Abstract
Purpose of Review
Herpesviruses are a leading cause of encephalitis worldwide. The article reviews the eight human herpesviruses with a focus on recent advances as they pertain to encephalitis.
Recent Findings
Notable recent updates include the development of multiplex polymerase chain reaction (PCR)-based panels, which have improved access to PCR tests, especially in rural and resource-limited areas. Despite unchanged treatment recommendations, research is ongoing into novel therapies. There have been recent advances in vaccines, particularly for varicella zoster virus (VZV) which may impact neurologic complications. Finally, the recent discovery of an association between herpes encephalitis and post-infectious autoimmune encephalitis has had a critical impact on the fields of infectious and autoimmune neurology, though there remains much to learn.
Summary
Most herpesviruses are neurotropic and must be considered on the differential diagnosis for infectious encephalitis. This article describes recent advances in the diagnosis, treatment, complications, and management of these infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herpesviruses are the most commonly diagnosed infectious encephalitides in Western countries [1]. While the epidemiology of encephalitis in tropical countries is more often driven by specific outbreaks, herpesviruses are the most frequently identified pathogen outside these epidemics [1,2,3]. In this article, we review the eight herpesviruses as they pertain to acute encephalitis, with a focus on recent advances. Several notable updates that are highlighted include the implementation and ramifications of new multiplex polymerase chain reaction (PCR) panels, evolving work in antiviral treatments and vaccinations, and the recognition of postinfectious autoimmune encephalitis. Nervous system involvement of herpesviruses beyond encephalitis (of which there are many) is outside the scope and not covered in this review.
Herpes Simplex Virus 1 (HSV1)
HSV1 is the leading cause of sporadic infectious encephalitis worldwide in all age groups [1,2,3,4]. HSV1 is a double-stranded deoxyribonucleic acid (DNA) virus with seropositivity rates around 40% in adults in industrialized nations and as high as 80–90% in the developing world [5]. An important epidemiologic shift over the past 20 years has been the rise in cases of HSV1 associated genital infections [6, 7]. Likely due to this shift, HSV1 has recently overtaken herpes simplex virus 2 (HSV2) as the leading cause of neonatal encephalitis [7,8,9].
Primary infection with HSV1 typically causes orolabial lesions, after which the virus establishes latency in the trigeminal and other cranial sensory ganglia. While primary infection can lead encephalitis, particularly in neonates and children, most cases of adult herpes encephalitis occur via reactivation of latent virus [10]. Adults with herpes simplex encephalitis (HSE) classically present with symptoms localizing to the frontal and temporal lobes including confusion, behavioral changes, impaired consciousness, aphasia, and seizures. Prodromal symptoms including fever and headache are common [5, 10]. Mortality rates in the era before effective antiviral therapy were as high as 70% [5]. Even with antiviral treatment, only 38% of patients return to their premorbid level of function [5]. Neonates with HSE typically present 16–17 days postpartum with nonspecific symptoms including lethargy, irritability, temperature instability, and poor oral intake [4]. Cutaneous lesions may be a helpful diagnostic clue in the neonatal population but are only present in 35% of cases [4].
While brain biopsy was the historic gold standard for diagnosis, the development of PCR-based testing on cerebrospinal fluid (CSF) has eliminated the need for biopsy in most cases [5]. CSF HSV PCR testing is considered exquisitely sensitive and specific (96–98% and 95–99%, respectively) [11]. False negatives may occur if testing is performed in the first few days of symptoms; thus, when suspicion for HSE is high and the initial PCR is negative, the Infectious Disease Society of America (IDSA) recommends retesting 3–7 days later [11]. Empiric antiviral therapy should not be delayed if CSF HSV PCR testing is not immediately available as several days of treatment are unlikely to result in a negative CSF PCR. However, CSF HSV PCR is typically negative after 10–14 days of acyclovir therapy [12]. False negatives may also be seen when CSF contains potential PCR inhibitors such heme products, bilirubin, immunoglobulins, or anticoagulants [13]. Despite the high reported sensitivity, there have been many recent case reports of repeated false negatives, even following the IDSA guidelines to retest after 4–7 days. Unfortunately, most of these cases had fatal outcomes [13,14,15,16]. Based on these findings, it may be worthwhile to consider brain biopsy or completion of empiric antiviral treatment in patients with a high clinical suspicion and negative testing. The HSV PCR may be sent alone or as part of a multiplex test such as the BioFire® FilmArray® Meningitis/Encephalitis Panel. Several studies have reported slightly higher false negative rates of HSV with the multiplex panels compared to singleplex HSV PCR tests [17•]. This small increase in false negatives may be outweighed by the faster turnaround time and ease of use, particularly in rural or resource limited settings where traditional PCR tests must be sent to reference laboratories, whereas multiplex panels can be performed in-house [18, 19].
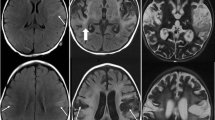
Neuroimaging is another critical diagnostic tool for HSE. Magnetic resonance imaging (MRI) is the preferred modality, with abnormalities seen in over 90% of PCR-proven cases. In resource-limited settings where MRI is difficult to obtain, computed tomography (CT) may be considered, though sensitivity is much lower, especially early in the disease course [12]. The most frequent MRI finding is unilateral or bilateral T2 hyperintensity within the temporal lobes, though frontal lobe involvement is also quite common. The lesions may demonstrate heterogeneous contrast enhancement, diffusion restriction, and/or hemorrhage [12, 20].
When HSE is suspected, empiric antiviral treatment should be started as soon as possible. The recommended regimen is high dose intravenous (IV) acyclovir (10 mg/kg of ideal body weight every 8 hours) for a total of 14–21 days [11]. Acyclovir has numerous limitations including nephrotoxicity, challenges in dosing, drug shortages, high rates of poor outcomes despite treatment, and increasing resistance—which several recent studies have attempted to address. One small case series demonstrated successful treatment of two patients with a continuous infusion of acyclovir, which may be preferred in some settings to dosing every 8 hours, but experience is very limited [21]. There has also been extensive research attempting to improve the oral bioavailability of acyclovir. While there was some encouraging data that the prodrug valacyclovir given orally every 8 hours might be adequate to treat HSE in resource-limited settings, experience and data are limited, and extreme caution is advised [22••, 23]. Similarly, high dose oral acyclovir and alternative formations including the use of absorption enhancers and novel drug delivery systems are being investigated with some promising data, but further research is needed before clinical implementation [22••]. Acyclovir resistance is a growing concern, particularly among immunocompromised patients, where prevalence of acyclovir-resistant HSV infections may be as high at 10% [24]. The data on the optimal management in for acyclovir-resistant strains is limited to case reports, but several agents have been successfully used including foscarnet, cidofovir, and brincidofovir [5]. In the past several years, promising data has emerged for antiviral agents with novel mechanisms against HSV. However, these remain in the preclinical research phase or are in early clinical trials for non-central nervous system (CNS) HSV infections [25,26,27,28,29, 30•]. The one active clinical trial in HSE is studying the impact of dexamethasone in conjunction with acyclovir on long-term neurocognitive outcomes, which is part of a larger movement to investigate immunomodulatory agents for HSE [31]. For now, monotherapy with IV acyclovir remains the gold standard for children and adults with HSE. Alternative agents, dosing or delivery methods are only recommended when IV acyclovir is not available or in refractory cases when resistance is suspected or proven [32, 33].
In addition to antiviral research, there has been a decades-long effort to develop a HSV vaccine without success. However, there are numerous active clinical and preclinical studies for HSV vaccines, including several utilizing the promising new technology of messenger ribonucleic acid (RNA)-based vaccines, which has been highly successful against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [34].
HSE and Autoimmune Encephalitis
A critical recent discovery was the recognition of post-infectious autoimmune encephalitis. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis following HSE was the first of these to be reported [35]. With increased awareness, it now appears this entity is not uncommon. Recent studies have demonstrated that 18–30% of patients with HSE go on to develop anti-NMDAR antibodies during or shortly after the viral encephalitis [35, 36]. The presence of these antibodies is associated with classic autoimmune encephalitis in most patients, though notably some patients who developed anti-NMDAR antibodies after HSE did not clinically develop autoimmune encephalitis at 12-month follow-up [36]. For those who did develop autoimmune encephalitis, the median time of onset after herpes encephalitis was 32 days, and the majority occurred within the first 2 months, but this ranged significantly (7–306 days) [36]. Several potential mechanisms for post-infectious autoimmune encephalitis have been proposed including (1) molecular mimicry, (2) neuronal damage leading to exposure of NMDAR to the immune system, (3) altered NMDAR expression due to HSV infection, and (4) modulation of the immune system due to HSV infection leading to inappropriate recognition of NMDAR [37]. Linnoila et al. recently developed a mouse model of post-infectious NMDAR encephalitis, opening the door to additional research [38••].
In addition to post-HSV NMDAR encephalitis, there are also case reports of post-HSV autoimmune encephalitis with antibodies against gamma-aminobutyric acid (GABA)-A receptors, glial fibrillary acidic protein (GFAP), and other unclassified neuronal autoantibodies [14, 39,40,41]. Other herpes viruses have also been implicated including varicella zoster virus (VZV) and Epstein-Barr virus (EBV) though less commonly [42, 43]. Clinicians should consider testing for autoantibodies in patients who develop new or worsening neurologic symptoms despite adequate treatment of viral encephalitis. At this point, there is insufficient data to recommend routine screening for autoantibodies in patients with herpesvirus encephalitis in the absence of a clinical syndrome concerning for autoimmune encephalitis. The optimal management of these patients remains unknown, though there is one retrospective, non-interventional study which is actively recruiting and may provide some insight [44]. Although limited data exists at present, small case series have shown benefit to treating with the same immunosuppressive regimens used in non-infection–triggered cases of NMDAR encephalitis [36]. Based on this, these authors advocate for the use of immunosuppressive therapy in this patient population, assuming active infection has been excluded as confidently as possible.
Herpes Simplex Virus 2
HSV2 is related to HSV1, though only 50% of the genome is shared by the two viruses and there are multiple clinical differences [45]. As above, HSV1 typically causes primary orolabial infection, establishes latency in the trigeminal ganglia, and can reactivate leading to encephalitis. HSV2 typically causes a primary genital infection, followed by latency in the sacral sensory ganglia, after which it can reactivate, usually in the form of meningitis. This difference between CNS manifestations of HSV1 and HSV2 does not appear to be due to the site of latency, but instead due to differences in the viral pathogenicity and the subsequent inflammatory response [6, 46].
The most common HSV2 CNS manifestation is self-limited viral meningitis [5]. HSV2 has also been shown to be a major cause of recurrent lymphocytic meningitis (also referred to as Mollaret meningitis) [47•]. Recent work has identified specific genetic variants associated with altered immune signaling pathways in patients with recurrent HSV2 meningitis, which may explain why these patients are predisposed to recurrent CNS reactivation [48]. The current treatment recommendation for HSV2 meningitis is IV acyclovir (5–10 mg/kg every 8 hours) until clinical improvement is observed followed by high-dose oral valacyclovir (1 g three times a day) for a total duration of antiviral treatment of 10–14 days [49]. A 2012 randomized trial demonstrated that suppressive valaciclovir does not prevent recurrent episodes and thus is not recommended [50]. Most patients with HSV2 meningitis have favorable outcomes [51•].
While much less common, HSV2 can lead to encephalitis, particularly among neonates, children, and immunosuppressed individuals [5, 51•]. HSV2 encephalitis most commonly affects the frontotemporal lobes (similar to HSV1), though isolated basal ganglia and/or brainstem encephalitis may also be seen [52, 53]. When encephalitis occurs, a full course of high-dose IV acyclovir is recommended (10 mg/kg every eight hours for 14–21 days) [49]. Unlike the fairly benign course of HSV2 meningitis, outcomes from HSV2 encephalitis are more variable, and permanent neurologic sequalae including epilepsy and acute retinal necrosis may occur [52, 54].
It is important to note that many recent studies related to HSE (including most of those discussed under the HSV1 section about diagnostic testing, management, and associated autoimmune encephalitis) include patients with both HSV1 and HSV2 encephalitis. While most patients in these studies had HSV1, some of these updates may pertain to HSV2 as well.
Varicella Zoster Virus
VZV is the third human herpesvirus (HHV3) with seropositivity rates between 80 and 92% [55, 56]. This historically occurred via primary varicella infection, which is highly contagious and spread via direct contact with vesicular fluid or inhalation of respiratory droplets [57]. Primary infection, also called “chicken pox,” causes a widely distributed vesicular rash 10–21 days post-exposure, after which the virus establishes latency in the dorsal root ganglia [58]. Similar to HSV1 and HSV2, CNS involvement of VZV is more commonly seen during reactivation, though can rarely occur during primary infection. The most commonly reported primary CNS manifestation is acute cerebellitis which is seen in around 1 per 4000 cases, predominantly in children [5].
When CNS infection occurs during reactivation, it leads to a wide variety of syndromes including meningitis, encephalitis, myelitis, retinitis, and vasculitis [5, 59, 60•]. VZV reactivation occurs in up to 50% of patients with latent infection, usually as an uncomplicated dermatomal rash referred to as shingles [60•]. During reactivation, however, the virus may invade the CNS, particularly in elderly or immunosuppressed patients [61•]. Several classic syndromes have been described. Herpes zoster ophthalmicus refers to reactivation in the V1 trigeminal nerve dermatome and can lead to multiple complications including upper cranial neuropathies, retinal necrosis, uveitis, and scleritis and has been associated with contralateral hemiparesis due to ischemic or hemorrhagic strokes [5]. Ramsay-Hunt syndrome refers to reactivation within the geniculate ganglion of the facial nerve with vesicles generally seen around the external ear and palate and may lead to facial or vestibulocochlear palsies [5]. Reactivation along the dermatomes of the trunk has been associated with radiculitis, peripheral nerve palsies, and myelitis [62]. However, it is important to recognize that many patients do not follow these classic syndromes and any of the CNS manifestations may be seen with dermatomal reaction at any site or in the absence of a dermatomal rash. Thus, it may be more appropriate to categorize these patients by the CNS manifestation regardless of dermatome involved.
In addition to direct meningeal or parenchymal infection, VZV has a vascular predilection, and it now appears that the majority of CNS VZV complications may, in fact, be related to VZV vasculopathy [61•]. VZV infection appears particularly critical in pediatric cerebrovascular disease, with one study demonstrating that 31% of pediatric strokes were associated with recent varicella infection [63]. Beyond ischemic strokes, VZV vasculopathy has also been associated with arterial dissection, aneurysm and subarachnoid hemorrhage, ischemic cranial neuropathies, ischemic myelopathy, venous sinus thrombosis, and temporal arteritis [64]. Clinically, VZV vasculopathy may be difficult to distinguish from VZV encephalitis and meningitis, but it is important to consider this entity as it may occur without detectable virus in the CSF as described below.
In the setting of clinical meningitis or encephalitis, a positive CSF VZV PCR confirms the diagnosis [5]. With the increased use of PCR testing, it appears that VZV meningitis and encephalitis may be much more common than previously thought. One recent study of 70 patients with meningitis or encephalitis found a positive VZV CSF PCR in 30% of cases [65]. A second retrospective study found that VZV accounted for 1% of all aseptic meningitis cases (notably higher than either HSV1 or HSV2) and 5% of all encephalitis cases (only slightly lower than the rate of HSV1 encephalitis) [51•]. In the case of CNS VZV vasculopathy, Nagel et al. demonstrated that CSF VZV PCR was only positive in 28% of cases of suspected VZV vasculopathy, whereas the VZV-specific immunoglobulin G (IgG) in the CSF was positive in 100% of cases [66]. However, subsequent studies have shown that elevated CSF VZV IgG may be seen in patients with prior systemic infection (due to either blood contamination of the CSF or passive transfer across the blood brain barrier) or as part of a generalized intrathecal inflammatory response [67,68,69]. Otto et al. demonstrated that an elevated VZV-specific antibody index (calculated as \(\frac{\left[\mathrm{CSF\ VZV\ IgG}\right]\mathrm{ x }[\mathrm{Serum total IgG}]}{\left[\mathrm{Serum\ VZV\ IgG}\right]\mathrm{ x }[\mathrm{CSF\ total\ IgG}]}\)) greater than 1.5 may distinguish CNS VZV reactivation from a generalized inflammatory response or prior systemic infection [67]. Thus, in patients with suspected CNS VZV infection, these authors recommend checking VZV PCR and VZV-specific antibody index, especially in cases of suspected vasculopathy.
For confirmed VZV encephalitis, IV acyclovir 10 mg/kg every 8 hours for 14 days is recommended, with consideration of longer courses or even long-term suppressive therapy in immunosuppressed patients [5]. For the case of VZV vasculopathy, the optimal treatment remains unknown. Most advocate for a 14-day course IV acyclovir (10–15 mg/kg every 8 hours) plus a short course of steroids (typically 3–5 days of prednisone 1 mg/kg/day), though this remains controversial [5, 64, 70].
A review of VZV infection would be incomplete without a discussion of vaccinations. A live attenuated varicella vaccine was first developed in the 1970s and became clinically available starting in the late 1980s [71]. The World Health Organization (WHO) currently recommends that varicella vaccination be implemented in all countries where varicella is an important public health concern but only if resources allow for sustained vaccination rates of > 80% since lower rates may be associated with increased morbidity and mortality among vulnerable populations [72]. Based on these recommendations, varicella vaccination has seen widespread implementation, though notably, this has not been the case in the majority of tropical countries [73,74,75]. The impact of varicella vaccination on CNS VZV infection is not known. However, it is critical to understand that the live attenuated virus in the vaccine is still capable of producing latent infection, and reactivation seems to occur at similar rates as seen in wild-type varicella exposure [76]. Thus, a history of varicella vaccination does not exclude the diagnosis of VZV CNS infection. In fact, there have been theoretical concerns about varicella vaccination increasing the rate of VZV reactivation (and thus possibly increasing the rate of CNS disease during reactivation as well), though real-world data has been mixed [73].
In 2006, the Food and Drug Administration (FDA) approved a live attenuated herpes zoster vaccine (Varivax®), which reduces VZV reactivation by around 60% [77]. This was followed by FDA approval of a new recombinant subunit inactivated shingles vaccine (Shingrix®) in 2017, which reduces VZV reactivations by greater than 95% [78]. Given the association of CNS VZV infection with reactivation, this reduction is likely associated with a similar reduction in CNS manifestations, though this has not been specifically studied. Shingrix® has now supplanted the live attenuated vaccine in the USA due to improved efficacy and safety [79]. It has also just received full FDA approval in the USA for administration for immunosuppressed patients over the age of 18 years old (previously only approved for over 50 years old) [80]. In addition, it is licensed or approved in 62 countries worldwide, though data on implementation is limited [81]. The WHO does not currently have recommendations regarding zoster vaccination due to its recent implementation and minimal data [72]. Future studies are needed to determine the efficacy of zoster vaccination against CNS manifestations specifically, which may perhaps lead to a more widespread effort to increase vaccination.
Epstein-Barr Virus
Similar to other human herpesviruses, EBV, also known as human herpes virus 4 (HHV4) is ubiquitous with primary infection typically occurring during childhood or adolescence. Most of these cases are asymptomatic or lead to infectious mononucleosis, although encephalitis can rarely occur [5, 82]. Following primary infection, EBV becomes latent within lymphocytes [83]. CNS infection due to reactivation is rare, but when it does occur, tends to present as a subacute encephalitis in profoundly immunosuppressed patients [5, 84]. A particularly high-risk group are transplant patients who are EBV seronegative at the time of transplant and receive an organ from an EBV seropositive donor [85]. In a patient with clinical and/or radiographic features of encephalitis, a positive CSF EBV PCR is highly suggestive of this diagnosis [5, 11]. However, it is important to note that due to EBV latency within the lymphocytes, a low-positive EBV PCR may be seen due to lysis of these cells and not true neuroinvasive infection [11]. While some advocate for the use of EBV serologies in the diagnosis of EBV encephalitis, these can be difficult to interpret as EBV encephalitis may occur in either primary EBV infection or reactivation and may occur in patients with such significant immunosuppression that they are unable to mount an antibody response to the virus. Thus, these authors recommend using PCR testing whenever available. Antiviral treatment remains controversial, though many advocate for 14–21 days of acyclovir or ganciclovir [5, 11, 82, 84]. Outcomes are favorable with 85% returning to baseline function [5].
While outside the scope of this review, it is important to note that EBV latency within lymphocytes may lead to EBV-associated lymphoproliferative disorders (EBV-LD), especially in immunosuppressed patients [83]. CNS EBV-LD may be clinically indistinguishable for subacute encephalitis; thus, clinicians should consider this entity in all patients with positive EBV CSF PCR, particularly those who do not have clinical or radiographic improvement after treatment of presumed EBV encephalitis.
Cytomegalovirus Virus (CMV)
CMV has variable seroprevalence with rates around 60% in the developed world and 100% in the developing world [86, 87]. Like EBV, it is rarely associated with neurologic manifestations in the immunocompetent host [5]. Primary infection is usually asymptomatic, followed by viral latency within the bone marrow hematopoietic progenitor cells [88]. Reactivation is generally limited to profoundly immunocompromised hosts, particularly those with advanced human immunodeficiency virus (HIV) infection or following hematopoietic stem cell transplantation (HSCT) [5, 89•]. CMV encephalitis has also been rarely reported in immunocompetent infants [90]. Patients often present with a subacute encephalitis and may have simultaneous retinitis, myelitis, and/or polyradiculitis [5, 89•]. MRI abnormalities are seen in one third of patients with CMV encephalitis, with the most common abnormalities being findings suggestive of ventriculitis (periventricular hyperintensities, subependymal enhancement, diffusion-restricting intraventricular material) [89•]. Diagnosis is made by positive CSF CMV PCR, and CSF may notably have polymorphonuclear pleocytosis and hypoglycorrhachia [89•]. The optimal treatment is unknown, but many advocate for treatment with ganciclovir, foscarnet, or both [5, 91]. Despite treatment, the prognosis of CMV encephalitis is generally poor [5].
Human Herpes Virus 6 (HHV6)
Primary HHV6 infection leads to the febrile syndrome roseola, and seroprevalence is around 80% [5]. CNS HHV6 disease is exceedingly rare, occurring only in profoundly T-cell depleted patients, as seen after HSCT [5]. Patients most commonly present with limbic encephalitis characterized by headaches, behavior changes, amnesia, and seizures [5]. There are no approved treatments, though case reports have shown some success with antiviral treatment including ganciclovir, foscarnet, cidofovir, and brincidofovir [92].
Of note, HHV6 is capable of chromosomal integration. When this occurs in a germ cell, all cells from subsequent offspring contain the viral DNA, which is seen in around 0.5–2% of the population [93•]. In these patients, PCR testing may reveal positive HHV6 simply due to lysis of their own cells. HHV6 is included in several of the now commonly used multiplex PCR panels, and with increased implementation of these panels, there have been increasing reports of HHV6 meningitis and encephalitis [94, 95•]. On further review, many of these positive results were clinically insignificant and likely reflected chromosomal integration [93•, 95•]. Thus, it is critical that these results be interpreted in the clinical context. In an immunocompetent patient, a positive HHV6 PCR test should generally be considered non-pathogenic, and alternative etiologies should be investigated.
Human Herpes Virus 7 and 8 (HHV7, HHV8)
HHV7 and HHV8 are included here for the sake of thoroughness, but neurological manifestations (including encephalitis and secondary CNS manifestations such as CNS metastases due to HHV8-associated Kaposi sarcoma) are limited to a few isolated case reports [96,97,98,99]. Based on the currently available data, these authors do not routinely recommend HHV7 and HHV8 testing for cases of clinical meningitis and encephalitis.
Conclusion
Herpesviruses are the most frequently diagnosed cause of infectious encephalitis worldwide and make up a significant portion of meningitis cases as well. Despite the many potential infectious etiologies of meningoencephalitis in tropical regions, HSV1 and HSV2 should remain high on the differential given their high incidence. VZV is likely more prevalent than previously thought. While zoster vaccination may lead to a reduction in VZV CNS infection, VZV remains a critical pathogen and should not be overlooked. Other herpes viruses should also be considered in the correct clinical context, especially in cases of immunosuppression. When available, PCR-based CSF testing is critical to establish these diagnoses, though clinicians should be careful to interpret the results in the clinical and epidemiologic context (as both false negatives and false positives can occur). Empiric treatment should not be delayed or stopped if the clinical suspicion is high. Research is ongoing into prevention, diagnostics, and optimal treatment of herpes virus encephalitides and the newly described post-infectious autoimmune encephalitis.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Boucher A, Herrmann JL, Morand P, Buzelé R, Crabol Y, Stahl JP, Mailles A. Epidemiology of infectious encephalitis causes in 2016. Méd Mal Infect. 2017;47:221–35.
Chaumont H, Roze E, Tressières B, Lazarini F, Lannuzel A. Central nervous system infections in a tropical area: influence of emerging and rare infections. Eur J Neurol. 2020;27:2242–9.
Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol J. 2008;5:134.
James SH, Kimberlin DW. Neonatal herpes simplex virus infection: epidemiology and treatment. Clin Perinatol. 2015; 42:47–59, viii.
Baldwin KJ, Cummings CL. Herpesvirus infections of the nervous system. Contin Minneap Minn. 2018;24:1349–69.
Zhu S, Viejo-Borbolla A. Pathogenesis and virulence of herpes simplex virus. Virulence. 2021;12:2670–702.
Wald A. Genital HSV-1 infections. Sex Transm Infect. 2006;82:189–90.
Corey L, Wald A, Celum CL. Quinn TC (2004) The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 1999;35:435–45.
Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Semin Perinatol. 2018;42:168–75.
Gnann JW, Whitley RJ. Herpes simplex encephalitis: an Update. Curr Infect Dis Rep. 2017;19:13.
Tunkel AR, Glaser CA, Bloch KC, et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;47:303–27.
Tyler KL. Update on herpes simplex encephalitis. Rev Neurol Dis. 2004;1:169–78.
Niksefat M, Guillen D, Moshayedi P, Rinaldo CR, Ojha A. Third time’s a charm: diagnosis of herpes simplex encephalitis after two negative polymerase chain reaction results. Heliyon. 2020; 6:e04247.
Mendez AA, Bosco A, Abdel-Wahed L, Palmer K, Jones KA, Killoran A. A fatal case of herpes simplex encephalitis with two false-negative polymerase chain reactions. Case Rep Neurol. 2018;10:217–22.
Matthews E, Alkhachroum A, Massad N, Letchinger R, Doyle K, Claassen J, Thakur KT. New-onset super-refractory status epilepticus: a case series of 26 patients. Neurology. 2020;95:e2280–5.
Roberts JI, Jewett GAE, Tellier R, Couillard P, Peters S. Twice negative PCR in a patient with herpes simplex virus type 1 (HSV-1) encephalitis. Neurohospitalist. 2021;11:66–70.
• Tansarli GS, Chapin KC. Diagnostic test accuracy of the BioFire® FilmArray® meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect . 2020;26:281–90. Are important updates regarding multiple PCR-based panels that discuss the diagnostic accuracy and clinical significance of results. These tests have many benefits but as utilization increases, clinicians must understand the limitations which these two papers nicely address.
Dien Bard J, Alby K. Point-Counterpoint: meningitis/encephalitis syndromic testing in the Clinical Laboratory. J Clin Microbiol. 2018;56:e00018-18.
Leber AL, Everhart K, Balada-Llasat J-M, et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J Clin Microbiol. 2016;54:2251–61.
Sili U, Kaya A, Mert A, HSV Encephalitis study group. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118.
O’Leary CK, Jones C, Bryant PA, Abo Y-N, Osowicki J, Gwee A. Feasibility of continuous infusions of acyclovir. Pediatr Infect Dis J. 2020;39:830–2.
•• Gurgel Assis MS, Fernandes Pedrosa TC, de Moraes FS, Caldeira TG, Pereira GR, de Souza J, Ruela ALM. Novel insights to enhance therapeutics with acyclovir in the management of herpes simplex encephalitis. J Pharm Sci. 2021;110:1557–71. Is an in-depth review of limitations of acyclovir and potential solutions. As a very commonly used medication (and the only currently recommended treatment for HSV/VZV encephalitis) but one that has numerous limitations, it is critical to research alternative therapies to overcome these limitations).
Bodilsen J, Nielsen H, Whitley RJ. Valaciclovir therapy for herpes encephalitis: caution advised. J Antimicrob Chemother. 2019;74:1467–8.
Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2010.
Liu Y, You Q, Zhang F, Chen D, Huang Z, Wu Z. Harringtonine Inhibits herpes simplex virus type 1 infection by reducing herpes virus entry mediator expression. Front Microbiol. 2021;12:2465.
Quenelle DC, Birkmann A, Goldner T, Pfaff T, Zimmermann H, Bonsmann S, Collins DJ, Rice TL, Prichard MN. Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis. Antiviral Res. 2018;149:1–6.
Li F, Song X, Su G, et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses. 2019;11:466.
Hou J, Zhang Z, Huang Q, Yan J, Zhang X, Yu X, Tan G, Zheng C, Xu F, He S. Antiviral activity of PHA767491 against human herpes simplex virus in vitro and in vivo. BMC Infect Dis. 2017;17:217.
Álvarez DM, Castillo E, Duarte LF, Arriagada J, Corrales N, Farías MA, Henríquez A, Agurto-Muñoz C, González PA. Current Antivirals and Novel Botanical Molecules Interfering With Herpes Simplex Virus Infection. Front Microbiol. 2020;11:139.
• Piret J, Boivin G. Immunomodulatory Strategies in Herpes Simplex Virus Encephalitis. Clin Microbiol Rev. 2020;33:e00105-e119. Reviews the detailed immunology behind HSV and associated encephalitis/CNS disease and addresses the potential for immunomodulatory strategies to improve outcomes.
Dexamethasone in Herpes Simplex Virus Encephalitis (DexEnceph). ClinicalTrials.gov Identifier: NCT03084783.
Intravenous Acyclovir Shortage Recommendations for Pediatrics.
McLaughlin MM, Sutton SH, Jensen AO, Esterly JS. Use of High-Dose Oral Valacyclovir During an Intravenous Acyclovir Shortage: A Retrospective Analysis of Tolerability and Drug Shortage Management. Infect Dis Ther. 2017;6:259–64.
Stanfield BA, Kousoulas KG, Fernandez A, Gershburg E. Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses. Viruses. 2021;13:1637.
H P, C F, M H, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;https://doi.org/10.1002/ana.23689.
Armangue T, Spatola M, Vlagea A, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–72.
Salovin A, Glanzman J, Roslin K, Armangue T, Lynch DR, Panzer JA. Anti-NMDA receptor encephalitis and nonencephalitic HSV-1 infection. Neurol Neuroimmunol Neuroinflamm. 2018;5:e458.
•• Linnoila J, Pulli B, Armangué T, Planagumà J, Narsimhan R, Schob S, Zeller MWG, Dalmau J, Chen J. Mouse model of anti-NMDA receptor post–herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm. 2019. https://doi.org/10.1212/NXI.0000000000000529. Outlines the critical work that was done to develop a mouse model of post-infectious NMDA encephalitis, which will open doors to ongoing research into the pathogenesis of this newly described condition.
Li J, Xu Y, Ren H, Zhu Y, Peng B, Cui L. Autoimmune GFAP astrocytopathy after viral encephalitis: A case report. Mult Scler Relat Disord. 2018;21:84–7.
Handoko M, Hong W, Espineli E, Saxena K, Muscal E, Risen S. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy Following Herpes Simplex Virus Encephalitis in a Pediatric Patient. Pediatr Neurol. 2019;98:85–6.
Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e245.
Schwenkenbecher P, Skripuletz T, Lange P, et al. Intrathecal Antibody Production Against Epstein-Barr, Herpes Simplex, and Other Neurotropic Viruses in Autoimmune Encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1062.
Danieli D, Moraes ACM, Alves MP, Dutra LA, Höftberger R, Barsottini OGP, Masruha MR. Anti-N-methyl-D-aspartate receptor encephalitis and Epstein-Barr virus: another tale on autoimmunity? Eur J Neurol. 2017;24:e46–7.
Autoimmune Encephalitis With Anti-NMDA Receptor Antibodies Following Herpetic Encephalitis (NMDARE-HSE). ClinicalTrials.gov Identifier:NCT04339127.
Corey L. Herpes Simplex Virus. In: Mandell’s Principles and Practice of Infectious Diseases, Sixth. 2005; Churchill Livingstone, New York.
Lind L, Studahl M, Persson Berg L, Eriksson K. CXCL11 production in cerebrospinal fluid distinguishes herpes simplex meningitis from herpes simplex encephalitis. J Neuroinflammation. 2017;14:134.
• Hait AS, Thomsen MM, Larsen SM, Helleberg M, Mardahl M, Barfod TS, Christiansen M, Brandt C, Mogensen TH. Whole-Exome Sequencing of Patients With Recurrent HSV-2 Lymphocytic Mollaret Meningitis. J Infect Dis. 2021;223:1776–86. Identifies genetic mutations associated with recurrent HSV2 meningitis, which is helpful in understanding risk factors for development but also in further defining the pathophysiology of this condition.
Goyal T, Ali I. Recurrent Herpes Simplex Virus 2 Lymphocytic Meningitis in Patient with IgG Subclass 2 Deficiency. Emerg Infect Dis. 2020;26:748–50.
Herpes - STI Treatment Guidelines. 2021; https://www.cdc.gov/std/treatment-guidelines/herpes.htm. Accessed 21 Dec 2021.
Aurelius E, Franzen-Röhl E, Glimåker M, Akre O, Grillner L, Jorup-Rönström C, Studahl M, HSV-2 Meningitis Study Group. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: a double-blind, randomized controlled trial. Clin Infect Dis. 2012;54:1304–13.
• Lee G-H, Kim J, Kim H-W, Cho JW. Herpes simplex viruses (1 and 2) and varicella-zoster virus infections in an adult population with aseptic meningitis or encephalitis: A nine-year retrospective clinical study. Medicine (Baltimore). 2021;100:e27856. Is a large retrospective study that helps shine light on positive CSF tests for HSV1, HSV2 and VZV – both helping update what is known about the epidemiology of these infections but also the clinical significance of positive results, the clinical syndromes (with clear separation into encephalitis versus meningitis) and patient outcomes.
Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol. 2008;65:596–600.
Liu F-Y, El Mouhayyar C, Mamtani R, Dammann F, Basein T. A case of herpes simplex 2 encephalitis with an unusual radiographic manifestation. IDCases 2020;21:e00884.
Hansen A-BE, Vestergaard HT, Dessau RB, et al. Long-Term Survival, Morbidity, Social Functioning and Risk of Disability in Patients with a Herpes Simplex Virus Type 1 or Type 2 Central Nervous System Infection, Denmark, 2000–2016. Clin Epidemiol. 2020;12:745–55.
Büchele KS, Correa DF, Tuyama M, et al. Seroprevalence of varicella antibodies in adults without clinical history of disease. Cad Saude Publica. 2020;36:e00149119.
Wiese-Posselt M, Siedler A, Mankertz A, Sauerbrei A, Hengel H, Wichmann O, Poethko-Müller C. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era. Germany BMC Infect Dis. 2017;17:356.
CDC. Chickenpox for HCPs. In: Cent. Dis. Control Prev. 2021; https://www.cdc.gov/chickenpox/hcp/index.html. Accessed 30 Dec 2021.
Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210.
Greenlee JE. Nervous System Complications of Systemic Viral Infections. Aminoffs Neurol Gen Med. 2014;857–883.
• Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella zoster virus. Curr Opin Infect Dis. 2020;33:273–8. Give important updates on the pathophysiology and clinical syndromes associated with CNS VZV infection.
• Kennedy PG, Mogensen TH. Determinants of neurological syndromes caused by varicella zoster virus (VZV). J Neurovirol. 2020;26:482–95. Give important updates on the pathophysiology and clinical syndromes associated with CNS VZV infection.
Kennedy PGE, Gershon AA. Clinical Features of Varicella-Zoster Virus Infection. Viruses. 2018;10:609.
Askalan R, Laughlin S, Mayank S, Chan A, MacGregor D, Andrew M, Curtis R, Meaney B, deVeber G. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–62.
Nagel MA, Gilden D. Update on Varicella Zoster Virus Vasculopathy. Curr Infect Dis Rep. 2014;16:407.
Alvarez JC, Alvarez J, Tinoco J, Mellado P, Miranda H, Ferrés M, Forero J, Álvarez C. Varicella-Zoster Virus Meningitis and Encephalitis: An Understated Cause of Central Nervous System Infections. Cureus 12:e11583.
Nagel MA, Forghani B, Mahalingam R, Wellish MC, Cohrs RJ, Russman AN, Katzan I, Lin R, Gardner CJ, Gilden DH. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73.
Otto C, Hofmann J, Finke C, Zimmermann M, Ruprecht K. The fraction of varicella zoster virus-specific antibodies among all intrathecally-produced antibodies discriminates between patients with varicella zoster virus reactivation and multiple sclerosis. Fluids Barriers CNS. 2014;11:3.
Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 1998;4:111–7.
Ambrose HE, Granerod J, Clewley JP, et al. Diagnostic Strategy Used To Establish Etiologies of Encephalitis in a Prospective Cohort of Patients in England▿. J Clin Microbiol. 2011;49:3576–83.
Guedes M, Filipe R, Costa A, Soares C, Sarmento A, Tavares M. Central nervous system varicella zoster vasculopathy in an immunocompromised patient. IDCases. 2018;15:e00483.
Adriana Lopez, Theresa Harrington, Mona Marin. 2021; Pinkbook: Varicella | CDC. https://www.cdc.gov/vaccines/pubs/pinkbook/varicella.html. Accessed 30 Dec 2021.
Varicella and herpes zoster vaccines: WHO position paper, June 2014 – Recommendations. Vaccine. 2016;34:198–199.
Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccines Immunother. 2018;15:645–57.
Ávila-Agüero ML, Beltrán S, del Castillo JB, et al. Varicella epidemiology in Latin America and the Caribbean. Expert Rev Vaccines. 2018;17:175–83.
Vos RA, Mollema L, van Boven M, et al. High varicella zoster virus susceptibility in Caribbean island populations: Implications for vaccination. Int J Infect Dis. 2020;94:16–24.
Hambleton S, Steinberg SP, LaRussa PS, Shapiro ED, Gershon AA. Risk of Herpes Zoster in Adults Immunized with Varicella Vaccine. J Infect Dis. 2008;197:S196–9.
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84.
Amlie-Lefond C, Gilden D. Varicella Zoster Virus: A Common Cause of Stroke in Children and Adults. J Stroke Cerebrovasc Dis. 2016;25:1561–9.
(2021) Fact sheet: Protect your patients with the new shingles vaccine | Herpes Zoster | CDC. https://www.cdc.gov/shingles/multimedia/shingles-factsheet-hcp.html. Accessed 30 Dec 2021.
Research C for BE and (2021) SHINGRIX. FDA.
Harbecke R, Cohen JI, Oxman MN. Herpes Zoster Vaccines. J Infect Dis. 2021;224:S429–42.
Cheng H, Chen D, Peng X, Wu P, Jiang L, Hu Y. Clinical characteristics of Epstein-Barr virus infection in the pediatric nervous system. BMC Infect Dis. 2020;20:886.
Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17:691–700.
Lau JSY, Low ZM, Abbott I, Shochet L, Kanellis J, Kitching AR, Korman TM. Epstein-Barr virus encephalitis in solid organ transplantation. New Microbiol. 2017;40:212–7.
Allen UD, Preiksaitis JK, AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13652.
Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II N Engl J Med. 1971;285:267–74.
Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20:202–13.
Forte E, Zhang Z, Thorp EB, Hummel M. Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response. Front Cell Infect Microbiol. 2020;10:130.
• Handley G, Pankow S, Bard JD, Yee R, Nigo M, Hasbun R. Distinguishing cytomegalovirus meningoencephalitis from other viral central nervous system infections. J Clin Virol. 2021;142:104936. Is an overview of CMV encephalitis with detailed clinical, radiographic and laboratory studies that provide insight into ways it can be distinguished from other viral encephalitides.
Guo Y, Jiang L. Cytomegalovirus encephalitis in immunocompetent infants: A 15-year retrospective study at a single center. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2019;82:106–10.
Tan BH. Cytomegalovirus Treatment. Curr Treat Options Infect Dis. 2014;6:256–70.
Prichard MN, Whitley RJ. The development of new therapies for human herpesvirus 6. Curr Opin Virol. 2014;9:148–53.
• Greninger AL, Naccache SN, Pannaraj P, Jerome KR, Dien Bard J, Ruderman JW. The Brief Case: Inherited Chromosomally Integrated Human Herpesvirus 6 (HHV-6) in the Age of Multiplex HHV-6 Testing. J Clin Microbiol. 2019;57:e02016-e2018. Explains the chromosomal integration of HHV6. This pathogen is included on many commonly used multiplex PCR panels and therefore it is critical for clinicians to understand the concept of chromosomal integration and the potential this has to cause clinically insignificant “positive” results.
Lumley SF, Pritchard D, Dutta A, Matthews PC, Cann K. Multiplex PCR reveals high prevalence of enterovirus and HHV6 in acellular paediatric cerebrospinal fluid samples. J Infect. 2018;77:249–57.
• Waldrop G, Zucker J, Boubour A, Radmard S, Green DA, Thakur KT. Clinical Significance of Positive Results of the BioFire Cerebrospinal Fluid FilmArray Meningitis/Encephalitis Panel at a Tertiary Medical Center in the United States. Arch Pathol Lab Med. 2021. https://doi.org/10.5858/arpa.2020-0380-OA. Are important updates regarding multiple PCR-based panels that discuss the diagnostic accuracy and clinical significance of results. These tests have many benefits but as utilization increases, clinicians must understand the limitations which these two papers nicely address.
Ongrádi J, Ablashi DV, Yoshikawa T, Stercz B, Ogata M. Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals. J Neurovirol. 2017;23:1–19.
Riva N, Franconi I, Meschiari M, Franceschini E, Puzzolante C, Cuomo G, Bianchi A, Cavalleri F, Genovese M, Mussini C. Acute human herpes virus 7 (HHV-7) encephalitis in an immunocompetent adult patient: a case report and review of literature. Infection. 2017;45:385–8.
Gorin FA, Bale JF, Halks-Miller M, Schwartz RA. Kaposi’s sarcoma metastatic to the CNS. Arch Neurol. 1985;42:162–5.
Said JW, Tasaka T, de Vos S, Koeffler HP. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus type 8 encephalitis in HIV-positive and -negative individuals. AIDS Lond Engl. 1997;11:1119–22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
Dr. Beckham receives research funding from Roche. Dr. Piquet has received research funding from the University of Colorado and Rocky Mountain MS Center; consulting fees from Genentech/Roche and Alexion. Dr. Piquet reports honorarium from MedLink and publication royalties from Springer. Drs. Matthews, Tyler, Chauhan, and Pastula have no relevant financial or nonfinancial interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on CNS Infections in Tropical Settings
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matthews, E., Beckham, J.D., Piquet, A.L. et al. Herpesvirus-Associated Encephalitis: an Update. Curr Trop Med Rep 9, 92–100 (2022). https://doi.org/10.1007/s40475-022-00255-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-022-00255-8