Abstract
Purpose of Review
The study aims to verify the advantages of nonsteroidal mineralocorticoid receptor blockers (MRBs) in the management of hypertension and cardiovascular and renal diseases, comparing with conventional MRBs.
Recent Findings
Based on the unique structures, the nonsteroidal MRBs have higher selectivity for mineralocorticoid receptors (MRs) and show no agonist activity for major steroid hormone receptors in contrast to steroidal MRBs. Today, there are two nonsteroidal MRBs, esaxerenone and finerenone, which completed phase 3 clinical trials. Series of clinical trials have shown that both agents achieve similar MR blockade with smaller doses as compared with steroidal MRBs, but have no off-target side effect such as gynecomastia. Esaxerenone has persistent blood pressure-lowering effects in various hypertensive populations, including essential hypertension and those with diabetes and/or chronic kidney disease, while finerenone has demonstrated reduction of the cardiovascular risk rather than blood pressure in patients with diabetes and chronic kidney disease.
Summary
Nonsteroidal MRBs are a more refined agent which contributes to appropriate MR blocking with minimized unpleasant adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compelling evidence highlights that blocking of mineralocorticoid receptors (MRs) could benefit to various hypertensive patients, regardless of the actual levels of peripheral aldosterone [1,2,3, 4•, 5,6,7,8]. Since the discovery in 1957 of a unique class of synthetic steroids, “spirolactones” which abolish aldosterone effects [9], several mineralocorticoid receptor blockers (MRBs) have been developed and proven to contribute to organ protection, particularly in heart failure (HF), diabetic kidney disease (DKD), and chronic kidney disease (CKD), beyond its antihypertensive effects [8, 10,11,12]. In the current situation, spironolactone (SC-9420) and eplerenone (SC-66110, CGP-30083) are two major steroidal MRBs supported by abundant clinical evidence from many momentous trials, while those studies also elucidate limitations of those steroidal MRBs in hypertension treatment, including both renal-related and off-target side effects [13,14,15].
For the last 6 decades, continuous efforts to achieve more safe and efficient inhibition of MRs have advanced towards development of a novel class of nonsteroidal MRBs, employing high-throughput screening [14, 16, 17]. Several compounds of nonsteroidal MRBs have proceeded with clinical trials, and in 2019, esaxerenone (CS-3150) was first marketed for hypertension in Japan [18]. Esaxerenone is a novel oral selective nonsteroidal MRB which has a longer half-life and higher selectivity for MRs than traditional MRBs [16, 19]. With the great selectivity for MRs, esaxerenone has demonstrated its solid effects on blood pressure (BP) without side effects related to sex steroid hormone receptors (SSHRs) such as gynecomastia [20, 21, 22••, 23••]. Those studies also reported a significant reduction of urinary albumin excretion in patients with DKD under esaxerenone treatment [23••, 24]. Finerenone (BAY 94–8862), another nonsteroidal MRB, has just been approved by the U.S. Food and Drug Administration (FDA) in July 2021. Several lines of evidence confirmed that finerenone can safely and efficiently reduce the cardiovascular and renal risk in patients with DKD, CKD, and HF [25, 26•, 27••]. Nonsteroidal MRBs are, therefore, in the spotlight as a new key player in hypertension management.
Herein, we review recent findings of nonsteroidal MRBs, particularly esaxerenone and finerenone, in the field of hypertension, their clinical impacts, and potential, comparing with traditional MRBs. In this article, we also argue what we could expect from nonsteroidal MRBs to provide, and remaining issues to be further investigated for our future practice.
Different Blocking Profiles of Steroid Hormone Receptors by Steroidal and Nonsteroidal MRBs Based on Their Structural Features
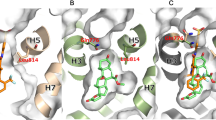
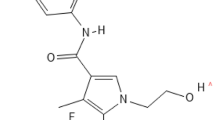
As traditional MRBs originated from synthetic agents with structural elements of progesterone [14], both spironolactone and eplerenone have a steroidal structure which enables them to smoothly pass across cell membrane as mineralocorticoids (Fig. 1). Those drugs inhibit mineralocorticoids from activating MR signaling by competitively binding to MRs in the cytoplasm, resulting in reduction of BP and inflammation (Fig. 2) [28]. Both MRBs also act as a weak agonist for MRs [16]. In contrast, nonsteroidal MRBs show unique mode of MR antagonism. For esaxerenone, the chemical structure is relatively large and quite distinct from those of traditional MRBs (Fig. 1) [16]. Reflecting the difference between their structures, the crystallization experiment demonstrated that esaxerenone shares the same binding site of MRs with other steroidal MRBs, but additionally extended into the binding pocket [29•]. This deep binding of esaxerenone leads to its very high affinity for MRs, compared with spironolactone and eplerenone: IC50 values are 3.7, 66, and 970 nM, respectively (Table 1) [16]. Moreover, esaxerenone does not have any agonist potency for human MRs due to the modified MR structure by esaxerenone which precludes coactivators from binding to MRs (Fig. 2) [29•]. Besides, finerenone also has a “bulky” structure with higher potency for MRs (a IC50 value of 18 nM) than the steroidal MRBs (Fig. 1) [17]. Intriguingly, finerenone has been reported not only to inhibit aldosterone-MR complex formation but also to decrease basal gene transcription as an inverse antagonist [30]. This reverse effect may result from the protrusion of MR helix 12 due to finerenone binding, which suppresses coactivator recruitment for MR signaling as esaxerenone [30]. From an aspect of pharmacokinetics, esaxerenone has a longer half-life time (18.6 h) than spironolactone and its major metabolites (up to 16.5 h), and eplerenone (up to 6 h), whereas finerenone has the shortest half-life time (around 2 h) among them (Table 1) [16, 31,32,33]. Despite the less lipophilic nature of the nonsteroidal MRBs than the others, esaxerenone and finerenone enter cells as steroidal MRBs, and exhibit antihypertensive effects, including natriuresis, and antiinflammatory and antifibrotic response in kidneys, heart, and blood vessels, by abrogating MR activation [14, 34, 35].
Molecular structures of steroids and mineralocorticoid receptor blockers. The structures of steroidal and nonsteroidal mineralocorticoid receptor blockers (MRBs) are shown, compared with aldosterone, a representative mineralocorticoid in human, and progesterone. Spironolactone was generated by mimicking the structure of progesterone, while eplerenone was developed to improve the selectivity of spironolactone for mineralocorticoid receptors. Nonsteroidal MRBs are relatively “bulky” and quite distinct molecules from those steroidal agents
Binding mode of mineralocorticoid receptor blockers to mineralocorticoid receptors. Mineralocorticoids (A) and mineralocorticoid receptor blockers (MRBs; B and C) bind to mineralocorticoid receptors (MRs) in the cytoplasm. (A) The complexes of a mineralocorticoid and a MR form a homodimer after translocation into the nucleus. Then, the homodimer complex binds to the hormone responsive element (HRE) of the target gene, leading to DNA transcription supported by co-activators (Co-A). (B) Steroidal MRBs prevent mineralocorticoids from binding to MRs, but themselves have partial agonist activity for MRs. Therefore, steroidal MRBs could also somewhat prompt transcription of the target gene. (C) Due to the “bulky” structure, nonsteroidal MRBs alter the shape of MR when binding. This modified MR complex precludes not only mineralocorticoid binding but also the recruitment of co-activators, resulting in inhibition of MR signaling
Of note, those nonsteroidal MRBs show different distribution patterns from the steroidal MRBs. Experiments using quantitative autoradiography indicated that both radio-labeled spironolactone and eplerenone show a higher accumulation of drug-equivalent concentrations in kidneys than in cardiac tissues in rodents [36], while radio-labeled esaxerenone and finerenone have balanced accumulation between kidneys and heart [37, 38]. However, what this distributional difference means in the real-world practice remains unknown, although a few animal studies have reported slight differences in cardiac protective effects between nonsteroidal and steroidal MRBs [34, 38]. For central nerve system, it was common across all examined MRBs that the radioactivity in brain was significantly lower than in blood.
In addition, nonsteroidal MRBs show greater selectivity for MRs compared with traditional MRBs. Due to the imitated structure for progesterone, spironolactone and its metabolites possess certain binding ability for various steroid hormone receptors [14]. Evaluation of the transcriptional activity of those steroidal receptors demonstrated that spironolactone blocks ligand binding to human glucocorticoid receptors (GR), androgen receptors (AR), and progesterone receptors (PR) with IC50 values of 2600, 640, and 180 nM, respectively (Table 1) [16]. Furthermore, spironolactone also acts as a moderate agonist for ARs and PRs, contributing to off-target side effects such as gynecomastia and irregular menstruation [15]. To compensate for those unfavorable nature of spironolactone, eplerenone was synthesized as a more selective MRB. In particular, IC50 values of eplerenone for ARs and PRs are increased to 42,000 and 7400 nM, respectively, while its IC50 value for MRs is also increased from 66 to 970 nM (Table 1) [16]. In contrast, esaxerenone and finerenone have very high affinity for MRs and no agonist or antagonist effect on the other steroid hormone receptors as in vitro experiments demonstrated [16, 17]. This very high selectivity for MRs is considered attributable to the side-chain rearrangement by nonsteroidal MRBs which could occur in MRs, but not in GRs, ARs, and PRs [29•]. Accordingly, nonsteroidal MRBs are expected to exert MR-blocking effects with relatively small doses, following less side effects, particularly in SSHR-associated ones.
Clinical Evidence of Nonsteroidal MRBs in Hypertension
BP-Lowering Effects
To date, in nonsteroidal MRBs, only esaxerenone has been launched as an antihypertensive agent in Japan. For monotherapy with esaxerenone, a phase 2 double-blind study enrolling 426 patients with essential hypertension (EH) without CKD showed the significant decrease of sitting BP after 12-week treatment compared with the placebo group: the least squares mean changes (95% confidence interval) in systolic and diastolic BP were − 7.0 (− 9.5, − 4.6)/ − 3.8 (− 5.2, − 2.4) mmHg in the placebo group, and − 10.7 (− 13.2, − 8.2)/ − 5.0 (− 6.4, − 3.6), − 14.3 (− 16.8, − 11.9)/ − 7.6 (− 9.1, − 6.2), and − 20.6 (− 23.0, − 18.2)/ − 10.4 (− 11.8, − 9.0) mmHg in the esaxerenone groups of 1.25, 2.5, and 5.0 mg once daily, respectively [20]. The BP-lowering effects were dose-dependent and similar even when esaxerenone was initiated in combination with a calcium channel blocker or an inhibitor of renin-angiotensin system (RAS) [21, 22••]. Of note, the subgroup analysis showed that BP reduction under esaxerenone is numerically greater in females, elderly, and those with lower levels of plasma renin activity [22••]. In comparison between esaxerenone and eplerenone, a phase 3 clinical trial, ESAX-HTN study, including 1001 adult patients with EH, demonstrated that esaxerenone (2.5 mg daily) is not inferior to eplerenone (50 mg daily) for the antihypertensive effect on clinic sitting BP [22••]. In this study, the proportion of the patients who achieved target BP levels (< 140/90 mmHg) were 31.5, 41.2, and 27.5% in the esaxerenone groups (2.5 and 5 mg daily) and the eplerenone group (50 mg daily), respectively [22••]. Ambulatory BP monitoring also showed similar BP trends between 2.5 mg/day of esaxerenone and 50 mg/day of eplerenone within daytime, while nighttime systolic BP was more dramatically improved in the esaxerenone group than the eplerenone group [39].
The antihypertensive effect of esaxerenone was further assessed for the following populations: CKD, DKD, and primary aldosteronism (PA). In hypertensive patients with CKD (estimated glomerular filtration ratio [eGFR] between 30 and 60 mL/min/1.73 m2), 5 mg/day of esaxerenone monotherapy significantly lowered BP by − 18.5 (− 23.7, − 13.3)/ − 8.8 (− 11.9, − 5.7) mmHg with no renal-related adverse effect [24]. Similar BP improvement was also observed with smaller doses of esaxerenone (median: 2.5 mg/day) in CKD patients under treatment with a RAS inhibitor [24]. In hypertensive type 2 DKD patients, add-on therapy of esaxerenone to a RAS inhibitor significantly decreased BP by − 13.7 (− 17.6, − 9.8)/ − 6.2 (− 7.8, − 4.6) mmHg, and reduced urine albumin-to-creatinine ratio (UACR) by about 30% [40]. The antihypertensive effects were consistent across subgroups of age and glycemic control. Finally, the efficacy of esaxerenone was evaluated in PA patients. In the study with 44 PA patients, esaxerenone was initiated at 2.5 mg/day and titrated to 5 mg/day [41•]. At the end of 12-week treatment, 93% (41/44) of study participants were treated with 5 mg/day of esaxerenone, and 68% had concomitant antihypertensive agents, a calcium channel blocker or an alpha blocker. Under esaxerenone treatment, BP was decreased by − 17.7 (− 20.6, − 14.7)/ − 9.5 (− 11.7, − 7.3) mmHg, resulting in 47.7% achievement of target BP levels (< 140/90 mmHg) in PA [41•]. Those findings indicate that esaxerenone possesses at least equivalent BP-lowering ability to other steroidal MRBs in various conditions, although all the clinical data shown here were obtained from Japanese cohorts.
In contrast, international trials targeting for chronic HF and CKD suggested that finerenone has a relatively weak effect on BP compared to esaxerenone. In the ARTS (MinerAlocorticoid Receptor antagonist Tolerability Study) trial, the reductions of systolic BP were significantly smaller in the finerenone group (the dose of 10 mg/day: mean ± standard deviation, − 4.2 ± 15.5 mmHg) than in the spironolactone group (the mean dose of 37 mg/day: − 10.1 ± 15.0 mmHg) [25]. Similarly, the decrease of systolic BP by finerenone was relatively small, but similar to eplerenone in the ARTS-HF (MinerAlocorticoid Receptor antagonist Tolerability Study- Heart Failure) trial: the least squares mean changes of systolic BP were − 2.365 (− 5.287, 0.558) mmHg in the eplerenone group (the average dose of 38.6 mg/day) and − 0.825 (− 3.929, 2.278), − 2.532 (− 5.630, 0.566), − 2.697 (− 5.757, 0.363), and − 2.397 (− 5.348, 0.554) mmHg in the finerenone groups (2.5 to 5, 5 to 10, 7.5 to 15, and 10 to 20 mg daily, respectively) [42]. Those results were consistent with those of its phase 3 trial, FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) [27••]. However, those studies were designed to evaluate the benefits of finerenone treatment on cardiac and renal outcomes, but not on BP. Due to the limited analysis on BP, the actual antihypertensive effect of finerenone should be further investigated.
Protective Effects on Cardiac and Renal Diseases
Several Japanese studies indicate the antialbuminuric effect of esaxerenone in DKD. In a study enrolling 51 patients with type 2 diabetes and albuminuria under a RAS inhibitor, esaxerenone initiation improved not only BP but also UACR during a 12-week observational period [40]. In this study, the dosage of esaxerenone was gradually titrated from 1.25 to 5 mg/day based on monitoring of serum potassium and eGFR, and at the end, 15.7, 47.1, and 37.3% of patients were treated with 1.25, 2.5, and 5 mg daily of esaxerenone. Overall, UACR decreased by 32.4% from baseline levels with improvement of urinary β2-microglobulin excretion [40]. Consequently, the ESAX-DN study, a more focused study on microalbuminuria, also demonstrated the long-term effect of esaxerenone on UACR in diabetes patients treated with a RAS inhibitor [23••]. Total 455 diabetes patients with microalbuminuria (UACR within 45 to 300 mg/g creatinine) were randomly allocated to the placebo and the esaxerenone groups, and in the latter group, esaxerenone was initiated at 1.25 mg/day and titrated to 2.5 mg/day. After 52-week treatment with esaxerenone, the UACR was significantly reduced by 58% from baseline values compared with the placebo group (8%) [23••]. Further analysis revealed that this antialbuminuric effect was independent of its BP-lowering effect. Moreover, of these, 22% of patients treated with esaxerenone achieved remission in UACR, which was more frequent than those with placebo (4%) [23••]. Similar improvement of UACR was also observed in a small study of 52 type 2 diabetes patients with macroalbuminuria (UACR > 300 mg/g creatinine): mean UACR decreased to less than half of baseline, and 52% of the study cases no longer had macroalbuminuria after esaxerenone treatment for 28 weeks [43]. From a cardio-protective aspect, a retrospective analysis reported the significant decrease of plasma B-type natriuretic peptide (BNP) levels by esaxerenone treatment in patients with HF [44]. Thus, clinical evidence on organ-protective benefits of esaxerenone is accumulating, but still limited. Future studies on hard outcomes such as CKD progression and the onset of cardiovascular disease are in great demand.
On the other hand, there are two large, randomized trials, ARTS and FIDELIO-DKD, which confirmed improved clinical outcome by finerenone treatment in HF with reduced ejection fraction (HFrEF) and/or DKD [25, 27••]. First, the ARTS study was initially conducted to assess the safety and tolerability of finerenone in patients with HFrEF and DKD, incorporating with 10 countries worldwide. Within the original study, finerenone treatment was associated with reductions of BNP, N-terminal pro-BNP, and UACR as was spironolactone treatment [25]. Furthermore, targeting for a composite outcome of any death, hospitalization for CVD, and emergency presentation for exacerbated HF, those who received once-daily administration of finerenone (10 mg once daily or more) had a lower incidence of the composite endpoint at day 90 than those treated with eplerenone (the average dose of 38.6 mg/day) in the ARTS-HF study [42]. Especially, comparing with the eplerenone group, the hazard ratio (HR) in the 10- to 20-mg group of finerenone was 0.56 (0.35, 0.90) at the composite outcome: HRs, 0.13 (0.02, 1.07), 0.56 (0.34, 0.93), and 0.58 (0.33, 1.02) for any death, cardiovascular hospitalization, and emergency presentation for worsening chronic HF, respectively [42]. In addition, the ARTS-DN study confirmed the dose-dependent improvement of UACR after finerenone initiation [45]. However, we must consider that the ARTS trial was not designed for comparison between finerenone and steroidal MRBs.
Recently, results of the FIDELIO-DKD trial have been announced [27••], advancing finerenone towards FDA approval. This phase 3 trial recruited adults with type 2 diabetes mellitus and CKD treated with a RAS inhibitor across the world, and finally, 5734 patients were randomly allocated to the finerenone or placebo groups. With a median follow-up period of 2.6 years, the finerenone group showed a lower incidence of the renal composite outcome, including kidney failure, over 40% decrease of eGFR and death from renal causes, than the placebo group (17.8% vs. 21.1%, respectively: HR, 0.82 [0.73, 0.93]) [27••]. Particularly, the sustained decrease of eGFR from baseline was less frequent in the finerenone group than in the placebo group (HR, 0.82 [0.72, 0.92]), while neither kidney failure nor death from renal causes was significantly different between them [27••]. For the cardiovascular arm of the trial, the finerenone group also had a lower incidence of the secondary composite outcome of death from CVD, nonfatal CVD, and hospitalization for HF compared with the placebo group (13.0% vs. 14.8%, respectively: HR, 0.86 [0.75, 0.99]) irrespective of a history of CVD [27••, 46]. In addition, subgroup analysis revealed that the incidence of new-onset atrial fibrillation or flutter is lower in the finerenone group than in the placebo group (3.2% vs. 4.5%, respectively: HR, 0.71 [0.53, 0.94]) [26•]. Those outcomes in the finerenone group were obtained with minimal changes of systolic BP from baseline: − 3.0 and − 2.1 mmHg at months 1 and 12 after finerenone initiation, respectively [27••]. Finerenone is, therefore, expected as an organ-protective agent rather than an antihypertensive one in type 2 diabetes.
Safety Profiles of Nonsteroidal MRBs: Renal-Related and Off-target Side Effects
Basically, decrease of eGFR followed by elevation of serum potassium is closely tied to the MR-blocking effect under MRB treatment. In patients with essential hypertension and preserved renal function (eGFR > 60 mL/min/1.73 m2), esaxerenone monotherapy lowered eGFR depending on its doses: the mean eGFR changes with standard deviation between baseline and week 12 were − 2.31 ± 6.85, − 3.69 ± 7.98, and − 6.36 ± 8.08 mL/min/1.73 m2 in the 1.25-, 2.5-, and 5-mg daily groups, respectively, while those of placebo and eplerenone (50 to 100 mg daily) were 0.06 ± 6.05 and − 2.11 ± 6.35 mL/min/1.73 m2, respectively [20]. Concurrently, serum potassium levels elevated under esaxerenone treatment by 0.2 to 0.3 mM at weeks 1 and 2, and then gradually decreased to nearly baseline levels. Finally, in the phase 2 trial, adverse events of hyperkalemia or renal dysfunction were reported in 0, 3.6, and 3.4% and 3.6, 0, and 3.4% of the esaxerenone groups of 1.25, 2.5, and 5 mg daily, respectively, compared with 2.3 and 1.1% of the placebo group [20]. Consequently, the phase 3 trial also confirmed that the incidence of renal-related adverse effects was not different between treatment of eplerenone and esaxerenone [22••]. With careful assessment of those parameters, esaxerenone could be safely used in patients with moderate renal dysfunction (eGFR between 30 and 60 mL/min/1.73 m2) and those who treated with a RAS inhibitor [24, 40], while when used in combination with a RAS inhibitor, esaxerenone use tended to more frequently cause serum potassium elevation (12.1%) and eGFR decrease (5.2%) [24].
Probably reflecting the mild effect on BP, finerenone in combination with other antihypertensives was associated with a lower incidence of hyperkalemia and worsening renal function than spironolactone in the patients with HFrEF and CKD: 4.5 and 10.4% in the 10-mg daily finerenone group, and 11.1% and 38.1% in the 25- to 50-mg daily spironolactone group, respectively [25]. On the other hand, in the ARTS-HF trial, the incidence of hyperkalemia (> 5.5 mM) and the degree of eGFR decline were similar between eplerenone and finerenone groups [42]. The prevalence of those adverse events by finerenone was compatible with the results of FIDELIO-DKD trial [27••]. Accordingly, renal-related side effects by nonsteroidal MRBs are considered almost equivalent to those of eplerenone, and maybe less frequent than those of spironolactone.
During trials of esaxerenone and finerenone, nonspecific symptoms, including nasopharyngitis, upper respiratory tract inflammation, and headache, were commonly observed across the study groups of the placebo, nonsteroidal MRBs, and other steroidal MRBs [20, 22••, 25, 42]. Expectedly and importantly, no adverse effect related to SSHRs was reported in the patients treated with nonsteroidal MRBs. In addition, there were no other significant differences of the safety profiles between nonsteroidal and steroidal MRBs. Liver function plays a key role for the metabolism of nonsteroidal MRBs as cytochrome P450 3A4 predominantly regulates its metabolic process. Nevertheless, evaluation of their pharmacokinetics suggested that those nonsteroidal MRBs could be safely used in mild to moderate hepatic impairment [47, 48].
Perspectives of MR Blocking Strategy
Recent studies have provided compelling evidence that aberrant MR activation is deeply involved in end-organ damage. Several factors such as sympathetic hyperactivity and obesity cause MR overactivation via aldosterone excess, leading to pro-inflammatory immune responses and vascular fibrosis [49, 50]. Without aldosterone, salt intake also induces Rac1-mediated MR activation in the target tissues, heart, and kidneys [51,52,53]. As another mechanism, glucocorticoids could act as an agonist for MRs in the condition where inactivation of cortisol by 11β-hydroxysteroid dehydrogenase type 2 is impaired by endogenous and exogenous factors [54]. Therefore, in hypertensive cases, MRBs could be initiated to abolish such MR-related organ damage regardless of aldosterone levels.
While accumulating evidence has established the solid position of spironolactone as an add-on drug in patients with resistant hypertension and CVD, SSHR-related adverse events often preclude them from continuing spironolactone. In the RALES (Randomized Aldactone Evaluation Study) trial enrolling severe HF patients, spironolactone use was associated with a higher risk for gynecomastia or breast pain in men compared with the placebo (10% vs. 1%, respectively) [55]. Similarly, such adverse events were reported in 10% of men treated with spironolactone for resistant hypertension [56]. In women, irregular menstruation is reported as the most common adverse effect of spironolactone [57]. In contrast, with decreased affinity for SSHRs, eplerenone less frequently cause those symptoms, but does not completely resolve them [58]. Also, due to the shorter half-life time and the lower potency compared with spironolactone, eplerenone needs to be taken twice daily [59], possibly leading to poor adherence.
As forementioned, the notable advantage of nonsteroidal MRBs is their outstanding selectivity for MRs (Table 1). Based on the unique binding mode, both esaxerenone and finerenone have no agonist activity for MRs and cause no sexual adverse effects. Furthermore, the results of several trials indicate that once daily administration of those MRBs leads to antihypertensive and organ-protective benefits at least equivalent to steroidal MRBs. Particularly, esaxerenone is considered useful as a standard antihypertensive drug in everyday practice. Therefore, the nonsteroidal agents enable us to extend MR-blocking therapy even to the patients who cannot pursue the treatment with conventional MRBs because of sexual side effects and/or poor adherence, resulting in better clinical outcomes of CVD. Besides, it also seems worthy investigating additional benefits specific to nonsteroidal MRBs, e.g., action on arteriosclerosis and cerebrovascular disease. Inversely, spironolactone may still serve as a key drug in certain endocrine-related conditions involving overactivation of other steroid hormone receptors. Recent studies focusing on steroid profiles revealed disorganized production of multiple steroid hormones in endocrine disorders [60,61,62]. However, a common thing in both steroidal and nonsteroidal MRBs is that their effects are closely associated with renal function and serum potassium. We further need to determine proper selection of and management with MRBs depending on the patient’s condition.
Conclusion
As expected, nonsteroidal MRBs, esaxerenone and finerenone, have great potential in management of hypertension and its complications. Their distinctive structures allow themselves to rigidly bind to just MRs, but not other steroid hormone receptors. Consequently, with no off-target sexual symptoms, the intended effect of MR blocking can be efficiently achieved by initiation of nonsteroidal MRBs. Recent trials confirmed the antihypertensive effect of esaxerenone in both monotherapy and combination therapy, and the preventive effects of nonsteroidal MRBs on cardiac and renal damage. In addition, other candidates of nonsteroidal MRBs are being examined in rapid succession [63,64,65]. Although further examination on their clinical utility is required to enrich our understanding about those agents, nonsteroidal MRBs will contribute to lowered risks for CVD and CKD progression, particularly in the patients who are intolerant of steroidal MRBs. A new era is coming to reappraise our antihypertensive strategy using MRBs.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059–68. https://doi.org/10.1016/S0140-6736(15)00257-3.
Serenelli M, Jackson A, Dewan P, Jhund PS, Petrie MC, Rossignol P, et al. Mineralocorticoid receptor antagonists, blood pressure, and outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8(3):188–98. https://doi.org/10.1016/j.jchf.2019.09.011.
Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. https://doi.org/10.1056/NEJMoa1009492.
• Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, et al. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–75. https://doi.org/10.1016/S2213-8587(18)30071-8. This subanalysis of the PATHWAY-2 study indicates that spironolactone lowers blood pressure regardless of plasma aldosterone levels in resistant hypertension.
Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62(17):1585–93. https://doi.org/10.1016/j.jacc.2013.04.086.
Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–51. https://doi.org/10.2215/cjn.00240106.
Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28(11):1376–85. https://doi.org/10.1093/ajh/hpv031.
Cleland JGF, Ferreira JP, Mariottoni B, Pellicori P, Cuthbert J, Verdonschot JAJ, et al. The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart ‘OMics’ in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. 2021;42(6):684–96. https://doi.org/10.1093/eurheartj/ehaa758.
Kagawa CM, Cella JA, Van Arman CG. Action of new steroids in blocking effects of aldosterone and desoxycorticosterone on salt. Science. 1957;126(3281):1015–6. https://doi.org/10.1126/science.126.3281.1015.
Sun LJ, Sun YN, Shan JP, Jiang GR. Effects of mineralocorticoid receptor antagonists on the progression of diabetic nephropathy. J Diabetes Investig. 2017;8(4):609–18. https://doi.org/10.1111/jdi.12629.
Montalescot G, Pitt B, Lopez de Sa E, Hamm CW, Flather M, Verheugt F, et al. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: the randomized double-blind reminder study. Eur Heart J. 2014;35(34):2295–302. https://doi.org/10.1093/eurheartj/ehu164.
Ferreira JP, Rossello X, Eschalier R, McMurray JJV, Pocock S, Girerd N, et al. MRAs in elderly HF patients: individual patient-data meta-analysis of RALES, EMPHASIS-HF, and TOPCAT. JACC Heart Fail. 2019;7(12):1012–21. https://doi.org/10.1016/j.jchf.2019.08.017.
Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, et al. Safety profile of mineralocorticoid receptor antagonists: spironolactone and eplerenone. Int J Cardiol. 2015;200:25–9. https://doi.org/10.1016/j.ijcard.2015.05.127.
Kolkhof P, Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125–40. https://doi.org/10.1530/JOE-16-0600.
Kallistratos MS, Pittaras A, Theodoulidis I, Grassos C, Poulimenos LE, Manolis AJ. Adverse effects of mineralocorticoid receptor antagonist administration. Curr Pharm Des. 2018;24(46):5537–41. https://doi.org/10.2174/1381612825666190222144359.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34. https://doi.org/10.1016/j.ejphar.2015.06.015.
Bärfacker L, Kuhl A, Hillisch A, Grosser R, Figueroa-Pérez S, Heckroth H, et al. Discovery of BAY 94–8862: a nonsteroidal antagonist of the mineralocorticoid receptor for the treatment of cardiorenal diseases. ChemMedChem. 2012;7(8):1385–403. https://doi.org/10.1002/cmdc.201200081.
Duggan S. Esaxerenone: first global approval. Drugs. 2019;79(4):477–81. https://doi.org/10.1007/s40265-019-01073-5.
Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F, Ishizuka H. Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol. 2018;84(8):1821–9. https://doi.org/10.1111/bcp.13616.
Ito S, Itoh H, Rakugi H, Okuda Y, Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33(7):542–51. https://doi.org/10.1038/s41371-019-0207-x.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res. 2019;42(12):1932–41. https://doi.org/10.1038/s41440-019-0314-7.
•• Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75(1):51–8. https://doi.org/10.1161/hypertensionaha.119.13569. This paper demonstrates the solid antihypertensive effect of esaxerenone followed by no sexual adverse event in essential hypertension. The blood pressure-lowering effect of 2.5 mg daily esaxerenone was noninferior to that of 50 mg daily eplerenone.
•• Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol. 2020;15(12):1715–27. https://doi.org/10.2215/cjn.06870520. This paper shows that esaxerenone use dramatically reduces urinary albumin excretion in diabetic petients treated with renin-angiotensin system inhibitors.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res. 2021;44(5):489–97. https://doi.org/10.1038/s41440-020-00585-y.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63. https://doi.org/10.1093/eurheartj/eht187.
• Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM, et al. Finerenone reduces onset of atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol. 2021. https://doi.org/10.1016/j.jacc.2021.04.079. This subanalysis of the FIDELIO-DKD trial shows that finerenone has a preventive effect on new onset of atrial fibrillation in diabetic patients with chronic kidney disease.
•• Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29. https://doi.org/10.1056/NEJMoa2025845. This large randomized cohort elucidates that finerenone significantly reduces cardiac and renal risks with mild improvement of blood pressure in diabetic patients with chronic kidney disease.
Yang J, Young MJ. Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences. Curr Opin Pharmacol. 2016;27:78–85. https://doi.org/10.1016/j.coph.2016.02.005.
• Takahashi M, Ubukata O, Homma T, Asoh Y, Honzumi M, Hayashi N, et al. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett. 2020;594(10):1615–23. https://doi.org/10.1002/1873-3468.13746. This paper reveals the unique binding mode of esaxerenone to mineralocorticoid receptors.
Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290(36):21876–89. https://doi.org/10.1074/jbc.M115.657957.
Gardiner P, Schrode K, Quinlan D, Martin BK, Boreham DR, Rogers MS, et al. Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites. J Clin Pharmacol. 1989;29(4):342–7. https://doi.org/10.1002/j.1552-4604.1989.tb03339.x.
Cook CS, Berry LM, Bible RH, Hribar JD, Hajdu E, Liu NW. Pharmacokinetics and metabolism of [14C]eplerenone after oral administration to humans. Drug Metab Dispos. 2003;31(11):1448–55. https://doi.org/10.1124/dmd.31.11.1448.
Gerisch M, Heinig R, Engelen A, Lang D, Kolkhof P, Radtke M, et al. Biotransformation of finerenone, a novel nonsteroidal mineralocorticoid receptor antagonist, in dogs, rats, and humans, in vivo and in vitro. Drug Metab Dispos. 2018;46(11):1546–55. https://doi.org/10.1124/dmd.118.083337.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73. https://doi.org/10.1016/j.ejphar.2015.11.028.
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther. 2016;358(3):548–57. https://doi.org/10.1124/jpet.116.234765.
Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–61. https://doi.org/10.1093/eurheartj/ehaa736.
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017;47(12):1090–103. https://doi.org/10.1080/00498254.2016.1263766.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78. https://doi.org/10.1097/fjc.0000000000000091.
Kario K, Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, et al. Effect of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, on nocturnal hypertension: a post hoc analysis of the ESAX-HTN study. Am J Hypertens. 2021;34(5):540–51. https://doi.org/10.1093/ajh/hpaa155.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res. 2019;42(10):1572–81. https://doi.org/10.1038/s41440-019-0270-2.
• Satoh F, Ito S, Itoh H, Rakugi H, Shibata H, Ichihara A, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res. 2021;44(4):464–72. https://doi.org/10.1038/s41440-020-00570-5. This paper demonstrates the mineralocorticoid receptor-blocking effect of esaxerenone in primary aldosteronism.
Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14. https://doi.org/10.1093/eurheartj/ehw132.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021. https://doi.org/10.1007/s10157-021-02075-y.
Naruke T, Maemura K, Oki T, Yazaki M, Fujita T, Ikeda Y, et al. Efficacy and safety of esaxerenone in patients with hypertension and concomitant heart failure. Hypertens Res. 2021;44(5):601–3. https://doi.org/10.1038/s41440-020-00606-w.
Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94. https://doi.org/10.1001/jama.2015.10081.
Filippatos G, Anker SD, Agarwal R, Pitt B, Ruilope LM, Rossing P, et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143(6):540–52. https://doi.org/10.1161/circulationaha.120.051898.
Kurata A, Yoshida T, Inoue M, Ishizuka T, Nakatsu T, Shimizu T, et al. Pharmacokinetics and safety of single-dose esaxerenone in japanese subjects with mild to moderate hepatic impairment. Adv Ther. 2020;37(1):253–64. https://doi.org/10.1007/s12325-019-01121-2.
Heinig R, Lambelet M, Nagelschmitz J, Alatrach A, Halabi A. Pharmacokinetics of the novel nonsteroidal mineralocorticoid receptor antagonist finerenone (BAY 94–8862) in individuals with mild or moderate hepatic impairment. Eur J Drug Metab Pharmacokinet. 2019;44(5):619–28. https://doi.org/10.1007/s13318-019-00547-x.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. https://doi.org/10.1161/circresaha.116.305697.
Saxena PR. Interaction between the renin-angiotensin-aldosterone and sympathetic nervous systems. J Cardiovasc Pharmacol. 1992;19(Suppl 6):S80–8. https://doi.org/10.1097/00005344-199219006-00013.
Quinkler M, Zehnder D, Eardley KS, Lepenies J, Howie AJ, Hughes SV, et al. Increased expression of mineralocorticoid effector mechanisms in kidney biopsies of patients with heavy proteinuria. Circulation. 2005;112(10):1435–43. https://doi.org/10.1161/circulationaha.105.539122.
Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, et al. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol. 2012;23(6):997–1007. https://doi.org/10.1681/asn.2011070734.
Ayuzawa N, Nagase M, Ueda K, Nishimoto M, Kawarazaki W, Marumo T, et al. Rac1-mediated activation of mineralocorticoid receptor in pressure overload-induced cardiac injury. Hypertension. 2016;67(1):99–106. https://doi.org/10.1161/hypertensionaha.115.06054.
Morris DJ, Latif SA, Hardy MP, Brem AS. Endogenous inhibitors (GALFs) of 11beta-hydroxysteroid dehydrogenase isoforms 1 and 2: derivatives of adrenally produced corticosterone and cortisol. J Steroid Biochem Mol Biol. 2007;104(3–5):161–8. https://doi.org/10.1016/j.jsbmb.2007.03.020.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001.
Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839–45. https://doi.org/10.1161/01.HYP.0000259805.18468.8c.
Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169–91. https://doi.org/10.1007/s40257-016-0245-x.
Yamamoto M, Seo Y, Ishizu T, Nishi I, Hamada-Harimura Y, Machino-Ohtsuka T, et al. Comparison of effects of aldosterone receptor antagonists spironolactone and eplerenone on cardiovascular outcomes and safety in patients with acute decompensated heart failure. Heart Vessels. 2019;34(2):279–89. https://doi.org/10.1007/s00380-018-1250-1.
Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15(8):709–16. https://doi.org/10.1016/s0895-7061(02)02957-6.
Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773–81. https://doi.org/10.1016/s2213-8587(20)30218-7.
Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62(3):514–24. https://doi.org/10.1373/clinchem.2015.251199.
Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115–24. https://doi.org/10.1016/j.cca.2017.05.002.
Wada T, Inagaki M, Yoshinari T, Terata R, Totsuka N, Gotou M, et al. Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin Exp Nephrol. 2021;25(2):120–30. https://doi.org/10.1007/s10157-020-01963-z.
Whittaker A, Kragh ÅM, Hartleib-Geschwindner J, Albayaty M, Backlund A, Greasley PJ, et al. Safety, tolerability, and pharmacokinetics of the mineralocorticoid receptor modulator AZD9977 in healthy men: a phase i multiple ascending dose study. Clin Transl Sci. 2020;13(2):275–83. https://doi.org/10.1111/cts.12705.
Bakris G, Pergola PE, Delgado B, Genov D, Doliashvili T, Vo N, et al. Effect of KBP-5074 on blood pressure in advanced chronic kidney disease: results of the BLOCK-CKD study. Hypertension. 2021;78(1):74–81. https://doi.org/10.1161/hypertensionaha.121.17073.
Acknowledgements
We would appreciate our colleagues at Tohoku University Hospital who dedicated their expertise in this field: Kei Omata MD, Yoshikiyo Ono MD, Ryo Morimoto MD, and Fumitoshi Satoh MD. We would also thank Mika Ainoya, Kumi Kikuchi, Akane Sugawara, Yasuko Tsukada, and Hiroko Kato for their secretarial assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization, paper review, and original draft preparation, Y.T.; review and editing, S.I.
Corresponding author
Ethics declarations
Conflict of Interest
Yuta Tezuka and Sadayoshi Ito declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tezuka, Y., Ito, S. The Time to Reconsider Mineralocorticoid Receptor Blocking Strategy: Arrival of Nonsteroidal Mineralocorticoid Receptor Blockers. Curr Hypertens Rep 24, 215–224 (2022). https://doi.org/10.1007/s11906-022-01177-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01177-6