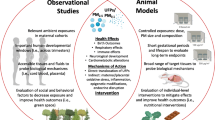
Abstract
Purpose of Review
To summarize the current knowledge of the pathophysiological implications and the clinical role of urban-related environmental exposures in pregnancy.
Recent Findings
The ongoing urbanization worldwide is leading to an increasing number of pregnant women being exposed to higher levels of urban-related environmental hazards such as air pollution and noise and, at the same time, having less contact with natural environments. Pregnancy represents a particular and vulnerable life period both for women and their children. Extensive physiological and metabolic changes, as well as changes to the cardiovascular and respiratory systems during pregnancy, could result in increased sensitivity to damage by environmental factors.
Summary
Exposure to air pollution and noise is associated with placental dysfunction and damage, which, in turn, could lead to maternal complications such as preeclampsia. In contrast, more contact with greenspace during pregnancy seems to mitigate these adverse impacts. These findings open up new challenges for our understanding of the potential effect of urban living on placental function and preeclampsia, and offer new clinical and research opportunities.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization. No Title. World Health Statistics 2018: Monitoring health for the SDGs 2018. https://www.who.int/gho/publications/world_health_statistics/2018/en/
United States Environmental Protection Agency. Air quality criteria for particulate matter: Environ. Prot. Agency, Natl. Cent. Environ. Assess. EPA, pp. 600/P-99/002aF-bF; 2004.
Xu F, Shi X, Qiu X, Jiang X, Fang Y, Wang J, et al. Investigation of the chemical components of ambient fine particulate matter (PM2.5) associated with in vitro cellular responses to oxidative stress and inflammation. 2020; https://doi.org/10.1016/j.envint.2020.105475.
Nadadur SS, Hollingsworth JW. In: Nadadur SS, Hollingsworth JW, editors. Air pollution and health effects. Molecular and integrative toxicology. 2015th ed. London: Springer-Verlag; 2015. p. 1–24.
European Environment Agency. Exposure of Europe’s population to environmental noise. 2019. https://www.eea.europa.eu/data-and-maps/indicators/exposure-to-and-annoyance-by-2/assessment-4
Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, et al. Auditory and non-auditory effects of noise on health. Lancet. 2014; https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61613-X/fulltext#articleInformation.
Münzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014;35(13):829–36.
World Health Organization. Urban green space interventions and health. 2017. http://www.euro.who.int/en/health-topics/environment-and-health/urban-health/publications/2017/urban-green-space-interventions-and-health-a-review-of-impacts-and-effectiveness.-full-report-2017
Twohig-Bennett C, Jones A. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res. 2018;166:628–37. https://doi.org/10.1016/j.envres.2018.06.030.
Kondo MC, Fluehr JM, McKeon T, Branas CC. Urban green space and its impact on human health. Int J Environ Res Public Health. 2018;15(3).
Dzhambov AM, Dimitrova DD, Dimitrakova ED. Association between residential greenness and birth weight: systematic review and meta-analysis. Urban For Urban Green. 2014. http://www.sciencedirect.com/science/article/pii/S1618866714000995;13:621–9.
Nieuwenhuijsen MJ, Dadvand P, Grellier J, Martinez D, Vrijheid M. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health. 2013;12. https://doi.org/10.1186/1476-069X-12-6.
Hypertension in Pregnancy: Executive Summary. Obstet Gynecol. 2013;122(5):1122–31.
Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. n.d.; http://www.sciencedirect.com/science/article/pii/S2210778912003686.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:1–15.
Godfrey KM, Barker DJ.Fetal programming and adult health. Public Health Nutrition. 2001; https://www.cambridge.org/core/product/3BB0394BBC80F4DC3B348ECD8EBC460D
Saenen ND, Martens DS, Neven KY, Alfano R, Bové H, Janssen BG, et al. Air pollution-induced placental alterations: an interplay of oxidative stress, epigenetics, and the aging phenotype? Clin Epigenetics. 2019;1. https://doi.org/10.1186/s13148-019-0688-zOverview of air pollution-induced placental molecular alterations observed in the ENVIRONAGE birth cohort and evaluate the existing evidence. In general, it showed that prenatal exposure to air pollution is associated with nitrosative stress and epigenetic alterations in the placenta.
Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. https://doi.org/10.1007/s10654-013-9762-6.
Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-Eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22.
Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64(3):494–500.
European Environment Agency. No Title. Air quality in Europe 2019. 2019; http://www.eea.europa.eu/publications/air-quality-in-europe-2019
Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ (Clin Res Ed). 2014;348:f7412.
Brook RD, Rajagopalan S, Pope CA, 3rd Brook JR, Bhatnagar A, Diez-Roux AV, et al. & American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78.
Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56(6):709–42.
Pope CA, 3rd Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116(1):108–15.
Meo SA, Suraya F. Effect of environmental air pollution. European Review for Medical and Pharmacological Sciences. 2015;http://www.europeanreview.org/article/10047
Ha S, Männistö T, Liu D, Sherman S, Ying Q, Mendola P. Air pollution and cardiovascular events at labor and delivery: a case-crossover analysis. Ann Epidemiol. 2017;27(6):377–83.
Weldy CS, Liu Y, Chang Y-C, Medvedev IO, Fox JR, Larson T, et al. In utero and early life exposure to diesel exhaust air pollution increases adult susceptibility to heart failure in mice. Part Fibre Toxicol. 2013. https://doi.org/10.1186/1743-8977-10-59.
Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: Methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102(2):182–90.
Olsson D, Mogren I, Forsberg B. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open. 2013;3(2):1–8.
Olsson D, Mogren I, Eneroth K, Forsberg B. Traffic pollution at the home address and pregnancy outcomes in Stockholm, Sweden. BMJ Open. 2015;5(8):1–8.
Wang Q, Zhang H, Liang Q, Knibbs LD, Ren M, Li C, et al. Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environ Pollut. 2018; http://www.sciencedirect.com/science/article/pii/S0269749117338502.
Choe S-A, Jun Y-B, Kim S-Y. Exposure to air pollution during preconceptional and prenatal periods and risk of hypertensive disorders of pregnancy: a retrospective cohort study in Seoul, Korea. BMC Pregnancy Childbirth. 2018;18:340. https://doi.org/10.1186/s12884-018-1982-z.
Dadvand P, Ostro B, Amato F, Figueras F, Minguillón MC, Martinez D, et al. Particulate air pollution and preeclampsia: a source-based analysis. Occup Environ Med. 2014; http://oem.bmj.com/content/71/8/570.abstract:–577.
•• Pedersen M, Halldorsson TI, Olsen SF, Hjortebjerg D, Ketzel M, Grandström C, et al. Impact of road traffic pollution on pre-eclampsia and pregnancy-induced hypertensive disorders. Epidemiology. 2017;28(1):99–106 This study found that ambient air pollution and road traffic noise increase risk of preeclampsia and pregnancy-induced hypertensive disorders among 72,745 singleton pregnancies (1997–2002) from the Danish National Birth Cohort.
Wu J, Wilhelm M, Chung J, Ritz B. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res. 2011; http://www.sciencedirect.com/science/article/pii/S0013935111000910.
• Mandakh Y, Rittner R, Flanagan E, Oudin A, Isaxon C, Familari M, et al. Maternal exposure to ambient air pollution and risk of preeclampsia: a population-based cohort study in Scania, Sweden. Int J Environ Res Public Health. 2020;17(5):1–16 Cohort of 43,688 singleton pregnancies that found that maternal exposure to ambient air pollution during gestation was associated with the risk of developing PE. The associations seemed more pronounced in pregnancies with SGA complications.
• Assibey-Mensah V, Glantz JC, Hopke PK, Jusko TA, Thevenet-Morrison K, Chalupa D, et al. Wintertime wood smoke, traffic particle pollution, and preeclampsia. Hypertension. 2020;75:851–8.
Payam D, Francesc F, Xavier B, Rob B, David M, Marta C, et al. Ambient air pollution and preeclampsia: a spatiotemporal analysis. Environ Health Perspect. 2013. https://doi.org/10.1289/ehp.1206430In 16.116 pregnancies, exposure to wintertime particulate pollution seems to have the greatest effect on maternal cardiovascular health during early pregnancy.
Ibrahimou B, Salihu HM, Aliyu MH, Anozie C. Risk of preeclampsia from exposure to particulate matter (PM2.5) speciation chemicals during pregnancy. J Occup Environ Med. 2014;56(12):1228–34.
Wesselink AK, Carwile JL, Fabian MP, Winter MR, Butler LJ, Mahalingaiah S, et al. Residential proximity to roadways and ischemic placental disease in a cape cod family health study. Int J Environ Res Public Health. 2017;14(7):5–7.
Madsen C, Håberg SE, Aamodt G, Stigum H, Magnus P, London SJ, et al. Preeclampsia and hypertension during pregnancy in areas with relatively low levels of traffic air pollution. Matern Child Health J. 2018;22:512–9. https://doi.org/10.1007/s10995-017-2417-6.
Kastury F, Smith E, Juhasz AL. A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Sci Total Environ. 2017; http://www.sciencedirect.com/science/article/pii/S0048969716319763.
Nemmar A, Vanbilloen H, Hoylaerts MF, PHM H, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in Hamster. Am J Respir Crit Care Med. 2001;164(9):1665–8.
Liu N, Miyashita L, Mcphail G, Thangaratinam S, Grigg J. Late breaking abstract - do inhaled carbonaceous particles translocate from the lung to the placenta? Eur Respir J. 2018; http://erj.ersjournals.com/content/52/suppl_62/PA360.abstract; This study provides the first evidence that inhaled PM translocate from the lung to the placenta.
Kaiglová A, Reichrtová E, Adamčáková A, Wsólová L. Lactate dehydrogenase activity in human placenta following exposure to environmental pollutants. Physiol Res. 2001;50(5):525–8.
Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part Fibre Toxicol. 2008;5:3. https://doi.org/10.1186/1743-8977-5-3.
Dutta A, Khramtsova G, Brito K, Alexander D, Mueller A, Chinthala S, et al. Household air pollution and chronic hypoxia in the placenta of pregnant Nigerian women: a randomized controlled ethanol Cookstove intervention. Sci Total Environ. 2018; http://www.sciencedirect.com/science/article/pii/S0048969717331509;619-620:212–20.
Maghbooli Z, Hossein-nezhad A, Adabi E, Asadollah-pour E, Sadeghi M, Mohammad-nabi S, Rad LZ, Hosseini AM, Radmehr M, Faghihi F, Aghaei A, Omidifar A, Aghababei Y, Behzadi H. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. Rosenfeld CS, editor. PLoS One. 2018; https://doi.org/10.1371/journal.pone.0199772
Wylie BJ, Matechi E, Kishashu Y, Fawzi W, Premji Z, Coull BA, et al. Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environ Health Perspect. 2016; https://pubmed.ncbi.nlm.nih.gov/27286442.
Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect. 2012;120(9):1346–52.
Neven KY, Saenen ND, Tarantini L, Janssen BG, Lefebvre W, Vanpoucke C, et al. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: an ENVIRONAGE cohort study. Lancet Planet Health. 2018;2:e174–83. https://doi.org/10.1016/S2542-5196(18)30049-4.
Qin Z, Hou H, Fu F, Wu J, Han B, Yang W, et al. Fine particulate matter exposure induces cell cycle arrest and inhibits migration and invasion of human extravillous trophoblast, as determined by an iTRAQ-based quantitative proteomics strategy. Reprod Toxicol. 2017;74:10–22. http://www.sciencedirect.com/science/article/pii/S0890623817300850
Familari M, Nääv Å, Erlandsson L, de Iongh RU, Isaxon C, Strandberg B, et al. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. Lin H-Y, editor. PLoS One. 2019. https://doi.org/10.1371/journal.pone.0218799
Deyssenroth MA, Peng S, Hao K, Lambertini L, Marsit CJ, Chen J. Whole-transcriptome analysis delineates the human placenta gene network and its associations with fetal growth. BMC Genomics. 2017. https://doi.org/10.1186/s12864-017-3878-0.
Liu Y, Wang L, Wang F, Li C. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Environ Health Perspect. 2016;124(10):746–51.
Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:22. https://doi.org/10.1186/1743-8977-10-22.
Lin S, Huo X, Zhang Q, Fan X, Du L, Xu X, et al. Short placental telomere was associated with cadmium pollution in an electronic waste recycling town in China. Lustig AJ, editor. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0060815.
van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PY, et al. Air pollution exposure and markers of placental growth and function: the generation R study. Environmental health perspectives, 2012;120(12):1753–1759. https://doi.org/10.1289/ehp.1204918.
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22.
Campbell S, Griffin DR, Pearce JM, Diaz-Recasens J, Cohen-Overbeek TE, Willson K, et al. New Doppler technique for assessing uteroplacental blood flow. Lancet [Internet]. 1983; https://linkinghub.elsevier.com/retrieve/pii/S0140673683919700.
Papageorghiou AT, Yu CKH, Bindra R, Pandis G, Nicolaides KH. Screening for pre-eclampsia and fetal growth restriction in twin pregnancies at 23 weeks of gestation by transvaginal uterine artery Doppler. Ultrasound Obstet Gynecol. 2002;20(6):535–40.
García B, Llurba E, Valle L, Gómez-Roig MD, Juan M, Pérez-Matos C, et al. Do knowledge of uterine artery resistance in the second trimester and targeted surveillance improve maternal and perinatal outcome? UTOPIA study: a randomized controlled trial. Ultrasound Obstet Gynecol. 2016;47(6):680–9.
Wang W, Zhong C, Huang L, Zhou X, Chen R, Wu J, et al. Prenatal NO2 exposure and ultrasound measures of foetal growth: a prospective cohort study in Wuhan, China. Occup Environ Med. 2017;74:oemed-2016.
Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, Margall N, et al. Smoking as a major risk factor for cervical cancer and precancer: results from the EPIC cohort. International journal of cancer, 2014;135(2):453–466. https://doi.org/10.1002/ijc.28666.
Contreras ZA, Heck JE, Lee P-C, Cui X, Hobel CJ, Janzen C, et al. Prenatal air pollution exposure, smoking, and uterine vascular resistance. Environ Epidemiol (Philadelphia, Pa). 2018; https://pubmed.ncbi.nlm.nih.gov/30627692.
Kempen, E. V., Casas, M., Pershagen, G., & Foraster, M. WHO Environmental Noise Guidelines for the European Region: A Systematic Review on Environmental Noise and Cardiovascular and Metabolic Effects: A Summary. International journal of environmental research and public health, 2018;15(2):379. https://doi.org/10.3390/ijerph15020379.
Vivanco-Hidalgo RM, Avellaneda-Gómez C, Dadvand P, Cirach M, Ois Á, Gómez González A, et al. Association of residential air pollution, noise, and greenspace with initial ischemic stroke severity. Environ Res. 2019. http://www.sciencedirect.com/science/article/pii/S0013935119305225;179:108725.
Pyko A, Andersson N, Eriksson C, de Faire U, Lind T, Mitkovskaya N, et al. Long-term transportation noise exposure and incidence of ischaemic heart disease and stroke: a cohort study. Occup Environ Med [Internet]. 2019;76(4):201 LP–7 LP Available from: http://oem.bmj.com/content/76/4/201.abstract.
Foraster M, Eze IC, Schaffner E, Vienneau D, Héritier H, Endes S, et al. Exposure to road, railway, and aircraft noise and arterial stiffness in the SAPALDIA study: annual average noise levels and temporal noise characteristics. Environ Health Perspect. 2020. https://doi.org/10.1289/EHP1136.
Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook FR, et al. Environmental stressors and cardio-metabolic disease: part II–mechanistic insights. Eur Heart J. 2016:ehw294. https://doi.org/10.1093/eurheartj/ehw294.
Steven S, Frenis K, Kalinovic S, Kvandova M, Oelze M, et al. Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension. Redox Biol. 2020; http://www.sciencedirect.com/science/article/pii/S2213231720302809.
Basner M, Müller U, Elmenhorst E-M. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34:11–23. https://doi.org/10.1093/sleep/34.1.11.
Schmidt FP, Basner M, Kröger G, Weck S, Schnorbus B, Muttray A, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J. 2013;34:3508–14. https://doi.org/10.1093/eurheartj/eht269.
Dzhambov AM, Dimitrova DD, Dimitrakova ED. Noise exposure during pregnancy, Birth Outcomes And Fetal Development: Meta-Analyses Using Quality Effects Model. Folia Med. 2014; https://content.sciendo.com/view/journals/folmed/56/3/article-p204.xml.
Ristovska G, Laszlo HE, Hansell AL. Reproductive outcomes associated with noise exposure - a systematic review of the literature. Int J Environ Res Public Health. 2014; https://pubmed.ncbi.nlm.nih.gov/25101773.
Nieuwenhuijsen MJ, Ristovska G, Dadvand P. WHO environmental noise guidelines for the European Region: a systematic review on environmental noise and adverse birth outcomes. Int J Environ Res Public Health. 2017; https://pubmed.ncbi.nlm.nih.gov/29048350This systematic review of previous systematic reviews and meta-analyses that suggested that there may be some suggestive evidence for an association between environmental noise exposure and birth outcomes.
Auger N, Duplaix M, Bilodeau-Bertrand M, Lo E, Smargiassi A. Environmental noise pollution and risk of preeclampsia. Environ Pollut. 2018; http://www.sciencedirect.com/science/article/pii/S0269749118300988. Study involving a population-based cohort comprising 269,263 deliveries on the island of Montreal, Canada between 2000 and 2013 that showed thatenvironmental noise pollution could be a novel risk factor for pregnancy-related hypertension, particularly more severe variants of preeclampsia.
Solano ME, Arck PC. Steroids, pregnancy and fetal development. Front Immunol. 2020; https://pubmed.ncbi.nlm.nih.gov/32038609.
Nakamura K, Sheps S, Clara AP. Stress and reproductive failure: past notions, present insights and future directions. J Assist Reprod Genet. 2008;25(2):47–62.
Yeager RA, Smith TR, Bhatnagar A. Green environments and cardiovascular health. Trends Cardiovasc Med. 2020. http://www.sciencedirect.com/science/article/pii/S1050173819300908;30:241–6.
Jendrossek M, Standl M, Koletzko S, Lehmann I, Bauer CP, Schikowski T, et al. Residential air pollution, road traffic, greenness and maternal hypertension: results from GINIplus and LISAplus. Int J Occup Environ Med. 2017;8(3):131–42.
Akaraci S, Feng X, Suesse T, Jalaludin B, Astell-Burt T. A Systematic review and meta-analysis of associations between green and blue spaces and birth outcomes. Int J Environ Res Public Health. 2020;17(8):2949 A systematic review and meta-analyses were conducted to synthesize thirty-seven studies on the association between residential green and blue spaces and pregnancy outcomes. Associations between green space and LBW and PTB were as hypothesized but not statistically significant.
Zhan Y, Liu J, Lu Z, Yue H, Zhang J, Jiang Y. Influence of residential greenness on adverse pregnancy outcomes: a systematic review and dose-response meta-analysis. Sci Total Environ. 2020; http://www.sciencedirect.com/science/article/pii/S004896972030930X. Meta-analysis of 36 studies with a total of 11,983,089 participants that showed that birth weight was significantly higher in highest level of greenness exposure group compared to lowest level group.
Choe S-A, Kauderer S, Eliot MN, Glazer KB, Kingsley SL, Carlson L, et al. Air pollution, land use, and complications of pregnancy. Science of the Total Environment. 645:1057-1064. https://doi.org/10.1016/j.scitotenv.2018.07.237.
Laurent O, Wu J, Li L, Milesi C. Green spaces and pregnancy outcomes in Southern California. Health Place. 2013; http://www.sciencedirect.com/science/article/pii/S1353829213001329.
Liao J, Chen X, Xu S, Li Y, Zhang B, Cao Z, et al. Effect of residential exposure to green space on maternal blood glucose levels, impaired glucose tolerance, and gestational diabetes mellitus. Environ Res. 2019.http://www.sciencedirect.com/science/article/pii/S0013935119303214;176:108526.
Young C, Laurent O, Chung JH, Wu J. Geographic distribution of healthy resources and adverse pregnancy outcomes. Maternal Child Health J [Internet]. 2016;20(8):1673–9.
Payam D, de Audrey N, Margarita T-M, Anna S, Marta C, Elmira A, et al. Surrounding greenness and exposure to air pollution during pregnancy: an analysis of personal monitoring data. Environ Health Perspect. 2012;120:1286–90. https://doi.org/10.1289/ehp.1104609.
Nieuwenhuijsen MJ, Khreis H, Triguero-Mas M, Gascon M, Dadvand P. Fifty shades of green: pathway to healthy urban living. Epidemiology. 2016;28(1):1.
Huang L, Fan L, Ding P, He Y-H, Xie C, Niu Z, et al. Maternal exercise during pregnancy reduces the risk of preterm birth through the mediating role of placenta. J Maternal-Fetal Neonatal Med [Internet]. 2019;32(1):109–16.
Dahlerup BR, Egsmose EL, Siersma V, Mortensen EL, Hedegaard M, Knudsen LE, et al. Maternal stress and placental function, a study using questionnaires and biomarkers at birth. PLoS One. 2018; https://pubmed.ncbi.nlm.nih.gov/30439989. This study showed associations between Pregnancy Related Anxiety and the adjusted fetal cortisol exposure.
Bijnens E, Zeegers MP, Gielen M, Kicinski M, Hageman GJ, Pachen D, et al. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environment international, 2015;79:1–7. https://doi.org/10.1016/j.envint.2015.02.008.
Funding
Payam Dadvand is funded by Ramón y Cajal fellowship (RYC-2012-10995) awarded by the Spanish Ministry of Economy and Competitiveness. Maria Foraster is supported by a Beatriu de Pinós fellowship [2018/8374/I] awarded by the Universities and Research Secretariat of the Catalan Ministry of Business and Knowledge. Maria Torres Toda is funded by a PFIS (Contrato Predoctoral de Formación en Investigación en Salud) fellowship (FI17/00128) awarded by Instituto de Salud Carlos III. We acknowledge support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preeclampsia
Rights and permissions
About this article
Cite this article
Zanini, M.J., Domínguez, C., Fernández-Oliva, T. et al. Urban-Related Environmental Exposures during Pregnancy and Placental Development and Preeclampsia: a Review. Curr Hypertens Rep 22, 81 (2020). https://doi.org/10.1007/s11906-020-01088-4
Published:
DOI: https://doi.org/10.1007/s11906-020-01088-4