retrospectively included [16]. One quart of the studied cohort had at least one episode of ARC. The authors evaluated the performance of Cockcroft-Gault (CG), Modification of Diet in Renal Disease (MDRD) study, and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. They concluded that these mathematical formulas were not adequate for calculating the doses of meropenem necessary to achieve a therapeutic level and that renal function should be measured rather than estimated, especially for those displaying ARC. In a retrospective, single-center study, among 74 patients admitted to a neurocritical ICU, Monteiro et al. showed a weak statistical correlation between measured and estimated methods, with underestimation of ARC, and concluded that these discrepancies were not clinically acceptable [17•].
Relatedly, in a post hoc analysis of an observational study in 80 neurocritical patients, 8 h-CLCR seems to translate into the most appropriate assessment of renal function in patients with aneurysmatic subarachnoid hemorrhage, after comparison with 14 mathematical Eqs. (18). Another retrospective, single-center, study including 82 critically ill patients (43% with severe acute coronavirus 2 respiratory syndrome (SARS-CoV-2)) showed the low concordance between the GFR estimated by the CKD-EPI formula and the 24 h-CLCR [19]. In a sub-study of an ICU multicenter randomized controlled trial, the performance of CG, MDRD, CKD-EPI, and Jelliffe equations was evaluated against measured urinary CLCR in 237 critically ill patients with different degrees of kidney function (38.4% had ARC based on 24 h-CLCR). The conclusion was that such equations had limited ability to adequately estimate 24 h-CLCR [20]. Identical conclusions were reached in a prospective observational study, encompassing 100 patients consecutively admitted to a medical ICU in Taiwan [21]. Cucci et al. performed a larger multicenter, retrospective study (383 ICU-admitted patients were included, providing 1708 8 h- or 24 h-CLCR paired measurements) and reported that among ARC patients, there was a low correlation (r = 0.24–0.28), a low to moderate accuracy (range 38–70%), and a high bias (range of − 58.5 to − 21.6) between CG and measured CLCR [22•].
In a retrospective cohort study, investigators showed that there was 25% discordance in drug dosing depending on the use of either estimated (CG) or measured renal function. In addition, 69% of the estimated values deviated ± 20% from the reference value (CLCR) [23•]. Similar conclusions were reached in a prospective cross-sectional study (145 ICU patients), showing that none of the used mathematical estimates accurately detects the ARC as accurately as 12 h-CLCR [24], as well as in another recent study investigating a cohort of 68 burn patients, even after using the new 2021 updated CKD-EPI Eq. [25•]. In a prospective, observational cohort study of critically ill Indigenous Australian and non-Indigenous patients, Tsai et al. included a total of 131 patients, showing a prevalence of ARC of 32%. CG and CKD-EPI equations showed limited agreement with measured CLCR [26]. A recent multicenter retrospective study investigated the agreement between 24 h-CLCR and CG, CKD-EPI, and MDRD; a total of 51.604 ICU days were included, with an ARC prevalence of 20% [27•]. The authors concluded that all the studied estimates were flawed in the critically ill and showed a tendency to significantly under-evaluate renal function. Identical conclusions were reached in another multicentric study involving 561 critically ill patients, showing no concordance between the estimation of GFR by the CKD-EPI formula and 4 h-CLCR [28•].
Recently, Huang et al., applying machine learning algorithms, developed and validated models for 1 day in advance daily prediction of CLCR in ICU setting [29]. Among the ten most predictive variables of the three models, seven were related to 24 h-CLCR on the previous day; however, unstable renal function incremented the attributable error. Taking into account the daily creatinine variation, a group of investigators studied the kinetic estimated GFR equation, based on two separated serum creatinine levels, in a cohort of 60 patients (180 paired samples with an ARC prevalence of 48%); they concluded that this “dynamic” formula is not a reliable alternative when compared to measured 24 h-CLCR [30].
Of interest, in 232 adult non-critically ill surgery patients with a significant proportion displaying ARC, a remarkable disagreement and low precision were present between estimated and measured renal function (8 h-CLCR), and studied equations underestimated renal function [31].
Mathematical equations for estimation of renal function are typically derived from non-ICU populations, such as patients with normal renal function or with mild dysfunction or normal individuals, and are not validated in the critically ill population. Therefore, in the ICU, any method of assessing kidney function that does not consider urine output should be considered unreliable. Of note, the 2020 Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock warn readers about the potentially inadequate results related to the use of estimates of renal function in the critically ill [6]. Similarly, for the optimization of β-lactam treatment in critical care patients, the French Society of Pharmacology and Therapeutics (2018) suggests estimating GFR by calculating urinary creatinine clearance instead of equations. [8].
In light of this information, we can state with some certainty that urinary creatinine clearance remains the more reliable, cost-effective, replicable, and biologically precise alternative compared to mathematical calculations for evaluating renal function at bedside and adequately detecting ARC in critically ill patients, particularly for the purpose of renal adjustment of antibiotic dosing. Although any time interval used for calculation of CLCR is adequate and informative, the nighttime evaluation within a time period of 8 h coinciding with nursing shifts can be the most pragmatic and probably associated with less workload. Furthermore, in our experience, it provides “fresh data” for decision-making involving pharmacokinetic issues during the morning medical round.
Epidemiology of ARC
Prevalence of ARC
In the last 5 years, few studies have evaluated ARC prevalence in septic patients. We included in this review populations that are at high risk of infection and sepsis, namely, critically ill, trauma, and burn patients. Several studies reinforced the relevant prevalence of ARC in the critically ill patient, underlining that its presence is ubiquitous around the world, as depicted in Table 1. We focused only on studies using measured CLCR, due to its higher reliability as mentioned above. A great variability in ARC prevalence was seen, ranging from 24.6 to 94%. These differences can be partially explained by the specific characteristics of the population, but also by the diversity of definitions used for ARC prevalence. Many authors have not yet defined with clarity the ideal method to identify ARC, which greatly hinders comparative analysis between studies. Generally speaking, ARC appeared to be more frequent in trauma and neurocritical patients.
Several studies have evaluated ARC prevalence in general critically ill patients, without specification of pathology, and some have analyzed its variation in time. In a retrospective, cohort study with 1328 critically ill patients, the adjusted prevalence of ARC was 47%, of which 624 (47%) had ARC during their stay at ICU, 272 (20.5%) had ARC throughout their stay that never resolved, 185 (13.9%) had ARC that resolved at some point during their ICU stay, and 167 (12.6%) had intermittent ARC that did not resolve during their stay [32]. Additionally, in the cases of an ICU stay ≥ 7 days, the ARC prevalence ranged from 22.1 to 24.9% over the first 7 days, and the median time to onset of ARC was 1 day, with more than 64% of patients developing ARC within 24 h. In a similar study that included 734 critically ill patients, the prevalence of ARC was 33.4%, with almost half of the cases showing ARC onset within the first 3 days of ICU admission. The median duration of ARC was 5 days and ended within 3 weeks in many cases [33]. In a retrospective, single-center study of 312 patients, Egea et al. reported an ARC prevalence during the ICU stay of 24.6%, with a maximum reached at day 6 (34.4%), decreasing from day 7 to day 12, remaining stable afterwards, around 20%; the cumulative incidence rate was near 60% at day 7 [34].
ARC prevalence in the specific population of critically ill patients with sepsis was evaluated in a prospective, single-center, observational study that encompassed 59 patients admitted to a surgical and trauma ICU and who had a diagnosis of severe sepsis [35]. An ARC prevalence of 61% was described. A smaller study by Tamatsukuri et al., on the other hand, observed a lower ARC prevalence of 35% in 17 patients with sepsis [36].
Unsurprisingly, patients with severe coronavirus disease (COVID-19) admitted to the ICU exhibit high prevalence of ARC, ranging from 25 to 72% [19, 37, 38•, 39, 40]. Male sex and young patients were predominant in the studied cohorts [38•, 39, 41]. The day of onset of ARC was variable, with some studies reporting an early onset (median first day being day 1 or 2 from ICU admission) [38•, 39], while others reported a much-delayed onset of ARC (median first day being day 13 to 28 from ICU admission) [37, 41, 42].
The large majority of studies have been conducted with neurocritical and/or trauma patients. A prospective observational study reported an ARC prevalence of 79% in 74 neurocritical patients with either traumatic brain injury (TBI) or subarachnoid hemorrhage, and this condition was sustained throughout the first 2 weeks after neurocritical ICU admission [17•]. In a prospective study to investigate ARC in 54 TBI patients, Dang et al. showed a prevalence of 50% [43]. Similarly, other investigators observed a prevalence of 82% in a cohort that encompassed 61 TBI patients admitted in an ICU. In this last study, it was also noted that ARC developed early after admission (29% of patients on day 1) [44]. In a prospective observational study conducted in a neurocritical ICU including a total of 80 patients, 94% of the participants with aneurysmal subarachnoid hemorrhage and 50% of those with intracerebral hemorrhage experienced ARC on at least 1 day during the ICU stay [45]. Damen et al. also reported, in a single-center, retrospective analysis, an ARC prevalence of 69.2% in neurocritical patients in a mix ICU population, with 17 patients (32.7%) demonstrating severe ARC (CLCR > 200 mL/min) [46]. Other studies in neurocritical setting showed a prevalence between 35 and 77% [47,48,49].
Regarding the critically ill burn patients, Mueller et al. reported in a retrospective, single-center study, that ARC occurred at least once in 66.3% of total 12 h-CLCR assessments (n = 163). Most patients were male (82%) and young [25•]. ARC also seems to be frequent in critically ill patients with malignancy, with a prospective observational study of 363 adult patients with solid and hematologic malignancies reporting an ARC prevalence of 32% on at least 1 day of the study days [50•]. Saito et al. also found a high prevalence of ARC in 133 patients with hematopoietic tumors, reporting that 41.4% of patients exhibited ARC [51].
Risk Factors for ARC
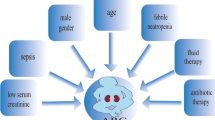
Since the first published studies on ARC in the critically ill patient, it was rapidly and consistently established an association between ARC and younger age, male gender, and trauma. Recent research continues to corroborate this association, but other risk factors have been reported.
As said above, one of the factors that has most consistently been linked to a high risk of ARC is age [32,33,34, 37, 38•, 41, 44, 47, 50•, 51,52,53,54,55], with most studies, including a recent systematic review, showing a difference of 10 to 20 years between patients with or without ARC [56]. Actually, ARC is significantly less frequent in patients over 50 years [32]. In a recent retrospective study, patients that developed ARC tended to be significantly younger as opposed to those that did not develop ARC (56 versus 68 years), with younger age being identified as an independent factor for development of ARC [32]. Likewise, in a retrospective cohort study involving 454 ICU admissions and 5586 8 h-CLCR, the investigators concluded that the probability of a patient showing ARC decreased 7% for each additional year of life [54].
Male sex has also been reported to be associated with ARC [19, 33, 39, 53, 54]. A retrospective cohort study, that included 734 patients from a mixed ICU, reported that male sex, along with younger age, was an independent factor for development of ARC [33]. In a mixed cohort of medical, neurocritical, and surgical critically ill patients, authors concluded that men seem to be three times more at risk than women for exhibiting ARC [54]. This association was also found on a multivariate analysis in other studies [32, 57,58,59]. On the contrary, Bing et al. performed a retrospective study with 324 patients admitted to a mixed ICU and reported that male sex was predominant but was not significantly associated with ARC after the multivariate logistic regression analysis (OR 1.946; 95% CI 0.90–3.945, p = 0.065) [60•].
The presence of trauma has also been described as an independent significant risk factor for ARC in critically ill patients [32, 54, 58, 60•]. In the study by Bing et al., trauma at admission was found to be a significant risk factor for ARC (OR 2.3; 95% CI 1.12–4.5, p = 0.02) [60•]. In another retrospective study that included 203 adult patients admitted to a trauma ICU (ARC prevalence of 50%), severe TBI was also found to be significantly associated with ARC after a multivariate analysis [58]. Other authors also showed that trauma admission was an independent risk factor for expressing ARC in the ICU, reporting an adjusted risk two times higher [54].
One study evaluated ARC in a specific cohort of critically ill obstetric patients. This was a retrospective study including 427 patients, with an ARC prevalence of 47.1%. Multivariate analysis identified a series of independent risk factors, including gestational age, fewer caesarean section, higher albumin level, severe preeclampsia, vasoactive drugs, infection, acute pancreatitis, and hypertriglyceridemia [61•].
Other risk factors for ARC found in multivariate analysis include African American race, lower serum creatinine concentration, neutrophil percentage, higher body mass index, absence of cardiovascular comorbidities, high blood glucose levels, enteral nutrition, antibiotic treatment, red blood cell transfusion, leukemia, use of vasopressors, and mechanically assisted ventilation [32, 43, 51, 57,58,59, 60•].
Pathophysiology of ARC—What We Know
There are few reports on how ARC occurs, as the pathophysiology behind this entity is still largely unknown. Recent publications suggest that rather than a fixed chain of events where one single alteration gives way to another, it seems to result from various processes occurring simultaneously.
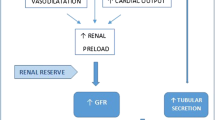
As a consequence of severe physiological stress related to sepsis or septic shock, the body appears to enter into an inflammatory hypermetabolic state in which pro-inflammatory mediators and cytokines are released [62, 63]. These compounds trigger profound metabolic and cellular changes that culminate into an increase in cardiac output and decrease in peripheral vascular resistance, which translates into increased renal blood flow and thus enhanced glomerular filtration. However, the increase in GFR seen in hyperinflammatory states may reflect a direct consequence of the inflammatory mediators as well, regardless of the hemodynamic changes they entail. In an experimental model of endotoxemia, after a lipopolysaccharide (LPS) derived from Escherichia coli was administered in healthy subjects, Beunders et al. [64] described an increased plasma concentration of pro-inflammatory cytokines, correlating with an increase in GFR (as measured by iohexol clearance). However, this increase in GFR did not appear to be dependent of perfusion pressure, as blood pressure was significantly lower compared to baseline during observation. In another recent study [65•], evaluating pathogenesis behind ARC at a transcriptional and metabolic level, the authors concluded that patients with ARC exhibited upregulation of L-arginine and L-glutamate, which indicated an increased consumption of arginine in critically ill patients with ARC. This in turn provides sufficient conditions for an increased production of nitric oxide (NO), ultimately increasing renal blood flow perfusion through NO-related inflammatory mediators. The authors also reported a direct regulation of GFR through N-methyl-D-aspartate receptor, which is regulated by glutamate. Finally, they concluded that the upregulation of cAMP leads to increased capillary permeability and extra-stromal precipitation.
Perhaps subjacent to the entire concept of ARC, closely linked to both the release of inflammatory mediators and a hypercatabolic state seen in sepsis, is the concept of renal functional reserve (RFR). RFR refers to the ability of the kidney to recruit previously dormant nephrons in times of biological stress, which results into increased renal blood flow and/or glomerular hyperfiltration. Although the exact mechanism behind the occurrence of RFR is yet unclear, recent studies reiterate the importance of protein loading and dilation of afferent glomerular arterioles after impaired renal auto-regulation, as well as complex interactions between tubuloglomerular feedback (TGF), the release of NO and vasodilator prostaglandins, and the metabolism of glucagon [66, 67].
Another mechanism behind ARC seems to be related to the severely catabolic state seen in these patients, which results into increased tissue destruction and excessive protein breakdown. Increased protein intake is thought to be associated with elicitation of RFR and thus enhanced GFR: an increase in the filtered load of amino acids reduces distal delivery of sodium chloride by increasing its tubular reabsorption, leading to inhibition of TGF, thus inducing afferent arteriolar vasodilation and consequently promoting hyperfiltration [66]. However, until recently, such a conclusion was derived from stable, healthy, and non-critical patients. In a recent retrospective single-center ICU study with around half of the cohort in sepsis [58], ARC was prevalent in approximately half of the patients admitted, who demonstrated marked protein catabolism (as evidenced by a worsened nitrogen balance), despite receiving a similar protein intake. Of note, the authors also found a significant association between ARC and increased protein intake (adjusted OR 2.06; 95% CI 1.09–3.91). Another study [68] concluded that patients with ARC presented a lower nitrogen balance and increased muscle loss despite receiving similar protein intake; patients with a higher protein intake had higher levels of CLCR. Whether a renoprotective nutrition (e.g., low-protein diet) would improve patient outcomes by reducing glomerular pressure and thus ARC (while perhaps promoting sarcopenia and muscle wasting) is less clear and warrants further investigation.
Tubular secretion seems to play a part as well in patients displaying ARC. A single-center, retrospective study [69•] attempted to compare GFR measured with iohexol plasma clearance and CLCR in critically ill patients with ARC. They concluded that half of the patients presenting ARC did not in fact have hyperfiltration and concluded that 6 h-CLCR appears to overestimate renal function by taking into account basal tubular excretion of creatinine. In a recent case report [70•], the same mechanism was evidenced. These findings suggest that ARC is due not only to increased glomerular filtration but also increased tubular secretion, at least to some extent. This is of particular importance, since it may influence renal elimination for drugs subject to these mechanisms, namely, some antimicrobials (β-lactams [71, 72], antiviral drugs [72], vancomycin [73]).
Additionally, one must consider the exogenous factors that contribute to ARC which do not result from the body’s own response to stimuli, but rather from medical intervention, such as aggressive fluid administration, use of vasopressor drugs, and inotropes. One study by Dhondt et al. [74] concluded that fluid resuscitation contributed more to the development of ARC than previously thought, after inducing a sepsis-like state in piglets through the continuous infusion (CI) of LPS from E. coli, except for one sham pig that only received the same amount of fluid treatment (0.9% sodium chloride solution, 6 mL/kg/h), and demonstrating that both groups displayed an elevated GFR over the time course of the study.
There are other factors that have been put forward when attempting to explain the pathophysiology behind ARC, such as a possible link between renal function and TBI [75], with the identification of elevated circulating atrial natriuretic peptide levels, and a significant correlation between neuromonitoring data (intracranial pressure, cerebral perfusion pressure, and the cerebrovascular pressure reactivity index) and ARC-presenting patients [76]. However, further studies are needed in order to shed light on this matter. The various mechanisms involved in the occurrence of ARC are summarized in Fig. 1.
Pathophysiology of ARC. Rather than one single process giving way to the other, the hyperinflammatory, hypercatabolic state seen in severe physiological stress (e.g., sepsis) sets in motion multiple simultaneous alterations. Mainly through the release of inflammatory mediators but also due to exogenous “iatrogenic” factors such as aggressive fluid administration, together they will trigger metabolic and cellular changes affecting glomerular filtration and tubular secretion, ultimately leading to ARC. cAMP, cyclic AMP; NO, nitric oxide; PGE2, prostaglandin-2; PVR, peripheral vascular resistance
Clinical Relevance of ARC in Sepsis
Several studies have been published evaluating the influence of ARC in pharmacokinetics (PK)/pharmacodynamics (PD) of antibiotics, not only with the newest antibiotics but also with some of the old antibiotics, showing that ARC has been recognized as an important factor for adjusting antibiotics’ doses. The three most reported antibiotics in recent years were vancomycin, meropenem, and piperacillin-tazobactam, which can be explained by the easy access to clinical data resulting from TDM. This is in accordance with current recommendations for routine TDM to be performed for aminoglycosides, β-lactam antibiotics, linezolid, teicoplanin, vancomycin, and voriconazole in critically ill patients [7].
Some of the published studies found in literature had the purpose of evaluating the association of ARC with subtherapeutic concentrations of antibiotics when using the standard dosage regimens, while others used population pharmacokinetic models and simulation to recommend dosage regimens for patients with ARC. Only few studies have explored the real impact of ARC on clinical outcomes, such as clinical cure, antimicrobial resistance, or mortality.
Association Between ARC and Underdosing of Antimicrobials
In the last years, several studies used TDM results to show that ARC leads to subtherapeutic plasma concentration of antibiotics. This has been extensively demonstrated for β-lactams. In a group of septic patients with ARC who received piperacillin-tazobactam, meropenem, cefepime, or ceftazidime, insufficient drug concentrations to treat infections due to Pseudomonas aeruginosa were observed in 55% of measurements [77]. In this study, the proportion of insufficient concentrations of meropenem and piperacillin increased with measured CLCR from 120 to 300 mL/min [77]. In another study with critically ill septic patients receiving high doses of β-lactams administered by CI, the rate of underdosing (< 4 × minimal inhibitory concentration, MIC) was significantly associated with CLCR, and a threshold for prediction was established at CLCR values ≥ 170 mL/min [78]. In a group of 62 critically ill patients receiving β-lactam antibiotics, the presence of ARC, compared to non-ARC, decreased the probability of target achievement, with 23% vs 69% (p < 0.01) for a target of 100% of time of free plasma concentration maintained above the MIC (fT > MIC) [21]. When a CI of ampicillin/sulbactam was administered to critically ill patients, the fourfold MIC breakpoint was not reached by 57% of patients with ARC [79]. A retrospective study showed that subtherapeutic piperacillin concentrations were more frequent in the group consisting of neurocritical patients compared to non-neurocritical patients (83% vs 46%), and the only risk factor identified for subtherapeutic piperacillin concentrations (< 80 mg/L) was measured CLCR, which was 173 and 99 mL/min, respectively [46].
Higher estimated GFR was associated with non-attainment of PK/PD target for meropenem, defined as plasma trough (Cmin) or steady-state concentration (Css) ≥ 10 mg/L [80]. Furthermore, the only significant predictor for not achieving the therapeutic PK/PD target of a free trough concentration 4 × MIC was ARC [81]. In this study, only 22% patients with ARC achieved the PK/PD target, while 64% non-ARC patients achieved the PK/PD target.
Regarding vancomycin, several recent studies have shown an association between ARC and PK/PD indices, including trough concentration, area under 24-h time-concentration curve (24 h-AUC), and AUC/MIC [57, 61•, 82]. Patients with ARC were more likely to have subtherapeutic vancomycin PK/PD indices [57]. In a group of critically ill obstetric patients, the initial trough concentration and 24 h-AUC of vancomycin in ARC patients were significantly lower than in non-ARC patients [61•]. The trough concentration among febrile neutropenic patients with ARC was significantly lower than for those without ARC [82]. Furthermore, in another study, the percentage of trough concentrations lower than 10 mg/L was 84.9% in the ARC group [83]. In a retrospective analysis of vancomycin TDM in patients undergoing neurosurgery, the trough concentration achievement rate in the ARC group was only 19.2% [84]. Using vancomycin trough plasma concentration/maintenance daily dose ratio to assess correlation with renal function showed that lower ratio was observed in patients with ARC compared to non-ARC group [85].
In contrast with other systemic azoles, fluconazole is hydrophilic and predominantly excreted by renal route. In a group of critically ill patients treated with fluconazole, decreased trough concentrations were significantly associated with ARC [86].
Recommended Dosage Regimens
Reviews on literature regarding the need for antibiotic dosage adjustments for ARC patients have recently been published [87••, 88••, 89••, 90••]. Most of the suggestions found in literature are based on population PK and simulation analysis, mostly with Monte Carlo simulations (MCS). There are some studies that analyze TDM results to determine the adequate dose accomplishing the desired targets. Increased doses, higher frequency, or prolonged infusion is frequently recommended in order to achieve PK/PD targets.
In the last 5 years, the vast majority of studies have evaluated β-lactams. New antibiotics with combinations of β-lactams and β-lactamase inhibitors, namely, ceftazidime/avibactam [91, 92], ceftolozane/tazobactam [93,94,95], and imipenem/cilastatin/relebactam [96,97,98,99], did not require dosage adjustment in patients with ARC. On the contrary, for other new antibiotics, there have been recommendations for dose adjustment. An extended infusion of 2 g q6h over 3 h of cefiderocol was recommended for patients with CLCR > 120 mL/min [100]. The dosage regimen recommended for ceftaroline in ARC patients was 600 mg as loading dose, followed by 1200 mg/day by CI [101].
Penicillins
Fournier et al. concluded that increased dosages of amoxicillin up to 2 g q4h over 2 h were necessary for patients with CLCR 200 mL/min [102].
Piperacillin-tazobactam was evaluated in several studies [35, 103,104,105], and different dosing regimens were proposed. For a PK/PD target of 100% fT > MIC and a MIC of 16 mg/L (for Pseudomonas aeruginosa), a dosage of 20 g/day of piperacillin was needed for patients with ARC [35, 103, 104]. Selig et al. suggested even higher doses of 28 g/day by CI for the same target in a population of burn and trauma patients [105].
Cephalosporins
Cefazolin was studied by Bellouard et al. [106]. Plasma concentrations of patients treated with CI for bacteraemia or infective endocarditis were used to establish a nomogram for optimal daily dose. Considering a target of 100% fT > 4 × MIC, a dose of 8 g/day was suggested for patients with CLCR 120 mL/min.
Different dosage adjustments for ARC patients were proposed for ceftriaxone, such as 2 g/day by CI [107] or 2 g q12h [108] considering the same target (100% fT > MIC and a MIC of 2 mg/L). Similar suggestions were made for a target defined as Cmin/MIC > 1 [109]. A much higher target (100% fT > 4 × MIC), with a lower MIC (0.5 mg/L), was proposed in a population with bacterial meningitis, justified by the need to reach adequate concentrations in cerebral spinal fluid for Streptococcus pneumoniae [110]. For these conditions, the dose of ceftriaxone suggested for patients with ARC was at least 78 mg/kg/day with a twice-daily regimen, which corresponds to 5.8 g/day in a patient with 75 kg. Dreesen et al. also considered a target of 100% fT > 4 × MIC and suggested 2 g q12h for a MIC value of 4 mg/L [111].
Cefepime dosage adjustment in patients with ARC has been suggested [112]. To achieve a target of 100% fT > MIC for a MIC of 8 mg/L, a loading dose of 4 g followed by CI of 7 g/day was needed.
Carbapenems
In recent times, several studies have proposed dosage regimens for meropenem in ARC [36, 113,114,115,116]. Different PK/PD targets were used varying from 40% fT > MIC to 100% fT > 4 × MIC for a MIC value of 2 mg/L. In all studies, administration of extended or CI was suggested as an alternative to intermittent dosing or as the only strategy. For intermittent administration of meropenem, dosing varied from 1 g q6h to 2 g q6h. Administration by extended infusion ranged from 1 g q8h over 3 h to 1 g q4h over 2 h. Doses suggested for CI varied from 2 to 8 g/day.
A randomized clinical trial was conducted to determine the best meropenem dosage regimen to achieve 50% fT > MIC in patients with ventilator-associated pneumonia and ARC divided in 3 groups [117•]. Prolonged meropenem infusion (1 g q8h over 6 h) reached better results than dose increase (2 g q8h over 3 h), in comparison to 1 g q8h over 3 h, with rates of achievement of 100%, 40%, and 13%, respectively.
Glycopeptides
One study with CI of vancomycin [118] recommended doses of 3500 mg/day and 4500 mg/day for ARC patients with CLCR 130–180 mL/min and > 181 mL/min, respectively. Another study using intermittent infusion of vancomycin [119] used a target trough level of 15 mg/L and proposed maintenance doses of 69 mg/kg/day for patients with ARC. For a patient of 70 kg, this would correspond to a daily dose around 4830 mg. Lower doses of 750 mg q8h were proposed using AUC 24-h 400–650 mg.h/L as target for patients with CLCR > 180 mL/min [120]; however, in this study, the probability of target attainment was only 62%. In a population of patients with hematological malignancies and ARC, for achieving a target exposure of 24 h-AUC of 400–600 mg.h/L at the steady state, daily doses ranging 2.5–3.25 g were recommended [121].
Aminoglycosides
Two studies evaluated amikacin, using as target Cmax/MIC > 8 after assuming a MIC of 8 mg/L, but different modelling approaches and covariates were used [122, 123]. Boidin et al. used an a priori control approach based on a nonparametric population PK model and body surface area (BSA) as a covariate, and for a median value of BSA of 1.9 m2, the optimal initial amikacin dose was higher than 3.4 g in patients with ARC [122]. Carrié et al. developed a population PK model with adapted body weight (ABW) as a covariate, and applying a MCS, 35 mg/kg ABW was recommended for a CLCR of 130 mL/min [123].
Fluoroquinolones
Two studies evaluated ciprofloxacin dose adjustments in ARC [124, 125]. Both studies suggested a dose of 600 mg q8h to reach the target of AUC/MIC > 125 in critically ill patients with ARC infected with pathogens with a MIC of 0.250 mg/L.
Oxazolidinones
Dosage adjustment of linezolid in critically ill patients with ARC was evaluated in two studies. Barrasa et al. administered linezolid as a CI, and the target was adjusted to Css > MIC [126]. An infusion rate of 75 mg/h (equivalent to 1800 mg/day) should be considered to ensure concentrations ≥ 2 mg/L. In the study of Wang et al., the therapeutic target comprised two pharmacodynamic indices (AUC/MIC > 80 and 85% T > MIC) [127]. For patients with ARC, a dose of 2400 mg 24-h CI was suggested.
Clinical Outcomes
Literature is scarce describing the influence of ARC on clinical outcomes of infected or septic patients receiving antibiotics (Table 2). After the first two studies published by Claus et al. [128] and Huttner et al. [129] that showed contradictory results, only a few more have explored this issue. Claus et al. found an association between ARC and antimicrobial therapeutic failure [128]. On the other hand, Huttner et al. did not observe an association between ARC and clinical failure of β-lactams administered to critically ill patients with severe infection [129]. The majority of the following studies did not show influence of ARC on clinical outcomes [95, 99, 130,131,132]. In these studies, different antibiotics were used, some with demonstrated influence of ARC on plasma concentrations, such as in the study of Udy et al., but other studies considered any antibiotic administered to the patient during the study period [131, 132], which may have included antibiotics that do not need dosage adjustment in ARC.
Carrie et al. found that ARC was associated with recurrent infection; however, there was no significant association with overall clinical failure [49]. There were subsequently two studies that showed a positive association between ARC and worst clinical outcomes [133•, 134], in which β-lactams and meropenem were evaluated.
These contradictory results may partially be explained by the variety of different definitions of ARC, method for its identification, population characteristics, and properties of antibiotics used. While some antibiotics have different dosage recommendations for patients with normal renal function and ARC, as mentioned previously, others do not need adjustments, and mixing these two types of antibiotics in the same study can be a confounding factor. Also, the majority of studies used estimates of CLCR, instead of measured CLCR, which lead to misidentification of patients and inconclusive results. Finally, standard dosage regimens may largely exceed the PK/PD targets for susceptible microorganisms with lower values of MIC, and even in the presence of ARC, therapeutic levels will be achieved.
Future Perspectives
Although there is an increased interest in ARC, there are still many issues requiring standardization, accuracy, or clarification:
-
1.
Although renal function should be interpreted as a continuum and as a dynamic concept, a unanimous definition of ARC, defining one consensual cut-off value of CLCR and the method used for its identification based only on measured CLCR instead of using mathematical estimates, would be valuable for standardization and coherent interpretation of distinct groups of research.
-
2.
With this in mind, it would become possible to carry out large multicenter studies in order to understand the true prevalence and risk factors for ARC in the ICU setting.
-
3.
Another subject that is still not well understood is the pathophysiology of ARC. Based on current knowledge, efforts should be made to clarify the underlying mechanisms and this way better identify the patients at risk of ARC.
-
4.
Most of the published literature on the influence of ARC on antibiotic therapeutic levels and recommended dosage regimens are based on studies using population PK and simulation analysis. There is an urgent need for more studies providing recommendations based on clinical data after antibiotic administration to ARC patients. Moreover, there is still a lack of evidence that subtherapeutic levels of antibiotics lead to worse outcomes in ARC patients. It would be of great value to conduct a large study with antibiotics that are evidently affected by ARC and analyze the influence of subtherapeutic levels on clinical outcomes, including clinical failure and antimicrobial resistance.
Conclusions
ARC is a well-recognized event with significant prevalence in the ICU around the world, with robust association with subtherapeutic levels of several antibiotics. However, there is still work to do on the correct identification of ARC patients through measured CLCR, understanding better the pathophysiology behind ARC, defining conditions for dose adjustments of antibiotics, and establishing an association with clinical outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Abdel El Naeem HEM, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anaesth. 2017;33(1):113–7.
Li L, Guan Z, Li R, Zhao W, Hao G, Yan Y, et al. Population pharmacokinetics and dosing optimization of metformin in Chinese patients with type 2 diabetes mellitus. Medicine (Baltimore). 2020;99(46): e23212.
Bilbao-Meseguer I, Barrasa H, Asín-Prieto E, Alarcia-Lacalle A, Rodríguez-Gascón A, Maynar J, et al. Population pharmacokinetics of levetiracetam and dosing evaluation in critically ill patients with normal or augmented renal function. Pharmaceutics. 2021;13(10).
Sime FB, Roberts JA, Jeffree RL, Pandey S, Adiraju S, Livermore A, et al. Population pharmacokinetics of levetiracetam in patients with traumatic brain injury and subarachnoid hemorrhage exhibiting augmented renal clearance. Clin Pharmacokinet. 2021;60(5):655–64.
Ong CLJ, Goh PSJ, Teo MM, Lim TP, Goh KKK, Ang XY, et al. Pharmacokinetics of levetiracetam in neurosurgical ICU patients. J Crit Care. 2021;64:255–61.
Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Iba T, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020). J Intensive Care. 2021;9(1):53.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46(6):1127–53.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care. 2019;23(1):104.
Reuter SE, Stocker SL, Alffenaar JC, Baldelli S, Cattaneo D, Jones G, et al. Optimal practice for vancomycin therapeutic drug monitoring: position statement from the anti-infectives committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit. 2022;44(1):121–32.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–247.
Dequin PF, Aubron C, Faure H, Garot D, Guillot M, Hamzaoui O, et al. The place of new antibiotics for Gram-negative bacterial infections in intensive care: report of a consensus conference. Ann Intensive Care. 2023;13(1):59.
Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299(17):915–9.
Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 49. New Zealand; 2010. p. 1–16.
Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15(3):R139.
Baptista JP, Neves M, Rodrigues L, Teixeira L, Pinho J, Pimentel J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J Nephrol. 2014;27(4):403–10.
Troisi C, Cojutti PG, Rinaldi M, Laici C, Siniscalchi A, Viale P, et al. Measuring creatinine clearance is the most accurate way for calculating the proper continuous infusion meropenem dose for empirical treatment of severe gram-negative infections among critically ill patients. Pharmaceutics. 2023;15(2).
• Monteiro E, Fraga Pereira M, Barroso I, Dias CC, Czosnyka M, Paiva JA, et al. Creatinine clearance in acute brain injury: a comparison of methods. Neurocrit Care. 2023. This article highlights the weak correlation between measured and estimated methods, concluding that measured creatinine clearance should be the preferred tool for renal function assessment of the neurocritical care setting.
Wells MA, Morbitzer K, Rhoney DH. Evaluation of the accuracy of standard renal function equations in critically ill patients with subarachnoid hemorrhage. Neurocrit Care. 2020;32(3):828–35.
Tomasa-Irriguible TM, Campos-Gómez A, Manciéo-Contreras JM, Sánchez-Satorra M, Philibert V, Bielsa-Berrocal L, et al. COVID- 19 and augmented renal clearance in critically ill patients. Ann Case Report. 2020;14: 495
Al-Dorzi HM, Alsadhan AA, Almozaini AS, A MA, Tamim H, Sadat M, et al. The performance of equations that estimate glomerular filtration rate against measured urinary creatinine clearance in critically ill patients. Crit Care Res Pract. 2021;2021:5520653.
Wu CC, Tai CH, Liao WY, Wang CC, Kuo CH, Lin SW, et al. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: a prospective observational study. Infect Drug Resist. 2019;12:2531–41.
• Cucci MD, Gerlach AT, Mangira C, Murphy CV, Roberts JA, Udy AA, et al. Performance of different body weights in the Cockcroft-Gault equation in critically ill patients with and without augmented renal clearance: a multicenter cohort. Pharmacotherapy. 2022. Multicentric retrospective study showing that CG performed poorly in ARC patients.
• Brown AR, Lavelle RI, Gerlach AT. Discordance of renal drug dosing using estimated creatinine clearance and measured urine creatinine clearance in hospitalized adults: a retrospective cohort study. Int J Crit Illn Inj Sci. 2020;10(Suppl 1):1–5. Retrospective cohort study showing 25% discordance between renal drug dosing based on mathematical estimates versus 8-h creatinine clearance.
Tolouian R, Hassanpour R, Sistanizad M, Kouchek M, Miri MM, Salarian S, et al. Using two predictor scoring systems together to increase the chance of identifying the augmented renal clearance phenomenon: a cross-sectional study. Iran J Kidney Dis. 2022;16(3):179–87.
• Mueller SW, Blass B, Molina KC, Gibson C, Krsak M, Kohler AD, et al. Augmented renal function in burn patients: occurrence and discordance with commonly used methods to assess renal function. J Burn Care Res. 2023. This retrospective, single-center study that encompassed 68 burn patients, reinforced the high prevalence of ARC in this population, occurring in 66.3% of total 12h-CLCR measurements (n = 163).
Tsai D, Udy AA, Stewart PC, Gourley S, Morick NM, Lipman J, et al. Prevalence of augmented renal clearance and performance of glomerular filtration estimates in Indigenous Australian patients requiring intensive care admission. Anaesth Intensive Care. 2018;46(1):42–50.
• Gijsen M, Wilmer A, Meyfroidt G, Wauters J, Spriet I. Can augmented renal clearance be detected using estimators of glomerular filtration rate? Crit Care. 2020;24(1):359. Multicentric retrospective study showing poor agreement between formulae estimating renal function (CG, CKD-EPI, MDRD) and 24h-CLCRCR.
• Tomasa-Irriguible TM, Sabater-Riera J, Pérez-Carrasco M, Ortiz-Ballujera P, Díaz-Buendía Y, Navas-Pérez A, et al. Augmented renal clearance. An unnoticed relevant event Sci Prog. 2021;104(2):368504211018580. Large multicentric study involving 561 critically ill patients showing no concordance between the estimation of GFR (CKD-EPI formula) and GFR calculation from the 4h-CLCR.CR.
Huang CY, Güiza F, Wouters P, Mebis L, Carra G, Gunst J, et al. Development and validation of the creatinine clearance predictor machine learning models in critically ill adults. Crit Care. 2023;27(1):272.
Carrié C, Rubin S, Sioniac P, Breilh D, Biais M. The kinetic glomerular filtration rate is not interchangeable with measured creatinine clearance for prediction of piperacillin underexposure in critically ill patients with augmented renal clearance. Crit Care. 22. England2018. p. 177.
Declercq P, Gijsen M, Meijers B, Schetz M, Nijs S, D’Hoore A, et al. Reliability of serum creatinine-based formulae estimating renal function in non-critically ill surgery patients: focus on augmented renal clearance. J Clin Pharm Ther. 2018;43(5):695–706.
Johnston BW, Perry D, Habgood M, Joshi M, Krige A. Augmented renal clearance: a retrospective, cohort study of urinary creatinine clearance in critically ill patients in the United Kingdom. J Int Med Res. 2021;49(5):3000605211015573.
Mikami R, Hayakawa M, Imai S, Sugawara M, Takekuma Y. Onset timing and duration of augmented renal clearance in a mixed intensive care unit. J Intensive Care. 2023;11(1):13.
Egea A, Dupuis C, de Montmollin E, Wicky PH, Patrier J, Jaquet P, et al. Augmented renal clearance in the ICU: estimation, incidence, risk factors and consequences-a retrospective observational study. Ann Intensive Care. 2022;12(1):88.
Carrié C, Legeron R, Petit L, Ollivier J, Cottenceau V, d’Houdain N, et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J Crit Care. 2018;48:66–71.
Tamatsukuri T, Ohbayashi M, Kohyama N, Kobayashi Y, Yamamoto T, Fukuda K, et al. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J Infect Chemother. 2018;24(10):834–40.
Rhoney DH, Brooks AB, Nelson NR. Augmented renal clearance: an under-recognized phenomenon associated with COVID-19. Crit Care Explor. 2022;4(2): e0617.
• Dhaese S, Peperstraete H, Hoste E, Van Biesen W, De Waele J. Augmented renal clearance in critically ill COVID-19 patients: forewarned is forearmed. J Crit Care. 2021;66:93–5. Prospective, observational study that reported the occurrence of ARC in at least one day in 72% of 129 critically ill COVID-19 patients. It also observed an early onset of ARC during the course of ICU stay.
Huang CY, Güiza F, Gijsen M, Spriet I, Dauwe D, Debaveye Y, et al. External validation of the augmented renal clearance predictor in critically ill COVID-19 patients. Antibiotics (Basel). 2023;12(4).
Molina Barragan AM, Pardo E, Galichon P, Hantala N, Gianinazzi AC, Darrivere L, et al. SARS-CoV-2 renal impairment in critical care: an observational study of 42 cases (Kidney COVID). J Clin Med. 2021;10(8).
Murt A, Dincer MT, Karaca C. Augmented renal clearance in COVID-19. Nephron. 2021;145(4):386–7.
Beunders R, van de Wijgert IH, van den Berg M, van der Hoeven JG, Abdo WF, Pickkers P. Late augmented renal clearance in patients with COVID-19 in the intensive care unit. A prospective observational study. J Crit Care. 2021;64:7–9.
Dang Z, Guo H, Li B, Zhen M, Liu J, Wei Y, et al. Augmented renal clearance in Chinese intensive care unit patients after traumatic brain injury: a cross-sectional study. Chin Med J (Engl). 2022;135(6):750–2.
Campassi ML, Repetto FG, Banegas Litardo DM, Castor R, Gómez G, Tiseyra B, et al. Incidence and determinats of augmented renal clearance in traumatic brain injury: a prospective observational study. J Crit Care. 2022;70: 154065.
Morbitzer KA, Jordan JD, Dehne KA, Durr EA, Olm-Shipman CM, Rhoney DH. Enhanced renal clearance in patients with hemorrhagic stroke. Crit Care Med. 2019;47(6):800–8.
Damen C, Dhaese S, Verstraete AG, Stove V, De Waele JJ. Subtherapeutic piperacillin concentrations in neurocritical patients. J Crit Care. 2019;54:48–51.
John G, Heffner E, Carter T, Beckham R, Smith N. Augmented renal clearance in patients with acute ischemic stroke: a prospective observational study. Neurocrit Care. 2023;38(1):35–40.
Lannou A, Carrié C, Rubin S, De Courson H, Biais M. Renal response after traumatic brain injury: a pathophysiological relationship between augmented renal clearance and salt wasting syndrome? Anaesth Crit Care Pain Med. 2020;39(2):239–41.
Carrie C, Bentejac M, Cottenceau V, Masson F, Petit L, Cochard JF, et al. Association between augmented renal clearance and clinical failure of antibiotic treatment in brain-injured patients with ventilator-acquired pneumonia: a preliminary study. Anaesth Crit Care Pain Med. 2018;37(1):35–41.
• Nazer LH, AbuSara AK, Kamal Y. Augmented renal clearance in critically ill patients with cancer (ARCCAN Study): a prospective observational study evaluating prevalence and risk factors. Pharmacol Res Perspect. 2021;9(2): e00747. This prospective study reported an ARC prevalence of 32% in patients with solid and hematological malignancies over the first 5 days of their ICU stay - the first study, to the best of our knowledge, to describe the incidence of ARC in critically ill patients with cancer.
Saito K, Kamio S, Ito K, Suzuki N, Abe K, Goto T. A simple scoring method to predict augmented renal clearance in haematologic malignancies. J Clin Pharm Ther. 2020;45(5):1120–6.
Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–54.
Luo Y, Wang Y, Ma Y, Wang P, Zhong J, Chu Y. Augmented renal clearance: what have we known and what will we do? Front Pharmacol. 2021;12: 723731.
Baptista JP, Martins PJ, Marques M, Pimentel JM. Prevalence and risk factors for augmented renal clearance in a population of critically ill patients. J Intensive Care Med. 2020;35(10):1044–52.
Nei AM, Kashani KB, Dierkhising R, Barreto EF. Predictors of augmented renal clearance in a heterogeneous ICU population as defined by creatinine and cystatin C. Nephron. 2020;144(7):313–20.
Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís M. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet. 2018;57(9):1107–21.
Zhao J, Fan Y, Yang M, Liang X, Wu J, Chen Y, et al. Association between augmented renal clearance and inadequate vancomycin pharmacokinetic/pharmacodynamic targets in Chinese adult patients: a prospective observational study. Antibiotics (Basel). 2022;11(7).
Dickerson RN, Crawford CN, Tsiu MK, Bujanowski CE, Van Matre ET, Swanson JM, et al. Augmented renal clearance following traumatic injury in critically ill patients requiring nutrition therapy. Nutrients. 2021;13(5).
Mulder MB, Eidelson SA, Sussman MS, Schulman CI, Lineen EB, Iyenger RS, et al. Risk factors and clinical outcomes associated with augmented renal clearance in trauma patients. J Surg Res. 2019;244:477–83.
• Bing E, Archambault K, Sananikone A, Nguyen KD, Fang YT, Jabamikos C, et al. Risk factors associated with augmented renal clearance in a mixed intensive care unit population: a retrospective study. Int J Clin Pharm. 2022;44(6):1277–86. This study involving 324 patients admitted to a mixed ICU reported age below fifty years old, lower serum creatinine, and trauma as independent risk factors for ARC.
• Tang L, Ding XY, Duan LF, Li L, Lu HD, Zhou F, et al. A regression model to predict augmented renal clearance in critically ill obstetric patients and effects on vancomycin treatment. Front Pharmacol. 2021;12: 622948. A retrospective, single-center study that reported a high ARC prevalence in critically ill obstetric patients and identified multiple independent risk factors for ARC in this specific population.
Xiao Q, Zhang H, Wu X, Qu J, Qin L, Wang C. Augmented renal clearance in severe infections-an important consideration in vancomycin dosing: a narrative review. Front Pharmacol. 2022;13: 835557.
Rico-Fontalvo J, Correa-Guerrero J, Martínez-Ávila MC, Daza-Arnedo R, Rodriguez-Yanez T, Almanza-Hurtado A, et al. Critically ill patients with renal hyperfiltration: optimizing antibiotic dose. Int J Nephrol. 2023;2023:6059079.
Beunders R, Schütz MJ, van Groenendael R, Leijte GP, Kox M, van Eijk LT, et al. Endotoxemia-induced release of pro-inflammatory mediators are associated with increased glomerular filtration rate in humans. Front Med (Lausanne). 2020;7: 559671.
• Wang Y, Luo Y, Yang S, Jiang M, Chu Y. LC-MS/MS-based serum metabolomics and transcriptome analyses for the mechanism of augmented renal clearance. Int J Mol Sci. 2023;24(13). Analytical research study evaluating the pathogenesis behind ARC at a transcriptional and metabolic level which identified the direct role of several inflammatory mediators.
Jufar AH, Lankadeva YR, May CN, Cochrane AD, Bellomo R, Evans RG. Renal functional reserve: from physiological phenomenon to clinical biomarker and beyond. Am J Physiol Regul Integr Comp Physiol. 2020;319(6):R690–702.
Ronco C, Bellomo R, Kellum J. Understanding renal functional reserve. Intensive Care Med. 2017;43(6):917–20.
Dreydemy G, Coussy A, Lannou A, Petit L, Biais M, Carrié C. Augmented renal clearance, muscle catabolism and urinary nitrogen loss: implications for nutritional support in critically ill trauma patients. Nutrients. 2021;13(10).
• Collet M, Hijazi D, Sevrain P, Barthélémy R, Labeyrie MA, Prié D, et al. Evaluation of glomerular filtration rate using iohexol plasma clearance in critically ill patients with augmented renal creatinine clearance: a single-centre retrospective study. Eur J Anaesthesiol. 2021;38(6):652–8. A retrospective study concluding that not all patients with ARC show hyperfiltration, and tubular secretion plays a part.
• Fransson M, Helldén A, Östholm Balkhed Å, Nezirević Dernroth D, Ha M, Haglund M, et al. Case report: Subtherapeutic vancomycin and meropenem concentrations due to augmented renal clearance in a patient with intracranial infection caused by. Front Pharmacol. 2021;12: 728075. A case report with an interesting therapeutic pathway reviewing the mechanisms behind ARC.
Udy AA, Jarrett P, Stuart J, Lassig-Smith M, Starr T, Dunlop R, et al. Determining the mechanisms underlying augmented renal drug clearance in the critically ill: use of exogenous marker compounds. Crit Care. 2014;18(6):657.
Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–29.
Kan WC, Chen YC, Wu VC, Shiao CC. Vancomycin-associated acute kidney injury: a narrative review from pathophysiology to clinical application. Int J Mol Sci. 2022;23(4).
Dhondt L, Croubels S, Temmerman R, De Cock P, Meyer E, Van Den Broeck W, et al. The development of a juvenile porcine augmented renal clearance model through continuous infusion of lipopolysaccharides: an exploratory study. Front Vet Sci. 2021;8:639771.
Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017;34(1):137–44.
Dias C, Gaio AR, Monteiro E, Barbosa S, Cerejo A, Donnelly J, et al. Kidney-brain link in traumatic brain injury patients? A preliminary report. Neurocrit Care. 2015;22(2):192-201.
Jacobs A, Taccone FS, Roberts JA, Jacobs F, Cotton F, Wolff F, et al. β-Lactam dosage regimens in septic patients with augmented renal clearance. Antimicrob Agents Chemother. 2018;62(9).
Carrié C, Petit L, d’Houdain N, Sauvage N, Cottenceau V, Lafitte M, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–9.
Passon SG, Schmidt AR, Wittmann M, Velten M, Baehner T. Evaluation of continuous ampicillin/sulbactam infusion in critically ill patients. Life Sci. 2023;320: 121567.
Tournayre S, Mathieu O, Villiet M, Besnard N, Brunot V, Daubin D, et al. Factors associated with meropenem pharmacokinetic/pharmacodynamic target attainment in septic critically ill patients treated with extended intermittent infusion or continuous infusion. Int J Antimicrob Agents. 2023;62(2): 106868.
Alsultan A, Dasuqi SA, Aljamaan F, Omran RA, Syed SA, AlJaloud T, et al. Pharmacokinetics of meropenem in critically ill patients in Saudi Arabia. Saudi Pharm J. 2021;29(11):1272–7.
Alzahrani AM, Hakami AY, AlAzmi A, Karim S, Ali AS, Burzangi AS, et al. Augmented Renal clearance and hypoalbuminemia-induced low vancomycin trough concentrations in febrile neutropenic patients with hematological malignancies. Cureus. 2022;14(9): e29568.
Yu YX, Lu J, Lu HD, Li L, Li JJ, Shi L, et al. Predictive performance of reported vancomycin population pharmacokinetic model in patients with different renal function status, especially those with augmented renal clearance. Eur J Hosp Pharm. 2022;29(e1):e6–14.
Chen Y, Liu L, Zhu M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J Int Med Res. 2020;48(10):300060520949076.
Mikami R, Imai S, Hayakawa M, Sugawara M, Takekuma Y. Clinical applicability of urinary creatinine clearance for determining the initial dose of vancomycin in critically ill patients. J Infect Chemother. 2022;28(2):199–205.
Van Daele R, Wauters J, Lagrou K, Denooz R, Hayette MP, Gijsen M, et al. Pharmacokinetic variability and target attainment of fluconazole in critically ill patients. Microorganisms. 2021;9(10).
•• Silva CM, Baptista JP, Santos I, Martins P. Recommended antibiotic dosage regimens in critically ill patients with augmented renal clearance: a systematic review. Int J Antimicrob Agents. 2022;59(5):106569. Systematic review of the literature on antibiotic dosage recommendations in patients with ARC.
•• Hefny F, Sambhi S, Morris C, Kung JY, Stuart A, Mahmoud SH. Drug dosing in critically ill adult patients with augmented renal clearance. Eur J Drug Metab Pharmacokinet. 2022;47(5):607–20. Extensive review on drug dosing recommendations in patients with ARC, including antibiotics.
•• Sistanizad M, Hassanpour R, Pourheidar E. Are antibiotics appropriately dosed in critically ill patients with augmented renal clearance? A narrative review. Int J Clin Pract. 2022;2022:1867674. Narrative review recommending dosing protocol for several antibiotics in ARC.
•• Shi AX, Qu Q, Zhuang HH, Teng XQ, Xu WX, Liu YP, et al. Individualized antibiotic dosage regimens for patients with augmented renal clearance. Front Pharmacol. 2023;14:1137975. Review providing antibiotic dosage regimens and dose optimization strategies for ARC patients.
Li J, Lovern M, Green ML, Chiu J, Zhou D, Comisar C, et al. Ceftazidime-avibactam population pharmacokinetic modeling and pharmacodynamic target attainment across adult indications and patient subgroups. Clin Transl Sci. 2019;12(2):151–63.
Stein GE, Smith CL, Scharmen A, Kidd JM, Cooper C, Kuti J, et al. Pharmacokinetic and pharmacodynamic analysis of ceftazidime/avibactam in critically ill patients. Surg Infect (Larchmt). 2019;20(1):55–61.
Nicolau DP, De Waele J, Kuti JL, Caro L, Larson KB, Yu B, et al. Pharmacokinetics and pharmacodynamics of ceftolozane/tazobactam in critically ill patients with augmented renal clearance. Int J Antimicrob Agents. 2021;57(4): 106299.
Sime FB, Lassig-Smith M, Starr T, Stuart J, Pandey S, Parker SL, et al. Population pharmacokinetics of unbound ceftolozane and tazobactam in critically ill patients without renal dysfunction. Antimicrob Agents Chemother. 2019;63(10).
Shorr AF, Bruno CJ, Zhang Z, Jensen E, Gao W, Feng HP, et al. Ceftolozane/tazobactam probability of target attainment and outcomes in participants with augmented renal clearance from the randomized phase 3 ASPECT-NP trial. Crit Care. 2021;25(1):354.
Bhagunde P, Patel P, Lala M, Watson K, Copalu W, Xu M, et al. Population pharmacokinetic analysis for imipenem-relebactam in healthy volunteers and patients with bacterial infections. CPT Pharmacometrics Syst Pharmacol. 2019;8(10):748–58.
Fratoni AJ, Mah JW, Nicolau DP, Kuti JL. Imipenem/cilastatin/relebactam pharmacokinetics in critically ill patients with augmented renal clearance. J Antimicrob Chemother. 2022;77(11):2992–9.
Patel M, Bellanti F, Daryani NM, Noormohamed N, Hilbert DW, Young K, et al. Population pharmacokinetic/pharmacodynamic assessment of imipenem/cilastatin/relebactam in patients with hospital-acquired/ventilator-associated bacterial pneumonia. Clin Transl Sci. 2022;15(2):396–408.
Roberts JA, Nicolau DP, Martin-Loeches I, Deryke CA, Losada MC, Du J, et al. Imipenem/cilastatin/relebactam efficacy, safety and probability of target attainment in adults with hospital-acquired or ventilator-associated bacterial pneumonia among patients with baseline renal impairment, normal renal function, and augmented renal clearance. JAC Antimicrob Resist. 2023;5(2):dlad011.
Kawaguchi N, Katsube T, Echols R, Wajima T. Population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection. Antimicrob Agents Chemother. 2021;65(3).
Chauzy A, Gregoire N, Ferrandière M, Lasocki S, Ashenoune K, Seguin P, et al. Population pharmacokinetic/pharmacodynamic study suggests continuous infusion of ceftaroline daily dose in ventilated critical care patients with early-onset pneumonia and augmented renal clearance. J Antimicrob Chemother. 2022;77(11):3173–9.
Fournier A, Goutelle S, Que YA, Eggimann P, Pantet O, Sadeghipour F, et al. Population pharmacokinetic study of amoxicillin-treated burn patients hospitalized at a Swiss tertiary-care center. Antimicrob Agents Chemother. 2018;62(9).
Klastrup V, Thorsted A, Storgaard M, Christensen S, Friberg LE, Öbrink-Hansen K. Population pharmacokinetics of piperacillin following continuous infusion in critically ill patients and impact of renal function on target attainment. Antimicrob Agents Chemother. 2020;64(7).
Besnard T, Carrié C, Petit L, Biais M. Increased dosing regimens of piperacillin-tazobactam are needed to avoid subtherapeutic exposure in critically ill patients with augmented renal clearance. Crit Care. 2019;23(1):13.
Selig DJ, Akers KS, Chung KK, Kress AT, Livezey JR, Por ED, et al. Comparison of piperacillin and tazobactam pharmacokinetics in critically ill patients with trauma or with burn. antibiotics (Basel). 2022;11(5).
Bellouard R, Deschanvres C, Deslandes G, Dailly É, Asseray N, Jolliet P, et al. Population pharmacokinetic study of cefazolin dosage adaptation in bacteremia and infective endocarditis based on a nomogram. Antimicrob Agents Chemother. 2019;63(10).
Leegwater E, Kraaijenbrink BVC, Moes DJAR, Purmer IM, Wilms EB. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J Antimicrob Chemother. 2020;75(6):1554–8.
Ollivier J, Carrié C, d'Houdain N, Djabarouti S, Petit L, Xuereb F, et al. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob Agents Chemother. 2019;63(3).
Heffernan AJ, Sime FB, Kumta N, Wallis SC, McWhinney B, Ungerer J, et al. Multicenter population pharmacokinetic study of unbound ceftriaxone in critically ill patients. Antimicrob Agents Chemother. 2022;66(6): e0218921.
Grégoire M, Dailly E, Le Turnier P, Garot D, Guimard T, Bernard L, et al. High-dose ceftriaxone for bacterial meningitis and optimization of administration scheme based on nomogram. Antimicrob Agents Chemother. 2019;63(9).
Dreesen E, Gijsen M, Elkayal O, Annaert P, Debaveye Y, Wauters J, et al. Ceftriaxone dosing based on the predicted probability of augmented renal clearance in critically ill patients with pneumonia. J Antimicrob Chemother. 2022;77(9):2479–88.
Al-Shaer MH, Neely MN, Liu J, Cherabuddi K, Venugopalan V, Rhodes NJ, et al. Population pharmacokinetics and target attainment of cefepime in critically ill patients and guidance for initial dosing. Antimicrob Agents Chemother. 2020;64(9).
Sjövall F, Alobaid AS, Wallis SC, Perner A, Lipman J, Roberts JA. Maximally effective dosing regimens of meropenem in patients with septic shock. J Antimicrob Chemother. 2018;73(1):191–8.
Burger R, Guidi M, Calpini V, Lamoth F, Decosterd L, Robatel C, et al. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life-threatening infections. J Antimicrob Chemother. 2018;73(12):3413–22.
Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother. 2018;73(5):1330–9.
Kumta N, Heffernan AJ, Cotta MO, Wallis SC, Livermore A, Starr T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of meropenem in neurocritical care patients: a prospective two-center study. Antimicrob Agents Chemother. 2022;66(8): e0014222.
• Razzazzadeh S, Darazam IA, Hajiesmaeili M, Salamzadeh J, Mahboubi A, Sadeghnezhad E, et al. Investigation of pharmacokinetic and clinical outcomes of various meropenem regimens in patients with ventilator-associated pneumonia and augmented renal clearance. Eur J Clin Pharmacol. 2022;78(5):823–9. Randomized clinical trial to determine the association between different dosage regimens and clinical outcomes in patients with ARC.
Vu DH, Nguyen DA, Delattre IK, Ho TT, Do HG, Pham HN, et al. Determination of optimal loading and maintenance doses for continuous infusion of vancomycin in critically ill patients: population pharmacokinetic modelling and simulations for improved dosing schemes. Int J Antimicrob Agents. 2019;54(6):702–8.
He J, Yang ZT, Qian X, Zhao B, Mao EQ, Chen EZ, et al. A higher dose of vancomycin is needed in critically ill patients with augmented renal clearance. Transl Androl Urol. 2020;9(5):2166–71.
Zhao S, He N, Zhang Y, Wang C, Zhai S, Zhang C. population pharmacokinetic modeling and dose optimization of vancomycin in Chinese patients with augmented renal clearance. Antibiotics (Basel). 2021;10(10).
Belabbas T, Yamada T, Egashira N, Hirota T, Suetsugu K, Mori Y, et al. Population pharmacokinetic model and dosing optimization of vancomycin in hematologic malignancies with neutropenia and augmented renal clearance. J Infect Chemother. 2023;29(4):391–400.
Boidin C, Bourguignon L, Cohen S, Roger C, Lefrant JY, Roberts JA, et al. Amikacin initial dose in critically ill patients: a nonparametric approach to optimize. Antimicrob Agents Chemother. 2019;63(11).
Carrié C, Delzor F, Roure S, Dubuisson V, Petit L, Molimard M, et al. Population pharmacokinetic study of the suitability of standard dosing regimens of amikacin in critically ill patients with open-abdomen and negative-pressure wound therapy. Antimicrob Agents Chemother. 2020;64(4).
Roberts JA, Alobaid AS, Wallis SC, Perner A, Lipman J, Sjövall F. Defining optimal dosing of ciprofloxacin in patients with septic shock. J Antimicrob Chemother. 2019;74(6):1662–9.
Gieling EM, Wallenburg E, Frenzel T, de Lange DW, Schouten JA, Ten Oever J, et al. Higher dosage of ciprofloxacin necessary in critically ill patients: a new dosing algorithm based on renal function and pathogen susceptibility. Clin Pharmacol Ther. 2020;108(4):770–4.
Barrasa H, Soraluce A, Usón E, Sainz J, Martín A, Sánchez-Izquierdo J, et al. Impact of augmented renal clearance on the pharmacokinetics of linezolid: advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int J Infect Dis. 2020;93:329–38.
Wang X, Wang Y, Yao F, Chen S, Hou Y, Zheng Z, et al. Pharmacokinetics of linezolid dose adjustment for creatinine clearance in critically ill patients: a multicenter, prospective, open-label, observational study. Drug Des Devel Ther. 2021;15:2129–41.
Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28(5):695–700.
Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92.
Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49(5):624–30.
Burnham JP, Micek ST, Kollef MH. Augmented renal clearance is not a risk factor for mortality in Enterobacteriaceae bloodstream infections treated with appropriate empiric antimicrobials. Plos One. 2017;12(7): e0180247.
Kawano Y, Maruyama J, Hokama R, Koie M, Nagashima R, Hoshino K, et al. Outcomes in patients with infections and augmented renal clearance: a multicenter retrospective study. Plos One. 2018;13(12): e0208742.
• Carrié C, Chadefaux G, Sauvage N, de Courson H, Petit L, Nouette-Gaulain K, et al. Increased β-lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: a before and after study. Crit Care. 2019;23(1):379. Study to compare the clinical outcome of ARC patients treated by conventional or increased β-lactam dosing regimens, using measured CLCRCR for identifying ARC.
Cojutti PG, Lazzarotto D, Candoni A, Dubbini MV, Zannier ME, Fanin R, et al. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: results from a prospective, monocentric, interventional study. J Antimicrob Chemother. 2020;75(10):3029–37.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
All authors (L. Baptista, I. Moura, C. M. Silva and J. P. Baptista) contributed to the writing and revision of the manuscript. All authors agreed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests related to this research to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baptista, L., Moura, I., Silva, C.M. et al. What is New in Augmented Renal Clearance in Septic Patients?. Curr Infect Dis Rep 25, 255–272 (2023). https://doi.org/10.1007/s11908-023-00816-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-023-00816-6