Abstract
Accurate diagnosis, risk stratification, and decisions about the need for and optimal duration of antibiotic therapy are cornerstones of the management of patients with respiratory infections. A growing body of evidence supports the use of procalcitonin, a marker of bacterial infection, in addition to conventional clinical parameters to improve diagnostic and prognostic assessment in patients with suspicion of respiratory infections. In addition, several randomized controlled trials indicate that procalcitonin may be used for clinical decision making about initiation and optimal duration of antibiotic therapy. For patients with respiratory infections, procalcitonin-guided antibiotic therapy resulted in less antibiotic use without any apparent adverse patient outcome. For other infections outcome studies are currently lacking. This review summarizes the results of recent investigations of procalcitonin in respiratory infections to provide physicians an overview of the utility and limitations of procalcitonin when used for bedside decision making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite recent advances in diagnostic and therapeutic measures, mortality and morbidity associated with respiratory infections remains unacceptably high [1]. Recent studies indicate that early and adequate antibiotic treatment is highly effective in reducing disease burden in patients with bacterial community-acquired pneumonia (CAP) [2]. However, identifying which patients with respiratory symptoms benefit from antibiotics is a recurrent challenge in both inpatient and outpatient settings. Blood and sputum cultures are seen as the gold standard for identifying bacterial pathogens, but they are difficult to obtain, take days to turn positive, and may not reveal the causative organism [3, 4]. In the absence of a timely and reliable gold standard, clinicians often treat patients empirically with antibiotics, either due to patient demand, as in the outpatient setting, or due to clinicians’ concern about the severity of illness, as in the emergency department or intensive care setting. The resultant overuse of antibiotics is costly, contributes to the growing problem of antibiotic resistance and exposes patients to the risk of side effects [5, 6]. Therefore, the accurate identification of which patients truly need antibiotics would benefit not only the patients by protecting them from unnecessary medications and side effects, but also society by decreasing selection pressure for drug resistance.
In recent years procalcitonin (PCT) has emerged as a promising marker for bacterial infection, with potential both for identifying which patients need antibiotics and for deciding how long to continue antibiotics once started. First identified in 1975 in a patient with pancreatitis [7], PCT has several features that argue for its use in clinical practice. First, in human cell culture models its level increases rapidly in response to LPS or IL-1β, common mediators of bacterial sepsis, but does not increase in response to interferon-γ, a common cytokine in viral infections [8]. In addition, unlike markers such as the erythrocyte sedimentation rate or C-reactive protein, it is unaffected by steroid administration [9, 10]. In a recent study of over a dozen biomarkers in patients with either bacterial or viral infections, PCT emerged as the marker with the highest sensitivity and specificity for bacterial infection [11•]. These characteristics have prompted studies of PCT in various clinical settings, from CAP to malaria to febrile neutropenia [12]. Numerous reviews and meta-analyses of PCT exist [12–14, 15•, 16–20, 21•]; the aim of the current review is to focus on recent developments in the use of PCT for antibiotic management, diagnosis, and prognosis of respiratory illnesses. The principle clinical settings discussed are: patients presenting from the community with suspicion of a lower respiratory tract infection (i.e. pneumonia or bronchitis); chronic obstructive pulmonary disease (COPD) flare; and the intensive care setting, including ventilator-associated pneumonia (VAP), aspiration pneumonia, and sepsis with and without a pulmonary focus (Table 1).
Procalcitonin to Guide Antibiotic Treatment of Respiratory Infections
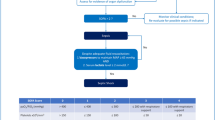
A promising role for PCT is the antibiotic management of respiratory infections; that is, determining which patients with nonspecific symptoms of a lower respiratory tract infection (cough, fever, etc.) should receive antibiotics and for how long. Since PCT levels increase with bacterial infection and decrease upon recovery, PCT can be used as a biomarker to guide antibiotic therapy in individual patients. Using a highly-sensitive PCT immuno-assay with a sensitivity of 0.06 ng/mL, antibiotic stewardship algorithms based on PCT values have been successfully implemented [21•, 22, 23•, 24•, 25•, 26, 27•, 28, 29•]. The salient PCT levels used in most PCT-guided therapy algorithms were 0.1, 0.25, and 0.5 ng/mL, with protocols adapted to the severity of illness and the clinical setting (Fig. 1) [21•]. Measured at the time of presentation these levels were used to guide the initiation of antibiotics; measured serially they indicate when it is safe to stop antibiotics. In outpatients and patients with moderate severity illness in the emergency department, PCT levels <0.1 ng/mL indicated that antibiotics were strongly discouraged; antibiotics were discouraged for levels <0.25 ng/mL, encouraged for levels >0.25 ng/mL, and strongly encouraged for levels >0.5 ng/mL. For reference, the PCT level in healthy people is less than 0.03 ng/mL while the level in a patient with a bacterial infection is at least one order of magnitude higher, sometimes a thousand-fold higher [30]. For higher severity patients in the intensive care unit the protocols were adapted so that a PCT level <0.5 ng/mL indicated that levels were back to “normal” and antibiotic therapy could be discontinued. Importantly, protocols for lower acuity patients used PCT to decide upon initiation and duration of antibiotic treatment; in higher acuity patients the focus was on the duration of therapy, to avoid the risk of withholding antibiotics in a potentially septic patient [2, 21•].
Antibiotic Management of Community Acquired Pneumonia and Bronchitis
Several randomized controlled trials (RCTs) have investigated PCT-based protocols to guide decisions about initiation and/or duration of antibiotic therapy in patients presenting from the community with symptoms of a lower respiratory tract infection. In these studies the benefit and harm were measured by clinical outcomes, assuming that if the patient recovered without antibiotics there was no relevant bacterial illness in need of antibiotic therapy. These RCTs have shown that using PCT to guide antibiotic use results in less antibiotic use without increasing rates of adverse events [22, 23•, 24•, 25•, 26]. These studies were conducted in emergency rooms (ERs), hospitals, and outpatient clinics. For example, in a multicenter RCT of patients presenting with symptoms of a lower respiratory tract infection to emergency departments in Switzerland, a PCT-based algorithm led to less antibiotic exposure (5.7 days in the PCT group vs. 8.7 days in the control group), fewer side effects (19.8% vs. 28.1%), and no increase in adverse outcomes (15.4% vs. 18.9%) [23•]. Most of the patients included in above study and other ER studies were admitted to the hospital and the studies were conducted in the European health care system, so until this year there was less data on patients evaluated in the ER but treated as outpatients. The most recent study, by Long et al., fills this gap. They demonstrated that a PCT-guided algorithm decreased antibiotic use among patients with community acquired pneumonia (CAP) presenting to an emergency room in Shanghai (5 days of antibiotics in the PCT group vs. 7 days in the control group); all patients in this trial were treated as outpatients [25•]. A PCT-guided antibiotic protocol was also successful for patients presenting with acute respiratory tract infections in a primary care setting [22, 24•]. Though an observational study suggested that outpatients, even those with pneumonia, generally had lower PCT values compared to hospitalized patients [31], two RCTs showed that using PCT to guide therapy in these patients can reduce antibiotic use markedly with similar time to recovery and without increasing adverse outcomes [22, 24•].
After the decision to initiate antibiotic therapy in a patient with symptoms of a respiratory infection comes the decision about optimal duration of therapy. Though there are some recommendations about how long to continue antibiotic therapy for patients with pneumonia due to specific pathogens (i.e. Legionella) or bronchitis, these guidelines are rather empiric and based on little efficacy data [32]. Christ-Crain et al. (2006) found that using serial PCT levels (at 4, 6, and 8 days after antibiotic initiation) to determine when to stop antibiotics for CAP led to a 55% reduction in antibiotic duration in the PCT-guided group without any change in patient outcome [28]. These results were confirmed in a large multicenter trial in Switzerland (PCT measured on days 3, 5, and 7) [23•], as well as a smaller trial in Denmark (single PCT measurement early in the hospital stay) [26]. In 2011, Saeed et al. broadened these results to patients with any suspected infection. Although the study was not randomized, they found that using PCT levels to indicate when to withhold antibiotics led to a 17% reduction in antibiotic use without apparent harm for patients [27•]. These findings have tantalizing applications for antimicrobial stewardship as well as control of healthcare costs.
Antibiotic Management of COPD Exacerbations
For exacerbation of chronic obstructive pulmonary disease (COPD) it would be extremely helpful to identify which patients would benefit from antibiotics and which would not. Although a recent post-hoc analysis of a RCT comparing doxycycline versus placebo for COPD exacerbation found a clinical response to antibiotics among patients with a low PCT level [33], results from prospective RCTs using PCT to guide antibiotic therapy are quite promising [34]. In a randomized trial of PCT-guided antibiotic therapy versus conventional treatment (i.e. antibiotics at the discretion of the attending physician) for patients admitted to the hospital with COPD exacerbation, Stolz et al. (2007) found that using PCT to determine whether or not to start antibiotics led to less antibiotic use overall without an increase in adverse outcomes and similar recovery times in lung function [35]. Subsequent RCTs overall including more than 550 COPD patients have confirmed these results [23•, 26, 34, 36].
Antibiotic Management in the Intensive Care Setting
PCT also shows promise for guiding antibiotic therapy in a critical care setting. In a randomized trial of 101 patients with ventilator-associated pneumonia (VAP), Stolz et al. (2009) found that using PCT to decide when to de-escalate antibiotics led to an increase in antibiotic-free days without causing an increase in mortality [29•]. Other studies have examined PCT-guided antibiotic use for all critically ill patients with suspected infection, not just respiratory infection. Saeed et al. (2011) found that using PCT to guide therapy in an intensive care unit (ICU) – including initiation, escalation, and discontinuation of antibiotics – led to less antibiotic use, which ultimately meant a lower cost of hospital stays [27•]. A meta-analysis including all ICU trials found that in addition to lower antibiotic consumption, cost can also be reduced without apparent harmful effects [37•]; however, the total number of patients studied is still too small to rule out a slight increased mortality risk from the PCT-guided strategy [15•, 37•]. A recent article urges caution before adopting PCT-guided therapy in the critical care setting, as occasionally patients with positive blood cultures will have low initial PCT levels [38]. Although these results contradict other studies that found increased PCT levels in most patients with bacteremia [39], initial antibiotic therapy should not be delayed in ICU patients with suspicion of infection and PCT should rather be used to guide duration of treatment. Although the available evidence from RCTs supports the use of PCT for de-escalation of antibiotic therapy for patients with infections, the same may not be true for escalation of antibiotic therapy when PCT levels increase, as demonstrated in a recent large sepsis trial [40•]. In this trial from Denmark, PCT-guided antimicrobial escalation in the ICU did not improve survival and did lead to organ-related harm (particularly kidney failure) and prolonged ICU stays.
Procalcitonin to Diagnose Respiratory Infections
Separate from the question of when and how long to prescribe antibiotics for patients with symptoms of a respiratory infection is the question of what specific type of infection a patient has. PCT has a less established role in this area, but potential applications are emerging.
Identifying Pathogens in Community Acquired Pneumonia
RCTs have demonstrated the role of PCT in guiding antibiotic use for CAP; other studies have examined its role in identifying which type of bacterial infection a patient has [41–43]. A recent Swiss study found that PCT was significantly higher in patients with CAP plus bacteremia (mostly S. pneumoniae) compared to patients with unknown CAP etiology [39]. A PCT cutoff of 0.25 ng/mL would have enabled a reduction of blood cultures by 37% while still identifying 96% of positive blood cultures. A study of German patients with CAP showed that PCT levels were significantly higher among patients with “typical” pneumonia (again, mostly S. pneumoniae) compared to patients with either “atypical” (Mycoplasma, Chlamydophila, or Legionella) or viral pneumonia [43]. However, there was a large degree of overlap in PCT levels between the groups. This suggests that PCT is helpful in estimating the risk of typical bacterial CAP, but is not specific enough to identify a given pathogen. Similarly, though a retrospective study in Austria found PCT levels significantly higher in patients with S. pneumoniae pneumonia compared to patients with Legionella pneumonia, the differences were not enough to generate either a sensitive or a specific test for identifying either pathogen [41].
PCT for Diagnosis in the Intensive Care Setting
As described above, PCT can be useful in guiding the duration of antibiotic therapy in VAP; however, it appears not to be as useful for the initial diagnosis of VAP. Though a first report found that PCT was helpful in identifying patients with VAP [44], other studies found very poor sensitivity and specificity for VAP [45] or other nosocomial pneumonia in the intensive care setting [46•]. A recent study examined PCT for distinguishing aspiration pneumonitis from aspiration pneumonia in intubated patients; there was no significant difference in PCT levels between patients with culture-positive bronchoalveolar lavage (i.e. aspiration pneumonia) from those with culture-negative lavage [47•]. So far, then, the role for PCT in the diagnosis of nosocomial pneumonias remains unclear.
A promising application for the intensive care setting is using PCT to estimate the risk for bacterial super-infection of influenza pneumonia. Three studies, from France, Spain, and Australia, examined PCT in critically ill patients during the 2009 H1N1 influenza pandemic [48•, 49, 50]. All three found that PCT levels were lower in patients with H1N1 alone, while patients with either standard bacterial pneumonia or with bacterial super-infection of viral pneumonia had elevated PCT levels. Therefore, PCT may be useful in determining which patients with severe influenza need antibiotics in addition to oseltamivir.
Procalcitonin in Determining Prognosis of Respiratory Infections
Respiratory infections are the leading cause of sepsis and death from infectious diseases in Western countries and health expenditures, in particular for inpatient management, are substantial [1]. Accurate prediction of outcome is a prerequisite for safe decision-making about hospitalization versus outpatient management, and can also help families make decisions for their critically ill loved ones. Several studies have investigated the potential of PCT as a prognostic marker.
Procalcitonin for Prognosis in CAP
In patients with CAP, many studies indicate that higher PCT levels correlate with more severe illness and risk of adverse outcome [51–55]. In that vein, a study from the ProHOSP group indicated that PCT levels were useful in predicting which patients with CAP would develop bacteremia [39]. However, few studies have shown that PCT offers prognostic information beyond that of standard measurements of pneumonia severity such as the Pneumonia Severity Index (PSI) or CURB-65 scores [56]. Kruger et al. (2008) found that PCT levels correlated with pneumonia severity and were significantly higher in non-survivors. In addition, PCT improved the prognostic potential of the CRB-65 score [57]. In contrast, Huang et al. (2008) found that PCT level did not improve upon CURB-65 or PSI scores for predicting 30-day mortality, except for patients who had high clinical scores yet low PCT levels. In that subgroup, low PCT connoted a mortality risk similar to that of patients with low clinical scores [58]. More recent work indicates that adding PCT to clinical risk scores improves their predictive potential only moderately [59•, 60•]. A Swiss study, finally, found that initial PCT levels did not improve clinical risk scores for mortality prediction [60•]; however, decreasing PCT levels over time correlated with a favorable outcome. In addition, the study found that PCT was more helpful to predict serious adverse events other than mortality (i.e. ICU admission, CAP-related complications). For these outcomes PCT significantly improved clinical risk scores.
Procalcitonin for Prognosis in COPD
There are fewer data on the use of PCT in the prognosis of COPD patients. In 116 patients with COPD exacerbations severe enough to require intubation, PCT levels were associated with increased mortality, even when controlling for clinical severity scores [61]. A recently published study of 318 Spanish patients with COPD found higher PCT levels in patients who died within thirty days of PCT measurement, but no significant relationship between PCT levels and two-year mortality [62]. Further work is needed to determine the use of PCT to indicate prognosis in COPD exacerbation outside the ICU setting.
Procalcitonin for Prognosis in VAP
Both Duflo (2002) and Luyt (2005) established that higher levels of PCT were associated with worse outcome in patients with VAP [63, 64]. More recently, Bloos et al. (2011) studied serial PCT levels in 175 ventilated patients with pneumonia, including 61 with VAP, in 10 hospitals in Europe, Canada, and the United States. They found that initial PCT levels were significantly higher in VAP non-survivors than in survivors; PCT levels performed similarly to APACHE II (Acute Physiology And Chronic Health Evaluation) scores in this study [65•]. A study of 101 patients with VAP in hospitals in the United States and Switzerland also found that non-survivors had higher levels of PCT at diagnosis. In this study, adding PCT and MR-proANP (mid-regional pro-atrial natriuretic peptide) levels to the SOFA (Sequential Organ Failure Assessment) score improved the AUC for that score from 0.768 to 0.895, though the change did not reach significance (p = 0.087), probably due to low power [66]. In addition, when Hillas et al. (2010) examined PCT levels on days 1, 4, and 7 of VAP in 45 patients in a Greek intensive care unit they found that, though PCT levels were higher in non-survivors, this difference was not significant when included as part of a multivariate prediction model. That said, an increasing PCT level from day one to day seven of VAP was a significant predictor of mortality in their multivariate model [67]. Overall, while higher PCT levels are associated with an increased risk of death in VAP, it is unclear if PCT contributes significant prognostic information beyond that provided by clinical indices such as APACHE II or SOFA.
Pediatric Populations
One area that deserves further study is the role of PCT in diagnosing bacterial respiratory infections in children. It is difficult to distinguish viral from bacterial pneumonia on radiological or clinical grounds, so — despite the frequency of viral infections in this age-group — children often get empiric antibiotics for respiratory infections. Some observational studies found that PCT levels were higher in children with severe bacterial infection compared to children with viral infection [68, 69], while other studies found no difference or only a marginal diagnostic advantage [70–76]. In light of these results, PCT’s role in diagnosing childhood respiratory infections remains unclear and further evidence is needed.
In terms of managing infections in children, a retrospective analysis suggests that withholding antibiotics from children with PCT levels <0.1 ng/mL would be safe [77]. Also, a RCT of using PCT to guide duration of antibiotics in neonatal sepsis suggested that, as in adults, PCT can reduce antibiotic use without increasing adverse outcomes [78•]. Further RCTs of PCT-guided therapy in children are needed.
Limitations of Procalcitonin
Though mentioned in context above, it is worth reiterating the areas and applications in which PCT has only limited usefulness: PCT may have low sensitivity and/or specificity (i.e. low “signal to background ratio”) to identify VAP or other nosocomial pneumonias, may not add to existing severity markers, and have not yet been adequately studied in pediatric populations to recommend its widespread use in clinical practice. In addition, some conditions produce a high PCT level without infection; any significant tissue injury – burns, mechanical trauma, surgery – will cause an elevated PCT level, as will pancreatitis [30]. This may partly be explained by translocation of bacteria from the intestines in severe conditions. Finally, sometimes patients with strong clinical suspicion of bacterial infection will have low PCT levels, and these patients should receive empiric antibiotic therapy, as no diagnostic test can rule out infection with a 100% accuracy [38]. Thus, no blood test should replace clinical impression and septic patients always warrant early antibiotics.
Emerging Applications
Given the promise of PCT in bacterial respiratory infections, researchers have explored its use in other areas as well, such as tuberculosis (TB) and febrile neutropenia. For TB, several studies have shown that PCT levels are lower in pulmonary TB than in CAP [79–81]. However, it appears that, as with other respiratory infections, a high PCT level is associated with a poor prognosis in TB [81]. No one has yet studied if PCT has a role in TB treatment. In febrile neutropenia, the stakes are high to identify and treat an infection quickly and appropriately. A study of oncology patients in Sweden found that a PCT level of >0.5 ng/mL had about a 60% sensitivity and 80% specificity for identifying febrile patients whose blood cultures would ultimately turn positive. Increasing the cut-off to 2 ng/mL increased the specificity to 95% [82]. Other studies have found similar results, and monitoring serial PCT levels may prove useful in anticipating bacteremia among neutropenic patients [83]. That said, a recent brief report out of France suggested that PCT was not helpful in predicting infection in neutropenic patients [84]. Clearly, more studies are needed to determine the role of PCT in oncology patients.
Conclusions
In conclusion, the current evidence supports a role for PCT in guiding antibiotic therapy for respiratory infections. There is good evidence that using PCT to determine when to prescribe antibiotics to patients presenting from the community with symptoms of a respiratory infection leads to less antibiotic use without affecting clinical outcomes. This concept has also been adapted by the 2012 surviving sepsis guidelines which now suggest the use of low procalcitonin to assist the clinician in the discontinuation of empiric antibiotics when no evidence of infection is found (grade 2C). Generally, the role of PCT as a diagnostic and prognostic marker in the intensive care setting is less well established. It may add to diagnosing bacterial infection in this setting and improves slightly existing prognostic indicators. It’s main role may be in determining the course of antibiotics in critically ill patients. Future work will clarify its role in this setting, as well as in pediatric populations and in non-respiratory infections.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Macfarlane JT, Colville A, Guion A, et al. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet. 1993;341(8844):511–4.
Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–96.
Reimer LG, Wilson ML, Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997;10(3):444–65.
Garcia-Vazquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164(16):1807–11.
Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis. 2004;10(3):514–7.
Harbarth S, Albrich W, Brun-Buisson C. Outpatient antibiotic use and prevalence of antibiotic-resistant pneumococci in France and Germany: a sociocultural perspective. Emerg Infect Dis. 2002;8(12):1460–7.
Canale DD, Donabedian RK. Hypercalcitoninemia in acute pancreatitis. J Clin Endocrinol Metab. 1975;40(4):738–41.
Linscheid P, Seboek D, Zulewski H, et al. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146(6):2699–708.
Muller B, Peri G, Doni A, et al. High circulating levels of the IL-1 type II decoy receptor in critically ill patients with sepsis: association of high decoy receptor levels with glucocorticoid administration. J Leukoc Biol. 2002;72(4):643–9.
de Kruif MD, Lemaire LC, Giebelen IA, et al. The influence of corticosteroids on the release of novel biomarkers in human endotoxemia. Intensive Care Med. 2008;34(3):518–22.
• Chalupa P, Beran O, Herwald H, et al. Evaluation of potential biomarkers for the discrimination of bacterial and viral infections. Infection. 2011. This small case-control study (81 patients) of numerous biomarkers demonstrated that PCT was the most sensitive and specific biomarker for distinguishing bacterial from viral infection.
Schuetz P, Christ-Crain M, Muller B. Procalcitonin and other biomarkers to improve assessment and antibiotic stewardship in infections–hope for hype? Swiss Med Wkly. 2009;139(23–24):318–26.
Tang H, Huang T, Jing J, et al. Effect of procalcitonin-guided treatment in patients with infections: a systematic review and meta-analysis. Infection. 2009;37(6):497–507.
Hatzistilianou M. Diagnostic and prognostic role of procalcitonin in infections. Sci World J. 2010;10:1941–6.
• Kopterides P, Siempos II, Tsangaris I, et al. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(11):2229–41. In a meta-analysis of seven RCTs (total 1131 patients), using a PCT-based algorithm in the ICU to guide duration of antibiotic therapy led to less antibiotic use without any apparent increase in adverse events. However, the number of patients studied is too small to rule out rare adverse events.
Schuetz P, Albrich W, Christ-Crain M, et al. Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010;8(5):575–87.
Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010;48(7):2325–9.
Limper M, de Kruif MD, Duits AJ, et al. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non-infectious fever. J Infect. 2010;60(6):409–16.
Christ-Crain M, Opal SM. Clinical review: the role of biomarkers in the diagnosis and management of community-acquired pneumonia. Crit Care. 2010;14(1):203.
Gilbert DN. Procalcitonin as a biomarker in respiratory tract infection. Clin Infect Dis. 2011;52 Suppl 4:S346–50.
• Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–31. This article summarizes all 14 RCTs (4467 patients) of PCT-guided antibiotic therapy for respiratory infections and sepsis in inpatient, emergency department, and outpatient settings, most of which have been conducted in Europe, and suggests algorithms for use in U.S.-based trials.
Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–7. discussion 7-8.
• Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. Jama. 2009;302(10):1059–66. This multicenter RCT of patients presenting to emergency departments with respiratory infections demonstrated that a PCT-guided algorithm results in less antibiotic use without an increase in adverse events.
• Burkhardt O, Ewig S, Haagen U, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–7. RCT of patients presenting to their primary care doctor with symptoms of a respiratory infection; PCT-guided algorithms for antibiotic treatment led to 40% less antibiotic use and similar clinical outcomes.
• Long W, Deng X, Zhang Y, et al. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology. 2011;16(5):819–24. RCT of 156 outpatients in China with community-acquired pneumonia; PCT-guided therapy led to less antibiotic use, shorter duration of antibiotic therapy, and similar clinical outcomes.
Kristoffersen KB, Sogaard OS, Wejse C, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission–a randomized trial. Clin Microbiol Infect. 2009;15(5):481–7.
• Saeed K, Dryden M, Bourne S, et al. Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. J Hosp Infect. 2011;78(4):289–92. Non-randomized trial of PCT to guide antibiotic use in 141 hospitalized patients with any suspected infection. This study is of interest because it includes cost analysis of PCT-guided therapy.
Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
• Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–75. A RCT of 101 patients with VAP demonstrates that using PCT to determine the duration of antibiotics leads to more antibiotic-free days without an increase in mortality.
Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36(3):941–52.
Holm A, Pedersen SS, Nexoe J, et al. Procalcitonin versus C-reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57(540):555–60.
Restrepo MI, Anzueto A. Antimicrobial treatment of community-acquired pneumonia. Clin Chest Med. 2005;26(1):65–73.
Daniels JM, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138(5):1108–15.
Tokman S, Schuetz P, Bent S. Procalcitonin-guided antibiotic therapy for chronic obstructive pulmonary disease exacerbations. Expert Rev Anti Infect Ther. 2011;9(6):727–35.
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19.
Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600–7.
• Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: A systematic review and an economic evaluation. Crit Care Med. 2011;39(7):1792–9.
Koeze J, Hendrix MR, van den Bergh FA, et al. In critically ill patients the procalcitonin level can be misleading. Crit Care. 2011;15(2):422.
Muller F, Christ-Crain M, Bregenzer T, et al. Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial. Chest. 2010;138(1):121–9.
• Jensen JU, Hein L, Lundgren B, et al.: Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial. Crit Care Med. 2011.
Bellmann-Weiler R, Ausserwinkler M, Kurz K, et al. Clinical potential of C-reactive protein and procalcitonin serum concentrations to guide differential diagnosis and clinical management of pneumococcal and Legionella pneumonia. J Clin Microbiol. 2010;48(5):1915–7.
Jereb M, Kotar T. Usefulness of procalcitonin to differentiate typical from atypical community-acquired pneumonia. Wien Klin Wochenschr. 2006;118(5–6):170–4.
Kruger S, Ewig S, Papassotiriou J, et al. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res. 2009;10:65.
Ramirez P, Garcia MA, Ferrer M, et al. Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur Respir J. 2008;31(2):356–62.
Luyt CE, Combes A, Reynaud C, et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34(8):1434–40.
• Dallas J, Brown SM, Hock K, et al. Diagnostic utility of plasma procalcitonin for nosocomial pneumonia in the intensive care unit setting. Respir Care. 2011;56(4):412–9.
• El-Solh AA, Vora H, Knight 3rd PR, Porhomayon J. Diagnostic use of serum procalcitonin levels in pulmonary aspiration syndromes. Crit Care Med. 2011;39(6):1251–6.
• Cuquemelle E, Soulis F, Villers D, et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011;37(5):796–800.
Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med. 2010;36(3):528–32.
Piacentini E, Sanchez B, Arauzo V, et al. Procalcitonin levels are lower in intensive care unit patients with H1N1 influenza A virus pneumonia than in those with community-acquired bacterial pneumonia. A pilot study J Crit Care. 2011;26(2):201–5.
Boussekey N, Leroy O, Georges H, et al. Diagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infection. 2005;33(4):257–63.
Boussekey N, Leroy O, Alfandari S, et al. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32(3):469–72.
Tseng JS, Chan MC, Hsu JY, et al. Procalcitonin is a valuable prognostic marker in ARDS caused by community-acquired pneumonia. Respirology. 2008;13(4):505–9.
Hirakata Y, Yanagihara K, Kurihara S, et al. Comparison of usefulness of plasma procalcitonin and C-reactive protein measurements for estimation of severity in adults with community-acquired pneumonia. Diagn Microbiol Infect Dis. 2008;61(2):170–4.
Okimoto N, Hayashi Y, Ishiga M, et al. Procalcitonin and severity of community-acquired pneumonia. J Infect Chemother. 2009;15(6):426–7.
Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50.
Kruger S, Ewig S, Marre R, et al. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31(2):349–55.
Huang DT, Weissfeld LA, Kellum JA, et al. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48 e2–58 e2.
• Menendez R, Martinez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587–91.
• Schuetz P, Suter-Widmer I, Chaudri A, et al. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37(2):384–92.
Rammaert B, Verdier N, Cavestri B, Nseir S. Procalcitonin as a prognostic factor in severe acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2009;14(7):969–74.
Lacoma A, Prat C, Andreo F, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:157–69.
Duflo F, Debon R, Monneret G, et al. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96(1):74–9.
Luyt CE, Guerin V, Combes A, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53.
• Bloos F, Marshall JC, Dellinger RP, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 2011;15(2):R88.
Boeck L, Eggimann P, Smyrnios N, et al. Midregional pro-atrial natriuretic peptide and procalcitonin improve survival prediction in VAP. Eur Respir J. 2011;37(3):595–603.
Hillas G, Vassilakopoulos T, Plantza P, et al. C-reactive protein and procalcitonin as predictors of survival and septic shock in ventilator-associated pneumonia. Eur Respir J. 2010;35(4):805–11.
Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–8.
Manzano S, Bailey B, Gervaix A, et al. Markers for bacterial infection in children with fever without source. Arch Dis Child. 2011;96(5):440–6.
Korppi M, Remes S, Heiskanen-Kosma T. Serum procalcitonin concentrations in bacterial pneumonia in children: a negative result in primary healthcare settings. Pediatr Pulmonol. 2003;35(1):56–61.
Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis. 2007;39(2):129–37.
Korppi M, Remes S. Serum procalcitonin in pneumococcal pneumonia in children. Eur Respir J. 2001;17(4):623–7.
Toikka P, Irjala K, Juven T, et al. Serum procalcitonin, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J. 2000;19(7):598–602.
Moulin F, Raymond J, Lorrot M, et al. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child. 2001;84(4):332–6.
Prat C, Dominguez J, Rodrigo C, et al. Procalcitonin, C-reactive protein and leukocyte count in children with lower respiratory tract infection. Pediatr Infect Dis J. 2003;22(11):963–8.
Don M, Valent F, Korppi M, Canciani M. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int. 2009;51(1):91–6.
Schutzle H, Forster J, Superti-Furga A, Berner R. Is serum procalcitonin a reliable diagnostic marker in children with acute respiratory tract infections? A retrospective analysis. Eur J Pediatr. 2009;168(9):1117–24.
• Stocker M, Fontana M, el Helou S, et al. Use of procalcitonin-guided decision-making to shorten antibiotic therapy in suspected neonatal early-onset sepsis: prospective randomized intervention trial. Neonatology. 2010;97(2):165–74.
Nyamande K, Lalloo UG. Serum procalcitonin distinguishes CAP due to bacteria, Mycobacterium tuberculosis and PJP. Int J Tuberc Lung Dis. 2006;10(5):510–5.
Kang YA, Kwon SY, Yoon HI, et al. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med. 2009;24(4):337–42.
Ugajin M, Miwa S, Shirai M, et al. Usefulness of serum procalcitonin levels in pulmonary tuberculosis. Eur Respir J. 2011;37(2):371–5.
Koivula I, Hamalainen S, Jantunen E, et al. Elevated procalcitonin predicts Gram-negative sepsis in haematological patients with febrile neutropenia. Scand J Infect Dis. 2011;43(6–7):471–8.
Koivula I, Juutilainen A: Procalcitonin is a useful marker of infection in neutropenia. Leuk Res. 2011.
Cornillon J, Bouteloup M, Lambert C. Evaluation of procalcitonin and CRP as sepsis markers in 74 consecutive patients admitted with prolonged febrile neutropenia. J Infect. 2011;63(1):93–5.
• Bafadhel M, Clark TW, Reid C, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139(6):1410–8.
Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–74.
Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505.
Disclosures
Dr. Schuetz was supported by a research grant from the Swiss Foundation for Grants in Biology and Medicine (Schweizerische Stiftung für medizinisch-biologische Stipendien, SSMBS, PASMP3-127684/1) and reports receiving support from BRAHMS Inc and Biomerieux to attend meetings and fulfill speaking engagements. Dr. Certain reports no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Certain, L., Schuetz, P. The Role of Procalcitonin in Respiratory Infections. Curr Infect Dis Rep 14, 308–316 (2012). https://doi.org/10.1007/s11908-012-0249-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-012-0249-5