Abstract
Encephalitis results in substantial morbidity and mortality and is a challenging syndrome for clinicians to manage. The clinical presentation is heterogeneous, there is a broad range of causative agents, and specific treatments for many etiologies are lacking. Over the past decade, a number of novel infectious and autoimmune etiologies of encephalitis have been identified. Despite such advances, however, up to 50 % of encephalitis cases typically remain without an identified etiology. Moreover, few new vaccines and therapies have been developed. Here, we discuss recent advances in encephalitis, with specific focus on several areas: (1) the changing demographics of West Nile virus in the United States and the implications for vaccine development, (2) challenges in the diagnosis of herpesviral infections in immunocompromised individuals, (3) the identification of a potential link between herpes simplex encephalitis and anti-NMDA receptor encephalitis, and (4) the delineation of prognostic factors related to outcome in individuals with encephalitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encephalitis affects 1 in 10,000 individuals annually and results in substantial morbidity and mortality worldwide. Many challenges confront clinicians in the management of patients with encephalitis, including lack of consensus case definitions and standardized diagnostic approaches [1]. Numerous large studies have found that a specific cause of encephalitis is typically identified in fewer than 50 % of cases [2–4], and progress in the field has been slowed by the rarity and heterogeneity of cases. Moreover, the field of encephalitis is in a state of considerable flux. A number of infectious etiologies have either emerged in new geographic areas or reemerged over the past decade. In addition, there has been increased recognition of autoimmune causes of encephalitis, with identification of novel autoantibodies and syndromes [5, 6].
Here, recent advances in the field of encephalitis are described. Four areas of focus will be discussed: (1) the resurgence of West Nile virus (WNV) as a major contributor to encephalitis in the United States in 2012, highlighting the difficulties of predicting outbreaks of viral disease and developing effective vaccines; (2) the increasing recognition of human herpesvirus-associated encephalitis occurring in the setting of immunosuppression; (3) the discovery of a potential link between viral infections and anti-NMDA receptor (anti-NMDAR) encephalitis, an autoimmune cause of encephalitis; and (4) the identification of prognostic factors in encephalitis, which may assist clinicians and families in the management of affected individuals.
Resurgence of West Nile Virus
WNV is the most common cause of acquired arboviral disease in the United States (U.S.). Notably, the demographics of WNV infection have changed dramatically over the past decade. The first identified case of WNV infection in the U.S. occurred during an outbreak of encephalitis in 1999 in New York [7]. Within several years, the virus had spread rapidly across the country, with large regional outbreaks in the central states in 2002 and the mountain states in 2003, resulting in nearly 3,000 cases of neuroinvasive disease each year. From 2004 to 2011, small outbreaks occurred each summer, but incidence generally decreased, with the fewest cases of WNV neuroinvasive disease—under 500 each year—reported in 2009 and 2011 [8•]. In 2012, however, a large multistate outbreak occurred, with more cases reported than in any year since 2003. Indeed, over 5,000 WNV cases and over 2,500 neuroinvasive cases were reported in 48 states, the District of Columbia, and Puerto Rico [9]. Most cases occurred from July to September, and incidence rates of WNV neuroinvasive disease were highest in South Dakota, North Dakota, Mississippi, Louisiana, and Texas. In Texas, although attack rates of WNV were higher in rural counties, the highest numbers of cases were reported from metropolitan cities, suggesting the need for broad surveillance [10]. Notably, WNV outbreaks are not confined to the U.S., since the virus has emerged in several areas and reemerged in others in recent years in central and eastern Europe and in countries surrounding the Mediterranean Sea [11].
Reasons for WNV outbreaks are not well understood, although a firm understanding of the ecology of WNV is likely to be crucial. WNV amplification depends on several factors, including numbers and distributions of reservoir animals (predominantly birds) and vectors (mosquitoes), as well as rate of viral replication in mosquitoes, all of which can be influenced by changes in weather patterns [12]. Notably, the average temperature in the summer of 2012 in the U.S. was the hottest on record for similar periods over the past century, suggesting a potential role for elevated temperatures in the potentiation of WNV. However, WNV outbreaks are not consistently related to heat waves, and many virus-endemic areas that experience heat waves do not have outbreaks [8•]. Overall, a greater understanding of ecological factors underpinning WNV transmission would allow for more effective predictive models of disease outbreaks.
The unpredictability of the epidemiology of WNV has also hampered attempts at further development of human vaccines, and there are currently no FDA-approved human vaccines against WNV. Four equine vaccines for WNV have been licensed, and several phase 1 and phase 2 trials have been conducted in humans [13]. A phase 3 trial with enough power to demonstrate efficacy would necessitate recruitment of a large numbers of patients at considerable, and possibly prohibitive, cost [8•]. Given the low incidence of WNV neuroinvasive disease in otherwise healthy individuals and the unpredictable nature of outbreaks, it is likely that future efforts at vaccine development will need to focus on high-risk groups in order to demonstrate efficacy [13].
Viral Encephalitis in the Immunocompromised Host
Infection by pathogens that cause encephalitis may result in a markedly different presentation and clinical course in immunocompromised, as compared with immunocompetent, individuals. Moreover, immunocompromised individuals are at risk for disease from unique, opportunistic pathogens. Below, we discuss recent findings on encephalitis caused by selected herpesviral infections in the immunocompromised host.
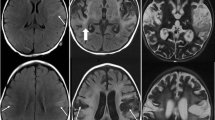
Prior case reports and small series have suggested that herpes simplex infections of the central nervous system (CNS) may result in an atypical presentation or more aggressive clinical course in the immunocompromised population [14–16]. In order to better characterize the clinical and neuroimaging features of herpes simplex encephalitis (HSE) in immunocompromised individuals, we performed a retrospective case–control review of patients diagnosed with HSE. We found that immunocompromised patients with HSE presented with fewer prodromal symptoms and with imaging evidence of more diffuse brain involvement extending beyond the temporal lobes and cingulate cortex [17•]. In addition, the absence of cerebrospinal fluid (CSF) pleocytosis was not uncommon. These findings likely reflect the inability of the host’s immune system to mount an adequate inflammatory reaction and to control the infection, and it is anticipated that this will translate into a worse prognosis. Indeed, we found that lack of CSF leukocytosis was a poor prognostic factor, and overall the odds ratio of death was 6 times higher in immunocompromised patients [17•]. These findings are particularly notable in the current setting of increased utilization of potent immunomodulatory and immunosuppressive therapies for a broad range of systemic and CNS autoimmune disorders [18] and highlight the need for clinicians to adopt a high index of suspicion for HSE in individuals on immunsuppression.
Human herpesvirus 6 (HHV6) comprises two variants, HHV6A and HHV6B, the latter of which has been firmly associated with human disease. HHV6B is the primary cause of the childhood disease roseola infantum and has also been associated with febrile seizures [19, 20]. Viral reactivation appears to occur preferentially in immunocompromised individuals, and there is a well-documented association with posttransplant acute limbic encephalitis. In this syndrome, anterograde amnesia and seizures typically begin several weeks after transplantation. MRI demonstrates bilateral nonenhancing T2 hyperintensities in the medial temporal lobes; there may be a mild CSF pleocytosis, and HHV-6 DNA can be amplified from the CSF of most patients [21, 22]. Guidelines recommend use of ganciclovir or foscarnet sodium either singly or in combination in these individuals [23].
Several studies have also noted that HHV6 may account for a substantial percentage of cases of focal encephalitis in immunocompetent adults and children [24–29]. In these cases, it remains unclear whether HHV-6 is causative or simply a “bystander,” since HHV6 reactivation frequently occurs during acute infections with other viruses and also in the setting of other neurologic conditions, including multiple sclerosis and temporal lobe epilepsy [30]. Indeed, HHV6 DNA has also been found in normal brain tissue and CSF, raising questions about the specificity of this finding [31]. Further confounding the issue of detection of HHV6 DNA in human samples is the recognition that HHV6 can stably integrate into the chromosomes of human cells. Thus, a positive PCR may either indicate actively replicating virus or chromosomal integration. Newer assays to distinguish chromosomal integration from reactivated virus may aid in the determination of whether a finding of HHV6 DNA is likely to be pathogenic [32, 33••].
Links Between Infections and Anti-NMDAR Encephalitis?
Infectious causes of autoimmune conditions have been proposed for over 100 years. A number of potential mechanisms, including molecular mimicry whereby a microbial antigen shares epitopes or structural similarities with self-antigens, the unmasking of cryptic epitopes in the setting of tissue damage, or breaking of immune tolerance, may trigger autoimmune disease in the setting of infection [34, 35]. Several well-established relationships, such as the association between Group-A-beta-hemolytic streptococcal infections and Sydenham’s chorea and that of Campylobacter jejuni infection and Guillain-Barre syndrome [36, 37], have strengthened the view that infections can contribute to autoimmune disease.
Recent evidence points to a potential relationship between infections and anti-NMDAR encephalitis. Increasingly recognized as an important cause of encephalitis worldwide, anti-NMDAR encephalitis is characterized by psychiatric changes, seizures, abnormal movements, and autonomic dysfunction and results from an antibody-mediated immune response against extracellular epitopes of the NMDA receptor [38]. In one study, the frequency of anti-NMDAR encephalitis in young individuals surpassed those of individual viral etiologies, including enterovirus and HSE [39]. In up to half of cases, an ovarian teratoma is present, and the syndrome is considered to develop as a paraneoplastic manifestation. In the remaining cases, however the etiology of anti-NMDAR encephalitis is unclear. Notably, many patients have prodromal symptoms that include headache, fever, nausea, vomiting, diarrhea, or upper respiratory symptoms, raising the possibility that the autoimmune process may be triggered by an infection [38]. Indeed, isolated cases of patients with positive mycoplasma or EBV serology or confirmed influenza infection developing anti-NMDAR encephalitis have been reported [40–42].
Recent evidence has linked the development of antibodies to the NMDAR to preceding or concurrent HSE. In a study of 44 patients with PCR-proven HSE, serum and CSF were analyzed for a large panel of autoantibodies. Surprisingly, NMDAR antibodies were detected in 30 % of patients (13 of 44) and comprised IgA, IgG, and IgM subclasses [43••]. Importantly, all three subclasses of NMDAR antibodies have been demonstrated to have pathogenic effects on neurons [44–46]. In some patients, NMDAR titers were undetectable at onset but increased over subsequent weeks, suggesting the initiation of a new B-cell response against the NMDAR in the setting of HSE. Although these findings suggest the possibility that HSE may trigger NMDAR encephalitis, in the relatively short follow-up time, none of the antibody-positive patients developed a syndrome compatible with NMDAR encephalitis. Thus, an alternate hypothesis is that neuronal damage in the setting of HSE results in the development of NMDAR autoantibodies as an epiphenomenon, having no relationship to the well-recognized clinical syndrome of anti-NMDAR encephalitis. At present, the data linking HSE and anti-NMDAR encephalitis are not conclusive, and this remains an area of intense investigation.
Predictors of Outcome in Encephalitis
In large studies, mortality rates in encephalitis range from 5 % to 10 %, with significant morbidity among survivors. Little, however, is known of prognostic indicators of outcomes in encephalitis. Some attempts have been made to address prognostic factors in subgroups of patients with encephalitis. In HSE, for example, it is well documented that a delay of greater than 2 days between hospital admission and initiation of acyclovir therapy is associated with worse prognosis, as are older age and poor level of consciousness at the time of initiation of therapy [47, 48]. In a prospective study of patients with acute infectious encephalitis, several factors, including older age, immunosuppression, and mechanical ventilation, were associated with death during hospitalization [49]. In patients with encephalitis involving the brainstem, elevated CSF protein and glucose were associated with a poorer outcome. Importantly, elevated CSF glucose was tightly associated with elevated serum glucose, suggesting that poor systemic glycemic control may drive increased CSF glucose in such patients, potentially providing a rationale for aggressive glucose control in affected patients [50].
More recently, prognostic factors in anti-NMDAR encephalitis were addressed in an observational study that included over 500 patients. Almost half had no improvement in the first month after initiation of immunotherapy or tumor removal, but improvements from severe to slight disability occurred within the first 24 months of treatment in 81 % of patients [51•]. Predictors of good outcome included early treatment with steroids and/or immunosuppression and lack of admission to an intensive care unit (ICU).
We recently examined outcomes in patients with acute encephalitis admitted to two hospitals affiliated with the Johns Hopkins Medical Institutions. This study differs from others in that all causes of encephalitis were included; moreover, only those patients who were admitted to the ICU during their hospital stay were studied. Among almost 500 patients identified with acute encephalitis, 103 had ICU stays of greater than 48 h. Of these patients, 28 had viral encephalitis, 10 had bacterial or fungal encephalitis, 17 had autoimmune causes of encephalitis, and the remaining 48 patients had encephalitis of unknown cause [52]. Multivariate regression analysis demonstrated that of the survivors, patients with autoimmune etiology were more likely to have a poorer outcome on hospital discharge, as assessed by modified Rankin Scale, than those with other etiologies. Our results are consistent with that seen in anti-NMDAR encephalitis, in which almost half of patients had no improvement in the first month after initiation of immunotherapy or tumor removal [51•]. Thus, both our study and that of Dalmau and colleagues suggest that patients with autoimmune encephalitis may experience substantial delays before any meaningful functional recovery. We also addressed predictors of in-hospital mortality and found an association with several conditions, including cerebral edema (odds ratio [OR] 18.6), status epilepticus (OR 8.16), and thrombocytopenia (OR 6.28), all of which are potentially reversible and modifiable. Importantly, these findings suggest that aggressive supportive therapies utilized in the critical care setting may improve outcomes in those with acute encephalitis.
Recently, we have begun examining predictors of outcome in encephalitis using hospital administrative data from the United States Agency for Healthcare Research and Quality (AHRQ) Healthcare Costs and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS). We have found that complications including intubation, acute respiratory failure, aspiration pneumonia, and sepsis are strong predictors of in-hospital mortality (Venkatesan & George, unpublished), consistent with a study in France of patients with infectious encephalitis [49]. Moreover, extremes of age and comorbidities, including HIV/AIDS, cancer, and other medical diseases, were also associated with increased mortality, in agreement with other studies [17•, 47, 49, 53–55]. In addition to identifying and substantiating a number of predictors in encephalitis, these findings lend support to the further exploration of large administrative data sets to define hospitalization rates, trends in etiologies, and outcomes in encephalitis.
Conclusions
Encephalitis continues to impose a significant burden on patients and society. A number of recent studies have furthered our understanding of demographics, clinical spectra, etiologies, and prognosis. However, novel approaches are clearly needed in order to advance diagnosis and treatment of encephalitis. New tools to uncover etiologies, including pathogen discovery efforts and the application of sophisticated methodologies to identify humoral and cell-mediated autoimmune disorders, will need to be brought to bear upon this important syndrome. Further therapeutic approaches will also need to be explored, with focus not only on the etiologic agent, but also on accompanying clinical manifestations that may be modifiable. Given the heterogeneity and unpredictability of disease, it is likely that future advances in encephalitis will involve collaborative, multicenter groups.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:114–28
Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998-2000. Clin Infect Dis. 2003;36:731–42.
Kolski H, Ford-Jones EL, Richardson S, et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994-1995. Clin Infect Dis. 1998;26:398–409.
Rantalaiho T, Farkkila M, Vaheri A, Koskiniemi M. Acute encephalitis from 1967 to 1991. J Neurol Sci. 2001;184:169–77.
Irani SR, Vincent A. Autoimmune encephalitis – new awareness, challenging questions. Discov Med. 2011;11:449–58.
Rosenfeld MR, Dalmau JO. Paraneoplastic disorders of the CNS and autoimmune synaptic encephalitis. Continuum (Minneap Minn). 2012;18:366–83.
Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14.
• Petersen LR, Fischer M. Unpredictable and difficult to control–the adolescence of West Nile virus. N Engl J Med. 2012;367:1281–4. This editorial highlights some of the major challenges in defining the epidemiologyof West Nile virus, predicting outbreaks, and developing vaccines.
Centers for Disease Control and Prevention. West nile virus and other arboviral diseases - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:513–7.
Nolan MS, Schuermann J, Murray KO. West Nile virus infection among humans, Texas, USA, 2002-2011. Emerg Infect Dis. 2013;19:137–9.
Reiter P. West Nile virus in Europe: understanding the present to gauge the future. Euro Surveill. 2010;15:19508.
Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–83.
De Filette M, Ulbert S, Diamond M, Sanders NN. Recent progress in West Nile virus diagnosis and vaccination. Vet Res. 2012;43:16.
Price R, Chernik NL, Horta-Barbosa L, Posner JB. Herpes simplex encephalitis in an anergic patient. Am J Med. 1973;54:222–8.
Grover D, Newsholme W, Brink N, Manji H, Miller R. Herpes simplex virus infection of the central nervous system in human immunodeficiency virus-type 1-infected patients. Int J STD AIDS. 2004;15:597–600.
Schiff D, Rosenblum MK. Herpes simplex encephalitis (HSE) and the immunocompromised: a clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum Pathol. 1998;29:215–22.
• Tan IL, McArthur JC, Venkatesan A, Nath A. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology. 2012;79:2125–32. This case–control study includes the largest series of patients with HSE in immunocompromised patients and is the first to directly compare the presentation between immunocompromised and immunocompetent states.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15.
Hall CB, Long CE, Schnabel KC, et al. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N Engl J Med. 1994;331:432–8.
Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–76.
MacLean HJ, Douen AG. Severe amnesia associated with human herpesvirus 6 encephalitis after bone marrow transplantation. Transplantation. 2002;73:1086–9.
Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156–65.
Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–27.
de Ory F, Avellon A, Echevarria JE, et al. Viral infections of the central nervous system in Spain: a prospective study. J Med Virol. 2013;85:554–62.
Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–77.
Xu Y, Zhaori G, Vene S, et al. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J. 1996;15:1018–24.
Ward KN, Thiruchelvam AD, Couto-Parada X. Unexpected occasional persistence of high levels of HHV-6 DNA in sera: detection of variants A and B. J Med Virol. 2005;76:563–70.
Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–67.
McCullers JA, Lakeman FD, Whitley RJ. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis. 1995;21:571–6.
Meyding-Lamadé U, Strank C. Herpesvirus infections of the central nervous system in immunocompromised patients. Ther Adv Neurol Disord. 2012;5:279–96.
Luppi M, Barozzi P, Maiorana A, et al. Human herpesvirus-6: a survey of presence and distribution of genomic sequences in normal brain and neuroglial tumors. J Med Virol. 1995;47:105–11.
Ward KN, Leong HN, Thiruchelvam AD, Atkinson CE, Clark DA. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol. 2007;45:1298–304.
•• Pellett PE, Ablashi DV, Ambros PF, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22:144–55. This is an important and timely review on current methodologies and challenges in distinguishing HHV6 chromosal integration from viral reactivation.
Perl A. Pathogenesis and spectrum of autoimmunity. Methods Mol Biol. 2012;900:1–9.
Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94.
Dale RC, Brilot F. Autoimmune basal ganglia disorders. J Child Neurol. 2012;27:1470–81.
Yuki N. Ganglioside mimicry and peripheral nerve disease. Muscle Nerve. 2007;35:691–711.
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–8.
Gable MS, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421–9.
Baltagi SA, Shoykhet M, Felmet K, Kochanek PM, Bell MJ. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 2010;11:179–84.
Xu CL, Liu L, Zhao WQ, et al. Anti-N-methyl-D-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol. 2011;11:149.
•• Prüss H, Finke C, Holtje M, et al. N-methylD-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902–11. This retrospective analysis of 44 CSF PCR positive HSE patients found that 13 patients (30%) had antibodies to the NMDA receptor in either serum or CSF and is the first to suggest a potential relationship between HSE and anti-NMDAR encephalitis.
Prüss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–9.
Prüss H, Höltje M, Maier N, et al. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012;78:1743–53.
Manto M, Dalmau J, Didelot A, Rogemond V, Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J Rare Dis. 2010;5:31.
Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35:254–60.
Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–9.
Mailles A, Stahl JP. Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis. 2009;49:1838–47.
Tan IL, Mowry EM, Steele SU, et al. Brainstem encephalitis: etiologies, treatment, and predictors of outcome. J Neurol. 2013;260:2312–9.
• Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. In this multicenter observational study, over 500 patients with anti-NMDAR encephalitis were followed for a median of 24 months, and treatment effects and outcomes were assessed.
Thakur KT, Motta M, Asemora AO, et al. Predictors of outcome in acute encephalitis. Neurology 2013;81:793–800.
Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–8.
McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry. 1997;63:321–6.
Emig M, Apple DJ. Severe West Nile virus disease in healthy adults. Clin Infect Dis. 2004;38:289–92.
Compliance with Ethics Guidelines
Conflict of Interest
Arun Venkatesan declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesan, A. Advances in Infectious Encephalitis: Etiologies, Outcomes, and Potential Links with Anti-NMDAR Encephalitis. Curr Infect Dis Rep 15, 594–599 (2013). https://doi.org/10.1007/s11908-013-0382-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-013-0382-9