Abstract
Purpose of the Review
This review aims to introduce animal models of portal hypertension in which targets and drugs can be tested and presents current advances in the field of preclinical and early clinical settings.
Recent Findings
The interest in this field has risen in recent years and many promising targets and potential drugs have been tested in preclinical and early clinical studies. Most of these targets are intrahepatic and aim to decrease hepatic stellate cell activity, as this cell type mediates both fibrosis and portal hypertension.
Summary
Liver cirrhosis with portal hypertension is a global health burden due to their complications. Besides that, there are only a few therapies available, those are ineffective in a large part of the patients. Therefore, novel targets and treatment options are vastly needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
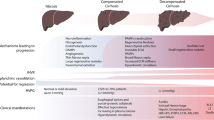
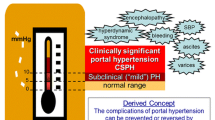
Liver cirrhosis is a global health care burden with more than 1.2 million death per year [1]. The main etiologies of cirrhosis are viral, alcoholic and non-alcoholic hepatitis. Even though the etiologies are diverse, the resulting hepatic pathophysiology is the same. Due to liver injury, damaged hepatocytes induce inflammation leading to activation of hepatic stellate cells (HSC). As a repair mechanism, activated HSC produce collagen to replace the damaged cells. In chronic liver injury, processes are uncontrolled and excessive amount of extracellular matrix is deposited in the liver. Activated HSCs proliferate and increase contractility [2, 3•]. Together with dysfunction of the liver sinusoidal endothelial cells (LSEC), pronounced HSC activity is responsible for increased liver stiffness resulting in augmentation of intrahepatic resistance. A hallmark in cirrhosis progression is then the onset of portal hypertension. The hepatic blood flow due to increased resistance leading to portal venous congestion and consequent increased portal pressure. Portal hypertension induces splanchnic angiogenesis impairing microcirculation and intestinal barrier permeability further aggravating the syndrome [4•]. This is associated with severe complications of cirrhosis, namely variceal bleeding, ascites, and infections [5], which increases the risk for decompensation of cirrhosis and progression towards acute-on-chronic liver failure (ACLF) [6•]. Especially ACLF shows high short-term mortality [7]. Therefore, not only understanding the mechanisms of portal hypertension but discovering targets and development and testing of drugs is important in this field [8].
Currently, mainly nonselective beta-blockers (NSBB) are used to reduce portal pressure. However, many patients do not respond to NSBBs. Therefore, novel targets and drugs have been investigated and some show promising results preclinical and early clinical studies. To guide this process, the portal hypertension special interest group of the AASLD recently proposed a framework to design preclinical studies and clinical trials to prioritize novel targets and pharmacological therapies [9•].
This review focuses on a few selected current concepts and novel targets or potential drugs to treat portal hypertension. Since the approaches are too diverse to include them all in one review, we have chosen to present the most promising approaches according to our experience. Additionally, this review introduces the key models for preclinical studies on portal hypertension.
Animal Models to Test Targets and Potential Drugs in Portal Hypertension
Most potential targets and drugs have not yet made their way into the clinic and others could not be translated into the human situation. Therefore, reliable models to reflect either cirrhotic or non-cirrhotic portal hypertension are of importance to discover and test targets and drugs. Even though targets and potential drugs can be tested in cell culture in initial experiments, animal models are relatively fast to test the complex interactions between different cells, organs, and side effects in the system and a large enough sample size can be easily generated to deliver reliable results.
Bile duct ligation is a well-established fast obstructive jaundice model of cholestatic cirrhosis and can be performed in mice and rats. Following the invasive ligation of the bile duct, mice and rats develop advanced fibrosis and portal hypertension after 14 days or 4 to 6 weeks, respectively [10•]. The model can be used to investigate advanced stages of chronic liver disease with hepatic inflammation, fibrosis, and portal hypertension. Thereby, the model is fast and highly reproducible.
Carbon tetrachloride (CCl4) intoxication mimics the toxic genesis of chronic liver disease and is especially used as a model for alcoholic liver disease. CCl4 is metabolized by hepatocytes deploy its hepatotoxic properties by the resulting toxic radicals, which initiate membrane degradation and induce inflammation leading to fibrogenesis in chronic experiments [11]. Effects of CCl4 intoxication can be potentiated by additional oral administration of barbiturates. Intoxication can be performed either by injection in a solution with oil or by inhalation and duration until advanced fibrosis and portal hypertension develops average 10 to 16 weeks depending on the method of administration [10•, 12]. Due to frequent subcutaneous or intraperitoneal injections during the initialization of liver fibrosis and portal hypertension, intoxication by injections entails higher risks of tissue necrosis and mortality than intoxication by inhalation, which is therefore preferred with respect to animal welfare. Intoxication by inhalation, however, needs to be performed in a protected setting due to the risks for the operator (effects on the central nervous system and carcinogenic properties) and nature (ozone-depleting).
Intoxication with thioacetamide (TAA) is another model of toxic liver disease. Hepatotoxic effects of TAA are caused by production of reactive oxygen species [13]. Similar to CCl4, TAA can be administered using different techniques, either by intraperitoneal injections or orally in the drinking water with weight adopted doses. Advanced fibrosis and portal hypertension develop in a comparable timeframe to CCl4 after 6 to 18 weeks. In later stages, however, the risk to develop cholangiocarcinoma increases. The main advantage of this model is the restriction of toxicity to the liver [12]. Similar to CCl4, it has to be handled carefully due to its carcinogenic properties.
Idiopathic portal hypertension is a non-cirrhotic vascular cause of portal hypertension affecting mainly young adults and histologically characterized by micro-thrombotic lesions in small portal venules [12, 14]. This syndrome can be imitated in rats by repetitive injection of microspheres in the ileocecal vein. The procedure needs to be performed weekly for 3 weeks and requires a laparotomy each time, which represents also one of its biggest disadvantages since only very skilled persons can perform this procedure. The microspheres cause micro-thrombotic insults in the liver, which lead to portal hypertension with hyperdynamic circulation [15].
The partial portal vein ligation (PPVL) is a model for non-cirrhotic portal hypertension and is usually performed in rats. A ligature is placed around the portal vein and a blunt needle. Thereby, the degree of portal hypertension can be adjusted by the used diameter of the needle. Removal of the needle leads to a calibrated stenosis of the portal vein. Distinct portal hypertension develops almost immediately after the procedure. In the following days, portal pressure slightly decreases due to the formation of portosystemic collaterals. Experiments using this model are usually performed 2 weeks after PPVL. This model can be used to study angiogenesis and hemodynamics independent of cirrhosis. Detailed step-by-step instruction has been published by us and others [10•, 12].
The gradual occlusion of the inferior vena cava (IVC) is a model for post-hepatic portal hypertension and leads to features that are comparable with Budd-Chiari syndrome. The occlusion is achieved by a hygroscopic ring that is placed around the IVC. The ring successively constricts by body fluid uptake leading to almost full constriction of the IVC after 4 weeks. The blockade of hepatic outflow leads to ascites formation and hepatomegaly [12, 16].
In all of these animals, hemodynamics can be assessed to identify the degree of portal hypertension and test the effects of treatment. While portal pressure can be measured directly and serves as the primary output, additional hemodynamic parameters can be assessed using the microsphere technique. Thereby, colored microspheres should be favored over radioactive ones, since they are less harmful. Using this technique, the blood flow in and the resistance of different organs can be calculated [10•].
Current Concepts in the Clinics
Inflammation not only contributes to the development of liver fibrosis and cirrhosis but also manipulates intra- and extrahepatic vascular function and thereby aggravates portal hypertension [17]. Strong correlations of inflammatory markers with hepatic venous pressure gradient (HVPG) have been demonstrated [18] besides an association of systemic inflammation with increased portal hypertension in cirrhosis [19]. Inhibition of caspase-mediated inflammation by emricasan improved MELD score by more than 2 points in half of the patients of a total of 74 patients as demonstrated recently in an open-label placebo-controlled trial [20•]. Furthermore, in 23 patients in an open-label uncontrolled trial, it was also well tolerated and decreased portal pressure in 12 of the patients with severe portal hypertension. The decrease was rather moderate (4 of 8 patients with more than 20%) [21•]. Overall, the effect of emricasan is modest and more data are necessary.
In cirrhosis, portal hypertension is linked to systemic inflammation caused by bacterial translocation facilitated from the impaired gut barrier [22]. Therefore, the microbiome is one potential target to reduce portal pressure and manipulation of the microbiome by antibiotic, probiotics, and prebiotics is investigated by several studies. However, so far, results are contradictory with regard to portal hypertension. Cirrhotic patients systemically receive antibiotics to improve overall survival [23, 24] and often NSBBs. NSSBs, besides their primary function to prevent rebleeding, have been shown to improve intestinal permeability in cirrhosis and consequently decreased bacterial translocation [25]. In several unblinded and non-randomized control trials treatment with Rifaximin, a semisynthetic broad-spectrum antibiotic approved for the treatment of hepatic encephalopathy improved HVPG and systemic hemodynamics and lowered the risk of decompensation. These studies included 30, 13, and 23 patients, respectively, and only the last study with 23 patients included non-randomized historical controls [26,27,28]. Of note, a double-blinded randomized control trial with 54 patients could not confirm these beneficial effects [29••]. Furthermore, Rifaximin seems to have only minor effects on bacterial composition, inflammation, and bacterial translocation [30]. Nevertheless, a combination of Rifaximin and Propanolol seem to be promising, probably due to additive effects of Rifaximin compared with NSBB monotherapy with decreased incidence of side effects due to lower NSBB dose than used normally as shown by an open-label randomized (2:1) trial in 73 patients [31]. Treatment with Norfloxacin, a synthetic broad-spectrum antibiotic active against Gram-positive and Gram-negative bacteria, reversed the hyperdynamic state, but effects on portal pressure were neglectable [32, 33]. However, Norfloxacin seems to improve survival in some cirrhotic patients, especially those with low ascitic fluid protein concentrations, probably due to decreased incidence of bacterial infection [34••].
Another approach could be to support the restoration of the bacterial composition in the intestine by probiotics. Supplementation with VSL#3, a live formulation of lyophilized of eight bacterial species, reduced the severity of liver disease by lowering the rate of hospitalization for hepatic encephalopathy or other complications of cirrhosis [35]. Additionally, one study could demonstrate the improvement of HVPG in 17 patients [36], while another study, again including 17 patients, found no effect [37]. Thereafter, the combination therapy seems more promising, adjunctive VSL#3 improved the response rate of propranolol with respect to HVPG and was safe and well tolerated in cirrhotic patients in a large randomized controlled trial with 94 patients [38].
Fecal transplantation could be a further approach. In a rodent model of non-alcoholic steatohepatitis with portal hypertension, transplantation of stool from healthy animals significantly decreased the portal pressure [39]. Furthermore, this concept has proven successful in a randomized controlled trial with 20 patients [40••].
Cirrhotic patients often develop coagulatory disorders and thrombocytopenia in advanced stages of cirrhosis [41, 42]. Anticoagulation therapy could be beneficial in cirrhosis with portal hypertension and indeed there are some recent hints showing improvement in portal hypertension. A few years ago, a non-blinded randomized controlled trial was performed in 70 outpatients with cirrhosis to investigate the effects of enoxaparin, a low–molecular weight heparin. Treatment for 12 months was safe and was efficient to prevent decompensation and improve survival [43]. Direct oral coagulants (DOAC) require less monitoring with similar bleeding risk as conventional coagulants [44]. A retrospective analysis demonstrated that anticoagulant therapy with DOACs in cirrhosis is safe and effective [45•]. Mechanistically, enoxaparin has been shown to decrease hepatic vascular resistance and portal pressure in experimental cirrhosis, mainly by decreasing HSC activity [46]. Similar results have been achieved using rivaroxaban in two experimental models of cirrhosis with portal hypertension [47]. Furthermore, vasodilatory effects may support these effects by increased eNOS activity [48, 49]. However, a recent study could not support the previous findings and could not show beneficial effects of enoxaparin on liver function, hepatic fibrosis, endothelial dysfunction, and portal hypertension [50]. Therefore, more data and future studies are needed to evaluate the potential of anticoagulant therapy in cirrhosis with portal hypertension.
Another approach targeting portal hypertension are phosphodiesterase type 5 (PDE5) inhibitors, which are in clinical use for erectile dysfunction, and clinical testing in portal and pulmonary hypertension [51]. In cirrhosis, PDE5 is upregulated in hepatic tissue, especially in perisinusoidal cells and the fibrotic septae [52]. Initial studies on PDE5 inhibition in experimental fibrosis showed beneficial effects on fibrosis and portal hypertension via decreased HSC activity [53,54,55]. Therefore, PDE5 inhibitors have been tested in the clinical setting in a phase-II study in compensated cirrhotic patients. A dose of 75-100 mg administered for 1 week decreased portal pressure without systemic side effects in an open-label trial with 30 patients [56].
β3-Adrenoceptor Agonists
β3-adrenoceptors participate in the regulation of vascular tone and are upregulated in hepatic and splanchnic tissue in cirrhotic patients with portal hypertension, as well as in animal models and therefore represent a potential therapeutic target. Stimulation of the β3-adrenoceptors by selective agonists decreased intrahepatic resistance and portal pressure in cirrhotic animals with only minor systemic effects [57, 58].
Statins
Statins are used for prevention and treatment of cardiovascular diseases in patients with high blood lipid levels. The primary mechanism of action is the interruption of cholesterol synthesis by inhibition of HMG-CoA reductase. They feature also additional effects, which make them potential drugs for the treatment of portal hypertension [59].
One of the so-called pleiotropic effects is the inhibition of small GTPase activity. Impaired HMG-CoA activity results in lower isoprenoid levels, pivotal components of cell membrane lipid anchors for small GTPases, such as RhoA and Ras, and thus decreased the activity of small GTPases due to hampered membrane binding [60,61,62]. Statins inhibit RhoA-dependent HSC activation and activity via Rho-kinase. Furthermore, statins improve hepatic endothelial dysfunction and thereby the communication between HSC and LSEC via Krüppel-like factor 2 (KLF2) and endothelial nitric oxide synthase (eNOS). Taken together, this leads to decreased fibrosis accumulation and intrahepatic resistance resulting in attenuation of portal pressure [63,64,65,66,67,68]. In cirrhotic rats with a single LPS injection, a vague approximate of ACLF, statins increased survival and prevented complications. Statin treatment reduced hepatic inflammation, improved liver function, and decreased portal pressure in these rats [69].
Recently, NCX 6560, a nitric oxide-releasing atorvastatin, was evaluated and compared with conventional atorvastatin. NCX 6550 decreased the incidence of hepatic and muscular toxicity, while the antifibrotic profile and improvement of portal hypertension were similar to conventional statins [70]. Approaches like this one may be promising to finally establish statins in the clinic for the treatment of portal hypertension in cirrhotic patients.
Additionally, statins improve portal hypertension by antiangiogenic extrahepatic effects. These effects are associated with RhoA and are mediated by the non-canonical hedgehog, which in turn leads to reduced vessel formation and accordingly decreases portal venous inflow [71]. This, however, applies only in the context of cirrhosis, while in non-cirrhotic portal hypertension, statin treatment aggravates the syndrome [71,72,73].
Due to potential hepatotoxic side effects of statins, evidence of their benefits in portal hypertension was primarily generated in experimental settings. In recent years, however, these concerns fade away since more and more studies prove their safety and overall tolerance in cirrhotic patients with portal hypertension, especially in those with concomitant cardiovascular diseases [74•, 75]. The first prospective study elucidating acute effects of statins in two small cohorts of cirrhotic patients (30 patients in total) was performed more than a decade ago. Although HVPG was not modified in these acute experiments, the authors could describe a decrease in hepatic resistance (around 14%) accompanied by the increased availability of hepatic nitric oxide (NO) products consequently increasing hepatic blood flow. Furthermore, no systemic side effects were identified in these patients [76]. More recently, in a triple-blinded randomized trial with 24 patients, simvastatin decreased portal pressure in patients receiving the drug for 3 months, while placebo did not. Interestingly, the response rate to simvastatin (55% of patients responded) was higher in patients with oesophageal varices and history of variceal bleeding. Again, no adverse events after statin treatment were recorded [77]. A larger double-blinded randomized control trial with patients receiving the standard prophylaxis to prevent variceal bleeding investigated the effects of additional statin administration for 24 months. Here, statins could not be related to decreased risk of rebleeding, but improved survival by decreasing the relative risk by 61%. In this trial, adverse events were reported in some patients [78••]. Two other independent trials investigating the effects of statins additionally administered to beta-blockers could confirm beneficial effects. In both trials combined, the therapy with statins and beta-blockers showed higher response and a stronger decrease in HVPG than single treatment with beta-blockers, especially in patients who did not respond to beta-blockers statins could improve HVPG. Therefore, the authors concluded that a combined therapy of beta-blockers and statins is promising in patients with portal hypertension [79, 80•].
Until now, however, most published studies are either retrospective or trials with small patient cohorts and need to be substantiated by larger randomized controlled studies, with some of those studies already started recruiting patients [81, 82]. For more detailed review of the literature, we recommend to read the publications by Abraldes et al. and Pose et al. [9•, 74].
Renin-Angiotensin System
The renin-angiotensin system (RAS) is a systemic regulatory circuit that regulates blood pressure, splanchnic vasodilation, and sodium retention. However, local RAS systems can be found in several tissues with functions that are independent of the systemic RAS.
In general, angiotensinogen, mainly produced by the liver, is converted to angiotensin I by renin and further cleaved by the angiotensin-converting enzyme I (ACE I) into angiotensin II. Angiotensin II is the regulatory peptide of the RAS and agonist of the angiotensin II receptor type 1 (AT1R). In chronic liver disease, angiotensin II and the AT1R are highly upregulated and contribute to liver fibrosis and portal hypertension [83, 84]. Therefore, those two components are historically the main targets for anti-hypertensive treatment and are already in clinical use for non-liver related diseases. Inhibitors of angiotensin II formation (ACE inhibitors) and AT1R antagonists (“sartans”) are in use for the treatment of arterial hypertension and chronic heart failure and were extensively investigated for their potential in chronic liver diseases. However, the effects on fibrosis are not more than modest with several systemic side effects [85, 86], especially shown in a placebo-controlled randomized double-blinded trial including 36 patients [87]. Even in combination with beta-blockers, the use of RAS inhibitors may still be not safe enough due to the high risk of side effects. This was confirmed in meta-analyses including three studies with 90 patients [88]. Genetic predisposition also seems to play a role in RAS-mediated portal hypertension. Patients with ACE I allele were found to have a higher HVPG and higher risk for variceal bleeding than patients with ACE D allele [89]. Additionally, the role of the angiotensin II receptor type II, another agonist of angiotensin II, to whom opposing effects to the AT1R are attributed has not been investigated so far in liver cirrhosis with portal hypertension. Therefore, manipulation of this classic RAS components has not yet found its way into the clinic.
Nevertheless, there is also an alternative RAS in which Ang1–7 is the key regulatory peptide. Substrates for Ang1–7 can be angiotensin I cleaved by NEP or angiotensin II cleaved by ACE II. Ang1–7 is the agonist of the mas proto-oncogene receptor (MasR) and features vasodilatory properties. In cirrhotic patients, Ang1–7 and the MasR are upregulated in the liver, as well as in splanchnic vessels. In extrahepatic vessels, Ang1–7 antagonizes AT1R signaling potently [90, 91]. Experimental manipulation of the system by MasR inhibition increased the portal pressure. On the other side, MasR stimulation induces eNOS release and thereby counteracts the pro-contractile AT1R [92]. Stimulation of the Ang1–7 agonist by the non-peptidic mimic AVE0991 decreased portal pressure without systemic side effects. Of note, AVE0991 had no effect on hepatic fibrosis [93].
Kinase Inhibitors
Since ACE inhibitors and AT1R blockers seem to be ineffective or cause severe side effects, downstream targets of the RAS may be potential targets to treat portal hypertension. Janus-kinase 2 (JAK2) links the RAS via the AT1R to the pro-fibrotic and pro-contractile RhoA/Rho-kinase pathway [94, 95]. JAK2 is highly upregulated in human cirrhosis, especially in HSC, and correlates with the severity of liver disease [95, 96]. Inhibition of JAK2 by the chemical compound AG490 successfully decreased hepatic vascular resistance and portal pressure in cirrhotic animals [96, 97]. Additionally, JAK2 inhibition decreases hepatic inflammation, angiogenesis, fibrosis, and activation of HSC, mainly via the RhoA/Rho-kinase pathway [95, 97]. This data was independently confirmed [97]. Due to its important role in fibrosis and portal hypertension, JAK2 inhibitors are in development for clinical use, especially since existing inhibitors like AG490 also have a high affinity to block other tyrosine kinases. The multikinase inhibitor ruxolitinib is approved in the USA and Europe for treatment of myelofibrosis and first studies in two experimental fibrosis models showed beneficial effects especially on inflammation and oxidative stress, both drivers of fibrogenesis and development of portal hypertension [98,99,100]. Due to the poor availability of data, so far, no clinical trials have been published. However, JAK2 inhibition seems promising and more studies need to be performed.
Multikinase Inhibitors
Since most of the JAK2 effects are mediated via the RhoA/Rho-kinase pathway, targeting this pathway directly is another approach to treat portal hypertension. The multikinase inhibitor sorafenib has been investigated in this regard and is approved for the treatment of advanced HCC, which shares several mechanisms with cirrhosis. In experimental models of portal hypertension, sorafenib decreased angiogenesis and vasoconstriction of splanchnic vessels [101,102,103]. However, in a small cohort of 13 cirrhotic patients, sorafenib (400 mg b.d.) showed only limited effects; only in 4 patients, HVPG decreased [104]. Regorafenib is a more potent multikinase inhibitor than sorafenib and may improve portal hypertension. In experimental models of cirrhotic and non-cirrhotic portal hypertension, acute and long-term treatment with regorafenib was able to blunt angiogenesis and improve portal hypertension. However, in long term–treated fibrotic animals, hepatotoxic side effects were observed [105].
FXR
The farnesoid X receptor (FXR) is also a promising target in chronic liver disease with portal hypertension. It is a transcriptional regulator of bile acid homeostasis and highly expressed in the liver and small intestine [106]. Previous studies have demonstrated its role in inflammation, liver fibrosis, and vascular homeostasis [107, 108]. Treatment with the selective semisynthetic FXR agonist obeticholic acid (OCA) reduced the hepatic resistance and portal pressure without systemic effects. This was related to increased intrahepatic eNOS activity [109] and mediated by dimethylaminohydrolase-1 which metabolizes the eNOS inhibitor asymmetric-dimethylarginine [110]. Furthermore, OCA has also anti-inflammatory properties as shown by in vitro experiments in Kupffer cells and LSEC, where it inhibits pro-inflammatory pathways via NF-κB. These anti-inflammatory properties downregulated HSC activity in experimental toxic fibrosis [111]. Several agonists and regulators of FXR have been tested in experimental models to demonstrate the impact of this pathway on portal hypertension, either by direct action on HSC or using the communication between HSC and LSEC. Dihydroartemisinin, a regulator of FXR expression, decreased HSC contraction in vivo and improved portal pressure in vitro [112, 113]. In another study, the non-steroidal FXR agonist PX20606 improved portal pressure, by reducing vascular remodeling and sinusoidal dysfunction, while hepatic fibrosis was also decreased [114]. Further, FXR and the bile acid receptor G protein bile acid receptor 1 were targeted in LSEC to improve endothelial dysfunction and as a consequence portal hypertension [115, 116].
So far, the only multicenter double-blinded placebo-controlled randomized clinical trial assessing obeticholic acid was performed in a cohort of 283 non-cirrhotic, non-alcoholic steatohepatitis patients, where it improved liver function and slightly decreased fibrosis after 72 weeks of treatment. Hemodynamics, however, were not assessed in this trial [117].
Targeted Approach
Since the mechanisms of contractility are contrary regulated in the liver and the splanchnic vascular region, more specific effects are desirable. Side effects may lay in the nature of kinase inhibitors since they have several targets and are often expressed in various tissues, where inhibition could cause unwanted effects. Cell-specific kinase inhibition might avoid those side effects. The Rho-kinase inhibitor Y-27632 decreased hepatic resistance in experimental cirrhosis, but showed also massive systemic side effects, since Rho-kinases are widely expressed in other organs too [67, 118]. Bound to a cell-specific carrier targeting activated HSC, Y-27632 was able to have the same beneficial effects, but without systemic side effects. Cell-targeted administration decreased hepatic fibrosis and portal pressure while it increased renal perfusion. These effects were associated with decreased contractility and collagen production by HSC [68, 119, 120].
Conclusions
Promising targets and potential drugs have been discovered in recent years. However, many drugs have failed after encouraging preclinical results due to extrahepatic side effects. Therefore, cell-targeted therapy could avoid unwanted side effects in the treatment of portal hypertension. These promising approaches have to demonstrate their beneficial potential in large randomized controlled clinical trials to find their way into the clinical routine treatment and to improve survival and quality of life of cirrhotic patients with portal hypertension.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165–72.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69.
• Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411 This review reflects and summarises the current knowledge about molecular insights in hepatic stellate cell biology.
• Sauerbruch T, Schierwagen R, Trebicka J. Managing portal hypertension in patients with liver cirrhosis. F1000Research. 2018;7:533. This review addresses clinical and practical issues in the treatment of portal hypertension.
Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141–56.
• Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primer. 2016;2:16041 This review provides a comprehensive overview of acute-on-chronic liver failure.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37, 1437.e1–9.
Zutshi Y. Liver disease treatments: the global market: PHM057C | BCC Research. 2015. Chapter 10.
• Abraldes JG, Trebicka J, Chalasani N, D’Amico G, Rockey DC, Shah VH, et al. Prioritization of therapeutic targets and trial design in cirrhotic portal hypertension. Hepatology. 2019;69:1287–99 This review summarises important considerations on potential targets and trial design in cirrhotic portal hypertension.
• Klein S, Schierwagen R, Uschner FE, Trebicka J. Mouse and rat models of induction of hepatic fibrosis and assessment of portal hypertension. Methods Mol Biol. 2017;1627:91–116 This overview provides a practical guide for preclinical testing in portal hypertension.
Slater TF, Cheeseman KH, Ingold KU. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos Trans R Soc Lond Ser B Biol Sci. 1985;311:633–45.
Königshofer P, Brusilovskaya K, Schwabl P, Reiberger T. Animal models of portal hypertension. Biochim Biophys Acta Mol Basis Dis. 2019 May 1;1865(5):1019–1030.
Chilakapati J, Korrapati MC, Hill RA, Warbritton A, Latendresse JR, Mehendale HM. Toxicokinetics and toxicity of thioacetamide sulfoxide: a metabolite of thioacetamide. Toxicology. 2007;230:105–16.
Khanna R, Sarin SK. Idiopathic portal hypertension and extrahepatic portal venous obstruction. Hepatol Int. 2018;12:148–67.
Klein S, Hinüber C, Hittatiya K, Schierwagen R, Uschner FE, Strassburg CP, et al. Novel rat model of repetitive portal venous embolization mimicking human non-cirrhotic idiopathic portal hypertension. PLoS One. 2016;11:e0162144.
Orloff MJ, Daily PO, Girard B. Treatment of Budd-Chiari syndrome due to inferior vena cava occlusion by combined portal and vena caval decompression. Am J Surg. 1992;163:137–42 discussion 142–143.
Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, et al. Inflammation and portal hypertension – the undiscovered country. J Hepatol. 2014;61:155–63.
Schwabl P, Mandorfer M, Steiner S, Scheiner B, Chromy D, Herac M, et al. Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment Pharmacol Ther. 2017;45:139–49.
Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724–34.
• Frenette CT, Morelli G, Shiffman ML, Frederick RT, Rubin RA, Fallon MB, et al. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clin Gastroenterol Hepatol. 2019;17:774–783.e4 This publication provides first clinical data on efficiency of pan-caspase inhibitors in advanced cirrhosis.
• Garcia-Tsao G, Fuchs M, Shiffman M, Borg BB, Pyrsopoulos N, Shetty K, et al. Emricasan (IDN-6556) lowers portal pressure in patients with compensated cirrhosis and severe portal hypertension. Hepatology. 2019;69:717–28 This publication provides first clinical data on effects of pan-caspase inhibitors in portal hypertension.
Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209.
Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–24.
Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–61.
Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–21.
Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992–9.
Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–8.
Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–5.
•• Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: a randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65:592–603 This randomized controlled trial on rifaximin is so far the only one publicated with portal hypertension as an endpoint.
Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: a randomized trial. J Gastroenterol Hepatol. 2018;33:307–14.
Lim YL, Kim MY, Jang YO, Baik SK, Kwon SO. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy for the reduction of portal pressure: an open randomized controlled pilot study. Gut Liver. 2017;11:702–10.
Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. a randomized trial. Ann Intern Med. 2003;139:186–93.
Hennenberg M, Trebicka J, Buecher D, Heller J, Sauerbruch T. Lack of effect of norfloxacin on hyperdynamic circulation in bile duct-ligated rats despite reduction of endothelial nitric oxide synthase function: result of unchanged vascular Rho-kinase? Liver Int. 2009;29:933–41.
•• Moreau R, Elkrief L, Bureau C, Perarnau J-M, Thévenot T, Saliba F, et al. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. gastroenterology. 2018;155:1816–1827.e9 This double-blind trial provides data on long-term effects of norfloxacin in patients with Child-Pugh class C cirrhosis.
Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37 e3.
Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504–12.
Jayakumar S, Carbonneau M, Hotte N, Befus AD, St Laurent C, Owen R, et al. VSL#3 ® probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. 2013;33:1470–7.
Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013;33:1148–57.
García-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A, et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology. 2018;67:1485–98.
•• Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017;66:1727–38 This randomized trial provides the proof of concept for fecal microbiota transplantation in cirrhosis.
Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46.
Pradella P, Bonetto S, Turchetto S, Uxa L, Comar C, Zorat F, et al. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894–900.
Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253–1260.e1–4.
Intagliata NM, Henry ZH, Maitland H, Shah NL, Argo CK, Northup PG, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci. 2016;61:1721–7.
• De Gottardi A, Trebicka J, Klinger C, Plessier A, Seijo S, Terziroli B, et al. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. 2017;37:694–9 This study presents the first large multicenter cohort on treatment with direct-acting oral anticoagulants in cirrhosis and provides a basis for prospective randomized clinical trials.
Cerini F, Vilaseca M, Lafoz E, García-Irigoyen O, García-Calderó H, Tripathi DM, et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64:834–42.
Vilaseca M, García-Calderó H, Lafoz E, García-Irigoyen O, Avila MA, Reverter JC, et al. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65:2031–44.
Yagüe S, Alvarez Arroyo V, Castilla A, González Pacheco FR, Llamas P, Caramelo C. Modulation of the effect of vascular endothelial growth factor on endothelial cells by heparin: critical role of nitric oxide-mediated mechanisms. J Nephrol. 2005;18:234–42.
Tasatargil A, Ogutman C, Golbasi I, Karasu E, Dalaklioglu S. Comparison of the vasodilatory effect of nadroparin, enoxaparin, dalteparin, and unfractioned heparin in human internal mammary artery. J Cardiovasc Pharmacol. 2005;45:550–4.
Fortea JI, Zipprich A, Fernandez-Mena C, Puerto M, Bosoi CR, Almagro J, et al. Enoxaparin does not ameliorate liver fibrosis or portal hypertension in rats with advanced cirrhosis. Liver Int. 2018;38:102–12.
Bremer HC, Kreisel W, Roecker K, Dreher M, Koenig D, Kurz-Schmieg AK, et al. Phosphodiesterase 5 inhibitors lower both portal and pulmonary pressure in portopulmonary hypertension: a case report. J Med Case Rep. 2007;1:46.
Schaffner D, Lazaro A, Deibert P, Hasselblatt P, Stoll P, Fauth L, et al. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J Gastroenterol. 2018;24:4356–68.
Loureiro-Silva MR, Iwakiri Y, Abraldes JG, Haq O, Groszmann RJ. Increased phosphodiesterase-5 expression is involved in the decreased vasodilator response to nitric oxide in cirrhotic rat livers. J Hepatol. 2006;44:886–93.
Choi S-M, Shin J-H, Kim J-M, Lee C-H, Kang K-K, Ahn B-O, et al. Effect of udenafil on portal venous pressure and hepatic fibrosis in rats. Arzneimittelforschung. 2011;59:641–6.
Halverscheid L, Deibert P, Schmidt R, Blum HE, Dunkern T, Pannen BHJ, et al. Phosphodiesterase-5 inhibitors have distinct effects on the hemodynamics of the liver. BMC Gastroenterol. 2009;9:69.
Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144–50.
Trebicka J, Hennenberg M, Schulze Pröbsting A, Laleman W, Klein S, Granzow M, et al. Role of beta3-adrenoceptors for intrahepatic resistance and portal hypertension in liver cirrhosis. Hepatology. 2009;50:1924–35.
Vasina V, Giannone F, Domenicali M, Latorre R, Berzigotti A, Caraceni P, et al. Portal hypertension and liver cirrhosis in rats: effect of the β3-adrenoceptor agonist SR58611A. Br J Pharmacol. 2012;167:1137–47.
Schierwagen R, Uschner FE, Magdaleno F, Klein S, Trebicka J. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol. 2017;312:G407–12.
Baba TT, Ohara-Nemoto Y, Miyazaki T, Nemoto TK. Involvement of geranylgeranylation of Rho and Rac GTPases in adipogenic and RANKL expression, which was inhibited by simvastatin. Cell Biochem Funct. 2013;31:652–9.
Veillard NR, Braunersreuther V, Arnaud C, Burger F, Pelli G, Steffens S, et al. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006;188:51–8.
Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–53.
Klein S, Klösel J, Schierwagen R, Körner C, Granzow M, Huss S, et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Investig J Tech Methods Pathol. 2012;92:1440–50.
Schierwagen R, Leeming DJ, Klein S, Granzow M, Nielsen MJ, Sauerbruch T, et al. Serum markers of the extracellular matrix remodeling reflect antifibrotic therapy in bile-duct ligated rats. Front Physiol. 2013;4:195.
Marrone G, Russo L, Rosado E, Hide D, García-Cardeña G, García-Pagán JC, et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J Hepatol. 2013;58:98–103.
Marrone G, Maeso-Díaz R, García-Cardena G, Abraldes JG, García-Pagán JC, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64:1434–43.
Zhou Q, Hennenberg M, Trebicka J, Jochem K, Leifeld L, Biecker E, et al. Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut. 2006;55:1296–305.
Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, et al. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220–7.
Tripathi DM, Vilaseca M, Lafoz E, Garcia-Calderó H, Viegas Haute G, Fernández-Iglesias A, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology. 2018;155:1564–77.
Rodríguez S, Raurell I, Torres-Arauz M, García-Lezana T, Genescà J, Martell M. A nitric oxide-donating statin decreases portal pressure with a better toxicity profile than conventional statins in cirrhotic rats. Sci Rep. 2017;7:40461.
Uschner FE, Ranabhat G, Choi SS, Granzow M, Klein S, Schierwagen R, et al. Statins activate the canonical hedgehog-signaling and aggravate non-cirrhotic portal hypertension, but inhibit the non-canonical hedgehog signaling and cirrhotic portal hypertension. Sci Rep. 2015;5:14573.
Hsu S-J, Wang S-S, Hsin I-F, Huang H-C, Lee F-Y, Lee J-Y, et al. Effects of simvastatin on the portal-systemic collateral vascular response to endothelin-1 and shunting degree in portal hypertensive rats. Scand J Gastroenterol. 2013;48:831–8.
Huang H-C, Wang S-S, Lee J-Y, Chen Y-C, Lee F-Y, Lin H-C, et al. Simvastatin effects on portal-systemic collaterals of portal hypertensive rats. J Gastroenterol Hepatol. 2010;25:1401–9.
• Pose E, Trebicka J, Mookerjee RP, Angeli P, Ginès P. Statins: old drugs as new therapy for liver diseases? J Hepatol. 2019;70:194–202 This review comprehensively summarises the evidence for use of statins in cirrhosis.
Souk K, Al-Badri M, Azar ST. the safety and benefit of statins in liver cirrhosis: a review. Exp Clin Endocrinol Diabetes. 2015;123:577–80.
Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–55.
Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47:957–63.
•• Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150:1160–1170.e3. This multicenter trial represents the most important clinical study on use of statins in portal hypertension so far.
Wani ZA, Mohapatra S, Khan AA, Mohapatra A, Yatoo GN. Addition of simvastatin to carvedilol non responders: a new pharmacological therapy for treatment of portal hypertension. World J Hepatol. 2017;9:270–7.
• Bishnu S, Ahammed SM, Sarkar A, Hembram J, Chatterjee S, Das K, et al. Effects of atorvastatin on portal hemodynamics and clinical outcomes in patients with cirrhosis with portal hypertension: a proof-of-concept study. Eur J Gastroenterol Hepatol. 2018;30:54–9. This open-label study provides important data on the use of atorvastatin in portal hypertension.
Statins And cirrhosis: reducing events of decompensation - full text view - ClinicalTrials.gov [Internet]. [cited 2019 Feb 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT03654053.
Efficacy of the combination of simvastatin plus rifaximin in patients with decompensated cirrhosis to prevent aclf development - full text view - ClinicalTrials.gov [Internet]. [cited 2019 Feb 21]. Available from: https://clinicaltrials.gov/ct2/show/NCT03780673.
Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117–25.
Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–76.
Heller J, Shiozawa T, Trebicka J, Hennenberg M, Schepke M, Neef M, et al. Acute haemodynamic effects of losartan in anaesthetized cirrhotic rats. Eur J Clin Investig. 2003;33:1006–12.
Heller J, Trebicka J, Shiozawa T, Schepke M, Neef M, Hennenberg M, et al. Vascular, hemodynamic and renal effects of low-dose losartan in rats with secondary biliary cirrhosis. Liver Int. 2005;25:657–66.
Schepke M, Werner E, Biecker E, Schiedermaier P, Heller J, Neef M, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389–95.
Wang J, Lu W, Li J, Zhang R, Zhou Y, Yin Q, et al. Hemodynamic effects of renin-angiotensin-aldosterone inhibitor and β-blocker combination therapy vs. β-blocker monotherapy for portal hypertension in cirrhosis: a meta-analysis. Exp Ther Med. 2017;13:1977–85.
Annicchiarico BE, Santonocito C, Siciliano M, Scapaticci M, Guarino D, Di Stasi C, et al. ACE I allele is associated with more severe portal hypertension in patients with liver cirrhosis: a pilot study. Dig Liver Dis. 2019;51:293–6.
Casey S, Herath C, Rajapaksha I, Jones R, Angus P. Effects of angiotensin-(1–7) and angiotensin II on vascular tone in human cirrhotic splanchnic vessels. Peptides. 2018;108:25–33.
Gaidarov I, Adams J, Frazer J, Anthony T, Chen X, Gatlin J, et al. Angiotensin (1–7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor. Cell Signal. 2018;50:9–24.
Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, et al. activation of the Mas receptor by angiotensin-(1–7) in the renin–angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874–884.e5.
Klein S, Herath CB, Schierwagen R, Grace J, Haltenhof T, Uschner FE, et al. hemodynamic effects of the non-peptidic angiotensin-(1–7) agonist AVE0991 in liver cirrhosis. PLoS One. 2015;10:e0138732.
Guilluy C, Brégeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med. 2010;16:183–90.
Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, et al. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334–48.
Klein S, Rick J, Lehmann J, Schierwagen R, Schierwagen IG, Verbeke L, et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66:145–55.
Wang D, Yin J, Dong R, Zhao J, Wang Q, Wang N, et al. Inhibition of Janus kinase-2 signalling pathway ameliorates portal hypertensive syndrome in partial portal hypertensive and liver cirrhosis rats. Dig Liver Dis. 2015;47:315–23.
Shaker ME, Hazem SH, Ashamallah SA. Inhibition of the JAK/STAT pathway by ruxolitinib ameliorates thioacetamide-induced hepatotoxicity. Food Chem Toxicol. 2016;96:290–301.
Hazem SH, Shaker ME, Ashamallah SA, Ibrahim TM. The novel Janus kinase inhibitor ruxolitinib confers protection against carbon tetrachloride-induced hepatotoxicity via multiple mechanisms. Chem Biol Interact. 2014;220:116–27.
Tan HK, Leow WQ, Chang PE. Ruxolitinib for the treatment of portal hypertension in a patient with primary myelofibrosis. Gastroenterology. 2017 May 4;(16)35073–9.
Hennenberg M, Trebicka J, Kohistani Z, Stark C, Nischalke H-D, Krämer B, et al. Hepatic and HSC-specific sorafenib effects in rats with established secondary biliary cirrhosis. Lab Investig. 2011;91:241–51.
Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–56.
Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865–73.
Pinter M, Sieghart W, Reiberger T, Rohr-Udilova N, Ferlitsch A, Peck-Radosavljevic M. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma--a pilot study. Aliment Pharmacol Ther. 2012;35:83–91.
Uschner FE, Schueller F, Nikolova I, Klein S, Schierwagen R, Magdaleno F, et al. The multikinase inhibitor regorafenib decreases angiogenesis and improves portal hypertension. Oncotarget. 2018;9:36220–37.
Higashiyama H, Kinoshita M, Asano S. Immunolocalization of farnesoid X receptor (FXR) in mouse tissues using tissue microarray. Acta Histochem. 2008;110:86–93.
Halilbasic E, Fuchs C, Traussnigg S, Trauner M. Farnesoid X receptor agonists and other bile acid signaling strategies for treatment of liver disease. Dig Dis. 2016;34:580–8.
Fuchs CD, Schwabl P, Reiberger T, Trauner M. Liver capsule: FXR agonists against liver disease. Hepatology. 2016;64:1773.
Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–98.
Mookerjee RP, Mehta G, Balasubramaniyan V, Mohamed FEZ, Davies N, Sharma V, et al. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J Hepatol. 2015;62:325–31.
Verbeke L, Mannaerts I, Schierwagen R, Govaere O, Klein S, Vander Elst I, et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci Rep. 2016;6:33453.
Xu W, Lu C, Zhang F, Shao J, Zheng S. Dihydroartemisinin restricts hepatic stellate cell contraction via an FXR-S1PR2-dependent mechanism. IUBMB Life. 2016;68:376–87.
Xu W, Lu C, Zhang F, Shao J, Yao S, Zheng S. Dihydroartemisinin counteracts fibrotic portal hypertension via farnesoid X receptor-dependent inhibition of hepatic stellate cell contraction. FEBS J. 2017;284:114–33.
Schwabl P, Hambruch E, Seeland BA, Hayden H, Wagner M, Garnys L, et al. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66:724–33.
Renga B, Cipriani S, Carino A, Simonetti M, Zampella A, Fiorucci S. Reversal of endothelial dysfunction by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1 dependent regulation of H2S generation and endothelin-1. PLoS One. 2015;10:e0141082.
Fiorucci S, Distrutti E. Targeting the transsulfuration-H2S pathway by FXR and GPBAR1 ligands in the treatment of portal hypertension. Pharmacol Res. 2016;111:749–56.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65.
Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, et al. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006;130:838–54.
van Beuge MM, Prakash J, Lacombe M, Gosens R, Post E, Reker-Smit C, et al. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Ther. 2011;337:628–35.
Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats | Scientific Reports [Internet]. [cited 2019 Feb 26]. Available from: https://www.nature.com/articles/s41598-019-38678-5.
Funding
The authors were funded by Deutsche Forschungsgemeinschaft (SFB TRR57) and Cellex Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any original data on human or animal subjects performed by any of the authors, but only review of published evidence.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Portal Hypertension
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schierwagen, R., Klein, S., Uschner, F. et al. Novel Targets and Drug Development in Portal Hypertension. Curr Hepatology Rep 18, 187–196 (2019). https://doi.org/10.1007/s11901-019-00462-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-019-00462-4