Abstract
Esaxerenone is a selective, nonsteroidal, high-affinity mineralocorticoid receptor antagonist recently approved in Japan for the treatment of hypertension. It has high oral biovailability, a large volume of distribution, and is primarly metabolized in liver and excreted in bile. Esaxerenone is an efficient antihypertensive, whether given alone or as add-on therapy. The antihypertensive effect is accompanied by renoprotective action, which is being further investigated in current clinical trials. Due to its relatively long half-life and high affinity for the mineralocorticoid receptor, esaxerenone is administered once daily and in low absolute doses. The safety of esaxerenone is considerable, since hyperkalemia is not frequent and, when it does appear, not sustained. Endocrine adverse events, which frequently occur with steroidal mineralocorticoid receptor antagonists, are extremely rare with esaxerenone. Although the risk of clinically significant drug–drug interactions is not high, esaxerenone treatment should start with low doses, with subsequent titration to achieve the optimal clinical effect, all while monitoring serum potassium and paying attention to concomitant therapy with drugs that may induce or inhibit esaxerenone metabolism. This review article offers comprehensive information about the pharmacodynamics and pharmacokinetics of esaxerenone in humans, which should help clinicians to more precisely tailor esaxerenone dosing regimens to their patients.

Similar content being viewed by others
References
Selye H. Production of nephrosclerosis by overdosage with desoxycorticosterone acetate. Can Med Assoc J. 1942;47(6):515–9.
Kolkhof P, Bärfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125–40.
Selye H. Protection by a steroid-spirolactone against certain types of cardiac necroses. Proc Soc Exp Biol Med. 1960;104:212–3.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341(10):709–17.
Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–75.
Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EAG, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283(5):H1802-1810.
Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990;67(6):1355–64.
Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–61.
Duggan S. Esaxerenone: first global approval. Drugs. 2019;79(4):477–81.
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica Fate Foreign Compd Biol Syst. 2017;47(12):1090–103.
Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F, Ishizuka H. Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol. 2018;84(8):1821–9.
Yamada M, Mendell J, Takakusa H, Shimizu T, Ando O. Pharmacokinetics, metabolism, and excretion of [14C]esaxerenone, a novel mineralocorticoid receptor blocker in humans. Drug Metab Dispos Biol Fate Chem. 2019;47(3):340–9.
Ito S, Itoh H, Rakugi H, Okuda Y, Yamakawa S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: a phase 2 randomized, placebo-controlled, double-blind study. J Hum Hypertens. 2019;33(7):542–51.
Kurata A, Furuie H, Ishizuka T, Nakatsu T, Shimizu T, Kato M, et al. Absolute bioavailability of esaxerenone and food effects on its pharmacokinetics after a single oral dose in healthy Japanese subjects: an open-label crossover study. Adv Ther. 2019;36(7):1618–27.
Yamada M, Ishizuka T, Inoue S-I, Rozehnal V, Fischer T, Sugiyama D. Drug–drug interaction risk assessment of esaxerenone as a perpetrator by in vitro studies and static and physiologically based pharmacokinetic models. Drug Metab Dispos Biol Fate Chem. 2020;48(9):769–77.
Kurata A, Yoshida T, Inoue M, Ishizuka T, Nakatsu T, Shimizu T, et al. Pharmacokinetics and safety of single-dose esaxerenone in Japanese subjects with mild to moderate hepatic impairment. Adv Ther. 2020;37(1):253–64.
Fukae M, Jamsen K, Shimizu T, Yin O, Kastrissios H, Yoshihara K. Population pharmacokinetic modeling of esaxerenone: a novel nonsteroidal mineralocorticoid receptor blocker. Abstract 8859. In: 28th Population Approach Group Europe (PAGE) Meeting; 2019 Jun 11–14; Stockholm, Sweden. Available from: https://www.page-meeting.org/default.asp?abstract=8859
Toyama K, Furuie H, Kuroda K, Ishizuka T, Okuda Y, Shimizu T, et al. Effects of repeated oral administration of esaxerenone on the pharmacokinetics of midazolam in healthy Japanese males. Eur J Drug Metab Pharmacokinet. 2021;46(5):685–94.
Kirigaya Y, Shiramoto M, Ishizuka T, Uchimaru H, Irie S, Kato M, et al. Pharmacokinetic interactions of esaxerenone with amlodipine and digoxin in healthy Japanese subjects. BMC Pharmacol Toxicol. 2020;21(1):55.
Kirigaya Y, Shiramoto M, Ishizuka T, Uchimaru H, Irie S, Kato M, et al. Effects of itraconazole and rifampicin on the single-dose pharmacokinetics of the nonsteroidal mineralocorticoid receptor blocker esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol. 2020;86(10):2070–9.
Yamada M, Ishizuka T, Inoue S, Rozehnal V, Fischer T, Sugiyama D. Drug–drug interaction risk assessment of esaxerenone as a perpetrator by in vitro studies and static and physiologically based pharmacokinetic models. Drug Metab Dispos. 2020;48(9):769–77.
Watanabe A, Ishizuka T, Yamada M, Igawa Y, Shimizu T, Ishizuka H. Physiologically based pharmacokinetic modelling to predict the clinical effect of CYP3A inhibitors/inducers on esaxerenone pharmacokinetics in healthy subjects and subjects with hepatic impairment. Eur J Clin Pharmacol. 2022;78:65–73. https://doi.org/10.1007/s00228-021-03194-x (Online ahead of print).
Yamada M, Inoue S-I, Sugiyama D, Nishiya Y, Ishizuka T, Watanabe A, et al. Critical impact of drug–drug interactions via intestinal CYP3A in the risk assessment of weak perpetrators using physiologically based pharmacokinetic models. Drug Metab Dispos Biol Fate Chem. 2020;48(4):288–96.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34.
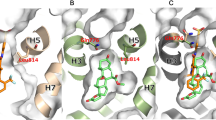
Takahashi M, Ubukata O, Homma T, Asoh Y, Honzumi M, Hayashi N, et al. Crystal structure of the mineralocorticoid receptor ligand-binding domain in complex with a potent and selective nonsteroidal blocker, esaxerenone (CS-3150). FEBS Lett. 2020;594(10):1615–23.
Chang W-T, Wu S-N. Characterization of direct perturbations on voltage-gated sodium current by esaxerenone, a nonsteroidal mineralocorticoid receptor blocker. Biomedicines. 2021;9(5):549.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73.
Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78.
Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther. 2016;358(3):548–57.
Li L, Guan Y, Kobori H, Morishita A, Kobara H, Masaki T, et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res Off J Jpn Soc Hypertens. 2019;42(6):769–78.
Bhuiyan AS, Rafiq K, Kobara H, Masaki T, Nakano D, Nishiyama A. Effect of a novel nonsteroidal selective mineralocorticoid receptor antagonist, esaxerenone (CS-3150), on blood pressure and renal injury in high salt-treated type 2 diabetic mice. Hypertens Res Off J Jpn Soc Hypertens. 2019;42(6):892–902.
Arai K, Morikawa Y, Ubukata N, Sugimoto K. Synergistic reduction in albuminuria in type 2 diabetic mice by esaxerenone (CS-3150), a novel nonsteroidal selective mineralocorticoid receptor blocker, combined with an angiotensin II receptor blocker. Hypertens Res Off J Jpn Soc Hypertens. 2020;43(11):1204–13.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN Study). Hypertens Dallas Tex 1979. 2020;75(1):51–8.
Kario K, Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, et al. Effect of the nonsteroidal mineralocorticoid receptor blocker, esaxerenone, on nocturnal hypertension: a post hoc analysis of the ESAX-HTN study. Am J Hypertens. 2021;34(5):540–51.
Rakugi H, Ito S, Itoh H, Okuda Y, Yamakawa S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens Res Off J Jpn Soc Hypertens. 2019;42(12):1932–41.
Satoh F, Ito S, Itoh H, Rakugi H, Shibata H, Ichihara A, et al. Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res Off J Jpn Soc Hypertens. 2021;44(4):464–72.
Naruke T, Maemura K, Oki T, Yazaki M, Fujita T, Ikeda Y, et al. Efficacy and safety of esaxerenone in patients with hypertension and concomitant heart failure. Hypertens Res Off J Jpn Soc Hypertens. 2021;44(5):601–3.
Satoh F, Morimoto R, Tezuka Y, Omata K, Yamanami H, Ono Y, et al. Blood pressure lowering effects and safety of esaxerenone, newly available mineralocorticoid receptor blocker. J Hypertens. 2021;39: e64.
Oshima A, Imamura T, Narang N, Kinugawa K. Renoprotective effect of the mineralocorticoid receptor antagonist esaxerenone. Circ Rep. 2021;3(6):333–7.
Ito S, Itoh H, Rakugi H, Okuda Y, Iijima S. Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction. Hypertens Res Off J Jpn Soc Hypertens. 2021;44(5):489–97.
Itoh H, Ito S, Rakugi H, Okuda Y, Nishioka S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: a single-arm, open-label study. Hypertens Res Off J Jpn Soc Hypertens. 2019;42(10):1572–81.
Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol CJASN. 2019;14(8):1161–72.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Esaxerenone (CS-3150) in patients with type 2 diabetes and microalbuminuria (ESAX-DN): phase 3 randomized controlled clinical trial. Clin J Am Soc Nephrol CJASN. 2020;15(12):1715–27.
Ito S, Kashihara N, Shikata K, Nangaku M, Wada T, Okuda Y, et al. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: a multicenter, single-arm, open-label phase III study. Clin Exp Nephrol. 2021;25(10):1070–8.
Fukae M, Jamsen K, Shimizu T, Yoshimura M, Yin O, Kastrissios H, Yoshihara K. M-014 Exposure-response analyses for efficacy and safety of esaxerenone, a novel nonsteroidal mineralocorticoid receptor blocker. A33. In: 10th American Conference on Pharmacometrics; 2019 Oct 20–23; Orlando, FL, USA. Available from: https://docplayer.net/156879773-Acop10-issn-acop10-orlando-fl-october-individual-abstracts-will-be-posted-online-post-meeting.html
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res Off J Jpn Soc Hypertens. 2021;44(4):371–85.
Mendell J, Kobayashi F, Shimizu T. Randomized, double-blind, single-dose, placebo-controlled crossover study to evaluate the effects of esaxerenone on QTc interval in healthy subjects. Clin Pharmacol Drug Dev. 2020;9(6):709–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This manuscript was partially funded by grant 175007 given by the Serbian Ministry of Education, Science and Technological Development.
Conflicts of Interest
Slobodan M. Janković and Snežana V. Janković declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data are included in the manuscript.
Code Availability
Not applicable.
Authors' Contributions
Slobodan M. Janković and Snežana V. Janković drafted, wrote, and revised the manuscript.
Rights and permissions
About this article
Cite this article
Janković, S.M., Janković, S.V. Clinical Pharmacokinetics and Pharmacodynamics of Esaxerenone, a Novel Mineralocorticoid Receptor Antagonist: A Review. Eur J Drug Metab Pharmacokinet 47, 291–308 (2022). https://doi.org/10.1007/s13318-022-00760-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00760-1