Abstract
Purpose of Review
With the widespread implementation of contemporary disease-modifying heart failure therapy, the rates of normalization of ejection fraction are continuously increasing. The TRED-HF trial confirmed that heart failure remission rather than complete recovery is typical in patients with dilated cardiomyopathy who respond to therapy. The present review outlines key points related to the management and knowledge gaps of this growing patient group, focusing on patients with non-ischaemic dilated cardiomyopathy.
Recent Findings
There is substantial heterogeneity among patients with normalized ejection fraction. The specific etiology is likely to affect the outcome, although a multiple-hit phenotype is frequent and may not be identified without comprehensive characterization. A monogenic or polygenic genetic susceptibility is common. Ongoing pathophysiological processes may be unraveled with advanced cardiac imaging, biomarkers, multi-omics, and machine learning technologies. There are limited studies that have investigated the withdrawal of specific heart failure therapies in these patients. Diuretics may be safely withdrawn if there is no evidence of congestion, while continued therapy with at least some disease-modifying therapy is likely to be required to reduce myocardial workload and sustain remission for the vast majority.
Summary
Understanding the underlying disease mechanisms of patients with normalized ejection fraction is crucial in identifying markers of myocardial relapse and guiding individualized therapy in the future. Ongoing clinical trials should inform personalized approaches to therapy.
Similar content being viewed by others
Introduction
The goals of therapy for heart failure (HF) with reduced ejection fraction (HFrEF) include alleviation of symptoms and improvements in mortality and cardiovascular outcomes [1, 2]. With the advent of effective disease-modifying therapies, left ventricular reverse remodeling (LVRR) is currently observed in at least 40% of patients with non-ischemic dilated cardiomyopathy (DCM) and is associated with improved prognosis [3]. Indeed, the trajectory of the left ventricular ejection fraction (LVEF) offers important insight into the clinical course and outcome of patients with HF [4]. A number of pivotal investigations in the last decade have shed light on the clinical profile and outcome of patients with previously reduced LVEF that improves to > 40%. Interest in this patient group has driven the creation of new subcategories of HF (Table 1). There is currently an agreement that HF with improved EF (HFimpEF) includes patients with a previous LVEF ≤ 40% that increases to > 40%, typically with the contribution of guideline-directed medical therapy (GDMT) and device therapy [3, 5].
In a subset of asymptomatic patients with HFimpEF, the LVEF and natriuretic peptides return to normal, and it has been a clinical and research conundrum whether this represents true HF recovery or remission (HFrEF in remission, HFrEFrem). The TRED-HF (therapy withdrawal in recovered DCM) trial demonstrated that complete withdrawal of pharmacological therapy is associated with relapse of HF in a substantial proportion of patients with normalized LVEF [6]. Nevertheless, the underlying biology, clinical course, and outcomes of patients with LVEF improvement/normalization are incompletely understood. The current review provides an update on issues related to the management of the subgroup of patients with normalized LVEF focusing on dilated cardiomyopathy (DCM).
The Continuum of Improving LVEF: Understanding the LVEF Trajectory
Ventricular dilation and reduction in LVEF are the principal features of DCM [7]. Left ventricular reverse remodeling involves an increase in LVEF, reflecting improvements in myocyte contractility, accompanied by regression of eccentric hypertrophy and fibrosis [8]. Factors that have been traditionally associated with LVRR include female sex, younger age, milder HF, shorter duration of the disease, and fewer comorbidities [9,10,11]. LVEF recovery may be partial (with an LVEF increasing to between 40 and 49%) or complete (normalized LVEF to ≥ 50%).
While 20–40% of patients are expected to transition to higher LVEF in just a few months, [9, 10, 12,13,14] evidence of LVRR may be observed as late as 2 years after initiation of therapy [15•]. An analysis of 4942 patients of the Swedish Heart Failure Registry revealed that 26% of patients with HFrEF at baseline showed an increase in LVEF to > 40% (with 10% to LVEF > 50%) and 25% of patients with HF with mildly reduced LVEF showed an increase in LVEF to > 50% [13•]. The exact rates of recovery are likely underestimated, considering that most relevant studies are based on a single repeat evaluation of LVEF. They are also likely to be greater now with the widespread implementation of highly effective contemporary GDMT and the use of medical devices [9, 10, 12, 14, 15
There are several key points to consider regarding patients with HFimpEF. Firstly, improvement or even normalization of LVEF does not guarantee the resolution of symptoms or return of biomarkers, such as natriuretic peptides, to normal [12, 12,13,14,15].
Secondly, the LVEF may deteriorate again, further supporting that in a substantial proportion of patients with normalized LVEF, there is a state of remission (HFrEFrem) rather than true permanent recovery [5, 6, 15,16,17]. Taken together, it appears that LVEF normalization does not reflect a return to the normal state but the transition to a lesser state of disease [3]. This two-way LVEF trajectory may depend on factors such as the root cause, disease duration, adherence to GDMT, or renewed exposure to cardiotoxicity or external stressors. In a retrospective analysis of 800 patients with non-ischaemic cardiomyopathy from the Trieste Heart Muscle Registry, an improved LVEF (LVEF > 40%) was documented in a remarkable 57% of the population over a median of 11-year follow-up period [15•]. The HFimpEF group was further divided into those with persistent and those with transiently improved LVEF. The latter represented 41% of the HFimpEF population, and the median time to relapse was 5 years despite continued medical therapy. Older age, lower LVEF, and longer disease duration at the time of improvement were associated with a greater risk of relapse. Nevertheless, the determinants of the duration of remission and the risk of recurrent LVEF drop have not been completely clarified.
Lastly, the prognosis of patients with improvements in LVEF continues to be investigated. Patients with HFimpEF have a better prognosis compared to those with persistent HFrEF and the degree of LVRR is associated with outcome [12,13,14, 16, 18]. The clinical course of HFimpEF appears distinct from that of HF with mildly reduced and preserved LVEF, despite the LVEF overlap [12, 18, 19]. Nevertheless, the overall survival appears worse than that of healthy controls and a proportion of patients experience adverse events in the long term, including heart failure hospitalizations and the need for advanced heart failure therapies. Current data are limited, and reliable predictors of recurrent HF remain unclear.
The TRED-HF Study: Key Points
The TRED-HF study was an open-label, pilot randomized controlled study with a single-arm crossover phase examining the effect of phased and complete withdrawal of pharmacological therapy in asymptomatic patients with DCM and normalized LVEF over a 6-month period [6]. It sought to answer the question of whether stopping all HF medications was safe once LVEF has normalized, and signs and symptoms of HF had resolved. In other words, it investigated whether this represented “complete” or “apparent” healing. The study included 51 patients with a mean age of 55 years (67% men) and previously reduced LVEF (≤ 40%, median LVEF of 25%), presenting with an LVEF of ≥ 50% along with normal LV end-diastolic volume at enrolment. The absence of symptoms and normal NT-proBNP levels confirmed disease remission as per current definitions. A stepwise reduction in medications was performed over 16 weeks. During the randomized phase, 44% of the patients who were assigned to medication withdrawal relapsed compared to none in the control group. Importantly, 26% relapsed within just 8 weeks of taking their last medication. When medications were withdrawn in the control group in the follow-on phase, a further 36% of patients relapsed. An adequate response to restarting therapy was observed in most patients, with an LVEF of > 50% documented in 85% of patients at the subsequent clinic visit.
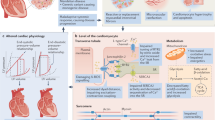
The TRED-HF trial, despite being a small pilot study, provided important insight into a previously unknown territory in the field of HF. The results confirmed that a significant proportion of patients are in remission and that full withdrawal of medical therapy should not routinely be performed. The study also highlighted that it is important to identify discriminators of sustained recovery as well as early markers of relapse. A normal LVEF and an asymptomatic status provide an incomplete assessment of disease status and the risk of recurrent HF. Interestingly, in participants who withdrew from therapy, there was evidence of an increase in the heart rate and blood pressure in the first few weeks after withdrawal, in keeping with neurohormonal (re-)activation and increased myocardial work. Patients who relapsed had a 10 beats/min greater rise in heart rate compared to those who did not relapse [20•]. Heart rate and heart rate change were associated with relapse. Imaging evidence of LV remodeling was evident from as early as 4 months after withdrawal (Fig. 1) [21, 22]. In those who relapsed, changes in LVEF preceded changes in NT-proBNP, suggesting that natriuretic peptides may not be good markers of early relapse.
Main changes in clinical and imaging parameters observed in patients who withdrew from therapy in TRED-HF based on available measurements. HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, LVEF left ventricular ejection fraction, LVEDVi left ventricular end-diastolic volume index, LVMi left ventricular mass index, GLS global longitudinal strain
How Can Disease Etiology and Underlying Disease Activity Inform Decision-Making?
Dilated cardiomyopathy results from a wide range of genetic and acquired causes [7]. An interaction between genetic predisposition and environmental factors may apply in a significant proportion of patients (“seed and soil” hypothesis, first used by Stephen Paget in 1889 to describe cancer metastasis) [5]. The underlying processes contributing to disease likely impact the rate and degree of LVRR and the future risk of relapse. In any given HFimpEF patient, the genetic susceptibility, repeat exposure to an external myocardial insult or stress and the effects of non-cardiac comorbidities should be taken into account. Predicting which patients with normalized LVEF are at increased risk of relapse (particularly after withdrawal of cardiac medications) appears to be a complex process. Several steps that need to be addressed in this context are outlined below.
Revisiting Specific Triggers of Cardiomyopathy
It has traditionally been considered that once an inciting factor of DCM is completely removed, the probability of satisfactory to full recovery is high [3]. Conditions such as tachycardia-induced, alcohol, and peripartum cardiomyopathy are typical examples. However, continuous advances have helped us understand that a dual or multiple-hit pathophysiology often applies. For instance, a rare genetic variant associated with DCM may be identified in a significant proportion of patients with alcohol cardiomyopathy, myocarditis, and anthracycline-induced cardiotoxicity [23, 24]. Polygenic risk resulting from common genetic variation is also likely to drive myocardial vulnerability [25]. Genetic variation affects the pathophysiological mechanisms related to the myocardial response to chemotherapeutic agents such as anthracyclines and trastuzumab [26, 27]. These observations may have implications in terms of targeted treatments and decisions regarding continuation of conventional therapy (Fig. 2). It may be tantalizing to withdraw medications in conditions attributed to a single resolved triggering factor, but this may not always be the case.
Suggested probability of LVEF improvement and normalization among different common aetiologies of dilated cardiomyopathy. Increasing evidence suggests that the underlying genetic background influences myocardial susceptibility to the respective trigger as well as clinical course and recovery. Other factors, including the presence/extent of fibrosis and co-morbidities, further affect myocardial response (spontaneous or following therapy). It is acknowledged that there is insufficient data comparing the clinical course of the various causes of dilated cardiomyopathy. The present figure may, therefore, require revision as new information emerges. TTNtv truncating variant in titin. (Created with Biorender.com)
A representative example of multifactorial etiology is peripartum cardiomyopathy, a condition with current rates of LVEF improvement ranging from 20 to 80% [28]. Time to recovery is variable and may be relevant to future risk of relapse. A tangible risk of deterioration even in the absence of pregnancy has been described [29]. Peripartum cardiomyopathy is now considered a complex syndrome involving vascular, hormonal, and inflammatory/autoimmune processes [30, 31]. Mitochondrial dysfunction may also play an important role [32]. Importantly, a genetic contribution has been identified in several studies that show a prevalence of 10–15% of truncating variants in titin (TTN, TTNtv) as well as genes associated with arrhythmic phenotypes [33, 34].
Genetic Cardiomyopathies
Genetic profiling informs the chances of observing LVRR and may contribute to decision-making for patients with HFimpEF/HFrEFrem [35,36,37,38]. Variants in genes of the nuclear envelope and cytoskeleton proteins are associated with lower rates of LVRR [36, 38, 39]. TTNtv are the most common variants in DCM and are often associated with a mild disease course and high rates of LVRR [39,40,41]. Whether the prevalence of rare variants in cardiomyopathy genes or the polygenic risk differs in patients with HFrEFrem compared to those with persistent HFrEF is a matter of investigation.
Recent data support gene-specific mechanisms of HF recurrence [42, 43]. For instance, in a retrospective analysis of 239 patients with TTNtv from the Maastricht DCM registry, both patient groups with and without TTNtv showed a similar sharp increase in LVEF at up to 2 years and similar rates of LVRR [42]. Subsequently, the LVEF slowly declined in those with TTNtv but remained stable in the non-TTNtv group. It has been suggested the ability of the heart to sustain energy demands may become less effective over time and this may be more marked in carriers of TTNtv [44]. Such results set the scene for further investigations with respect to myocardial adaptations in different genotypes. A better understanding of energetic and mechanical changes may identify the substrate for relapse in the presence of hemodynamic stressors.
Ongoing Disease Processes and the Role of Cardiac Imaging and Biomarkers
Data from the Penn Heart Study, where myocardial recovery was defined using an LVEF cut-off of > 50%, demonstrated persistent biomarker evidence of inflammation, neurohormonal activation, and myocardial injury in this population [12]. Combining data from clinical assessment as well as advanced imaging and biomarkers may enable the characterization of ongoing disease processes and prediction of the risk of relapse (Fig. 3) [4, 45].
Proposed pathway to applying precision medicine principles in heart failure patients in myocardial remission to reduce the risk of relapse. A combination of our understanding of the ongoing substrate of disease and the role of existing and upcoming therapies in targeting disease mechanisms is central to providing personalized care. HFrEF heart failure with reduced ejection fraction, LVEF left ventricular ejection fraction, ECM extracellular matrix, HTN hypertension, CAD coronary artery disease, GDMT guideline-directed medical therapy, QoL quality of life. (Created with Biorender.com)
Imaging data beyond LVEF may inform management. Global longitudinal strain has been shown to vary widely, denoting large heterogeneity of intrinsic myocardial function [46,47,48,49]. A significant proportion of HFimpEF patients have reduced GLS despite a normal LVEF, and this has been associated with a greater risk of relapse and worse outcomes [46,47,48,49,50]. A study in 206 patients with non-ischaemic cardiomyopathy and normalized LVEF (LVEF > 50%) showed that a GLS of < 16% was associated with a threefold increased risk of mortality and major adverse cardiac events [47•]. A recent retrospective study in 699 patients reported that left atrial reverse remodeling, defined as > 15% reduction in the left atrial end-systolic volume, was observed in ~ 60% of patients with improved LVEF and was associated with a lower risk of cardiovascular death and hospitalization [51]. In a small study of 96 patients with HFimpEF, diastolic dysfunction, defined as an elevated E/E′ (> 12.1), was associated with a higher risk of a similar endpoint [52].
The availability of cardiac magnetic resonance (CMR) has been increasing and CMR is recommended for assessment of DCM in current guidelines [1]. Evaluation for myocardial fibrosis by CMR provides important prognostic information in DCM [53, 54]. Evidence suggests that replacement myocardial fibrosis predicts lower rates of LVRR [55]. A retrospective review of 148 patients with recent-onset DCM showed that one-third of the population with initially improved LVEF (≥ 45%) presented with re-worsening of their LVEF which was associated with the degree of fibrosis on CMR [56]. Preliminary data from TRED-HF showed that there was expansion of the extracellular matrix during treatment withdrawal, suggestive of ongoing fibrotic activity [22].
Other domains of cardiac phenotyping are continuously evolving [57]. A number of omics technologies are available that can contribute to the biological characterization of these patients and various computational methods are being investigated for the utilization of large amounts of data to identify pathways of disease [58,59,60]. Techniques such as MR spectroscopy may further contribute to our understanding of ongoing energy deficits in these patients [61]. Molecular identification of fibrotic activity is further achieved with the use of collagen biomarkers and dedicated radiotracers, while therapies targeting fibrosis are concurrently under development [62,63,64].
Phenogrouping for Targeted Therapies
Mining of large datasets using machine-learning technologies is a novel method of grouping patients who share common features; these may reflect separate biologies and mechanisms of disease and may allow the use of targeted therapies [65,66,67]. In a study of 426 patients with DCM, clustering of clinical, imaging, genetic, and proteomic data identified three distinct DCM subgroups, including a profibrotic metabolic subtype characterized by extensive fibrosis, and increased prevalence of diabetes and kidney disease [66•]. Importantly, regression analysis identified a simple 5-variable model to assign patients to the relevant group. Similar investigations in patients with HFimpEF/HFrEFrem are limited. One study in 889 patients with improved LVEF to > 50% utilized an unsupervised clustering algorithm to group patients based on differences in 11 pre-defined variables [68]. The study identified 7 phenotypes of HFimpEF, signifying marked heterogeneity. In addition, one of the phenotypes was associated with an increased risk of relapse and greater mortality. This was a retrospective analysis of an administrative dataset, and the study results were not externally validated. Nevertheless, they set an example of how such methodologies may identify patient groups that benefit from different therapeutic strategies.
The Role of Current Disease-Modifying Therapies. What Is the Evidence?
Most HF trials in patients with an LVEF > 40% excluded patients with previous HFrEF, and no such trials have included asymptomatic patients with improved LVEF. The optimal HF drug regimen for these patients remains unknown. There is currently an agreement that GDMT should be maintained at maximal tolerated doses in patients with continued symptoms and signs of HF, irrespective of the LVEF [2, 3]. The TRED-HF trial showed that withdrawal of all HF medications in patients with HFrEFrem was associated with a high rate of relapse [6]. In this trial, all patients were on a renin-angiotensin-system (RAS) blocker and most on a beta-blocker. Less than half were on a mineralocorticoid receptor antagonist (MRA) and 12% were on a diuretic. No patients were on angiotensin-receptor neprilysin inhibitors (ARNi) or sodium-glucose cotransporter 2 inhibitors (SGTLT2i). Based on the results of the TRED-HF trial, the most recent iteration of the AHA/ACC/HFSA guidelines recommended that GDMT should be continued to prevent HF relapse even in asymptomatic patients with HFimpEF [2]. The recent ESC/HFA guidelines also recommend continued treatment [1]. It remains unclear whether GDMT should continue at the maximum dose or whether there may be a “simplified” regimen that can effectively maintain remission in specific subgroups.
Management of neurohormonal dysregulation and myocardial stress are crucial aspects of therapy [1]. The activity of traditional pathophysiological HF mechanisms in HFrEFrem patients is unclear and likely varies between patients as discussed above. The patient profiles and circumstances that allow the safe reduction and/or withdrawal of agents in asymptomatic patients with HFrEFrem are currently being scrutinized.
Loop Diuretics
It appears reasonable to reduce or stop loop diuretics in patients without evidence of congestion [69]. The ReBIC-1 (Rede Brasileira de Estudos em Insuficiência Cardíaca) trial confirmed that diuretic withdrawal is safe in stable HF [70]. The study enrolled 188 patients of a mean age of 59 years and with an LVEF ≤ 45% (mean LVEF 32%) who were on optimized HF treatment including low-dose loop diuretics. The participants were in NYHA class I or II, without congestion, and free of recent HF admissions. Diuretic withdrawal versus continued administration was not associated with worse self-reported dyspnoea or need for furosemide use during the 90-day period of the study.
Beta-Blockers
An analysis of the TRED-HF trial showed that rises in heart rate preceded overt myocardial dysfunction in patients who relapsed following medication withdrawal [20•]. It is reasonable to speculate that continued suppression of the sympathetic nervous system may be required to control myocardial work and stress and avoid HF recurrence. Older studies performed over 20 years ago had shown that withdrawal of beta-blockers was associated with worsening of LV function in patients with congestive HF and dilated cardiomyopathy respectively [71,72,73]. A retrospective analysis utilizing data from a national Japanese database investigated the association of beta-blocker use with myocardial relapse in HFimpEF patients with a current LVEF ≥ 40% [74]. Propensity score matching yielded a total of 1087 patient pairs (on and off beta-blocker therapy). The study showed that beta-blocker use was associated with a 23% lower risk of a decrease in LVEF ≥ 10% at 2 years of follow-up. These results suggest that beta-blockers may be an important pillar of maintaining remission.
Renin-Angiotensin Blockers and Angiotensin-Receptor Neprilysin Inhibitors
Inhibition of the RAS system with RAS blockers has a pivotal role in the treatment of HFrEF and withdrawal of therapy is associated with worsening symptoms and ventricular function [75]. As mentioned, all patients in TRED-HF were on a RAS blocker; however, the exact contribution of RAS inhibition to sustaining remission is unclear. ARNi provides prognostic benefits for patients with HF across the entire range of LVEF [76]. Updated analyses of the PARAGON-HF (Prospective Comparison of ARNi with ARB Global Outcomes in HF with Preserved Ejection Fraction) trial showed significant reductions in HF events in patients with LVEF > 45%, although, the benefit was more evident in patients with an LVEF at the lower end of the spectrum. Notably, it excluded patients with a previous LVEF of less than 40% [77]. There is no data investigating whether continuation or initiation of ARNi sustains remission more effectively in asymptomatic patients with normalized LVEF. Switching to a RAS blocker when there is evidence of remission may be an attractive strategy, considering the cost limitations for some insurance systems.
Mineralocorticoid Receptor Antagonists
The added benefit of MRA on LVRR in mild to moderately symptomatic patients with HF on appropriate background therapy has been questioned [78]. Whether ΜRAs need to be continued once the LVEF has normalized is unclear. In TRED-HF, of the 15 patients who were taking MRA at the start of the study and subsequently relapsed, 10 did not restart an MRA after the study and all managed to achieve remission again [6].
An open-label, controlled, prospective observational study from China examined the effects of withdrawal of spironolactone in 70 asymptomatic patients with idiopathic DCM and HFimpEF with an LVEF > 40% [79]. This was not randomized and spironolactone withdrawal versus continuation was based on an informed decision by the patient. The mean LVEF was 46% and NT-proBNP levels ranged from 298 to 1793 pg/L. The primary endpoint of myocardial relapse (defined as at least one of a > 10% LVEF reduction or > 15% LVESVi or a twofold rise in NT-proBNP concentration or clinical signs and symptoms of HF) was observed in 58% of patients in the withdrawal group and 13% in the continuation group over 12 months. Interestingly, 74% of patients who relapsed had clinical evidence of HF without reduction in LVEF. Νo deaths or adverse cardiovascular events were reported. The study should be interpreted in the context of a non-randomized design and a population of patients with ongoing evidence of HF.
Sodium-Glucose Cotransporter 2 Inhibitors
Similar to ARNi, large trials have shown that SGLT2i reduces cardiovascular death and HF admissions in symptomatic patients with HF across the entire range of LVEF [80]. Specifically, the DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction) investigated the prognostic benefit of dapagliflozin in patients with an LVEF of more than 40% and elevated natriuretic peptides [81]. The study enrolled 6263 patients with a mean age of 72 years, of which most were in NYHA class II–III. The study was unique in that it allowed for patients with a previous LVEF of < 40% to be included. In particular, 18% of patients fulfilled the criteria for HFimpEF [82]. The study showed an 18% reduction in cardiovascular death and worsening HF that appeared consistent in the HFimpEF group [81, 83, 84]. On the other hand, SGLT2i exhibits a number of properties (e.g., antifibrotic effects, metabolic modulation) that may target pathways associated with myocardial relapse [85].
Should Device Therapy Be Continued?
The benefit of ICDs in patients with DCM is currently debated [86]. Given the high incidence of LVRR, many advocate a longer period of contemporary GDMT (e.g., up to 9 months) before considering an ICD for primary prevention, thus allowing enough time for myocardial recovery [87, 88]. There is limited information regarding the effectiveness of ICDs in the setting of LVEF normalization [89]. Indeed, a significant proportion of patients with DCM are young and face risks of serious device complications in the medium- and long-term. Available information supports that the arrhythmic risk is reduced but not removed when LVEF improves [90, 91]. There is, therefore, a general consensus that generator change should be performed in patients with HFimpEF [3]. The presence of high-risk genetic variants, the extent of myocardial fibrosis on CMR, GLS, cardiac biomarkers such as natriuretic peptides and ST2 may identify those patients least likely to reverse remodel and most likely to benefit from an ICD [1, 38, 53, 92,93,94].
Cardiac resynchronization therapy (CRT) is associated with LVRR and improved outcomes, including lower rates of ventricular arrhythmias [95]. It is indicated in the presence of ventricular dyssynchrony assessed primarily by electrocardiography and there is consensus that it should be continued once LVEF recovers due to a high risk of recurrent HF in withdrawal studies [3, 96]. The STOP-CRT trial further investigated whether withdrawing neurohumoral blockade (RAS blockers and/or beta-blockers) is feasible in patients who respond well to CRT presenting with normalized LVEF [97•]. The rate of relapse was low suggesting that in these patients, drug withdrawal may be safe and feasible.
Involving the Patient and Shared Decision-Making
Heart failure patients perceive good quality of life as a major scope of their clinical management [98, 99]. Patients are often keen to explore the option of having their medications reduced (in terms of dose and/or quantity) despite the possible risk of relapse. Patients are often of young age and may not wish to continue taking medications long-term if there is no strong evidence of benefit [6]. Some are concerned about medication-related side effects which may affect quality of life [100]. For women who intend to become pregnant, discontinuing ACEi/ARB/ARNi, MRA, and SGLT2i is indicated. In addition, reducing the number of medications may provide financial relief depending on patient contribution by country. This could be the case when switching from an ARNi to an ACEi, should the risk of HF morbidity remain similar. Patient preference should be considered also with respect to adherence to complex medication regimens.
Unanswered Questions and Future Research
The TRED-HF study provided randomized data to support the continuation of at least some therapy in patients with HFrEFrem. However, several questions remain. Uncovering predictors of myocardial relapse will be key for the management of this patient group (Table 2). The optimal medical HF regimen for patients with HFrEFrem to reduce the risk of HF recurrence remains unclear. Appropriate patient phenotyping is required for a personalized approach. Understanding the underlying pathophysiology in an individual patient may help guide streamlined therapies targeting individual mechanisms of relapse. The TRED-HF 2 trial is an open-label randomized trial that will examine whether it is safe and feasible to withdraw MRA and/or SGLT2i in patients with DCM and HFrEFrem who are on background therapy with a RAS blocker and beta-blocker. The main outcome will be HF relapse while imaging and biomarker information will be acquired during the study. The study protocol of another randomized study has been published that will investigate whether halving neurohormonal blockers (RAS blockers or ARNi and beta-blockers) is non-inferior to the original dose in terms of protection from HF relapse/hospitalization [101].
Conclusions
Management of patients with HFrEFrem will be an increasingly common scenario. Clarifying the underlying disease processes and the best clinical management of these patients must be a major priority. The accumulating evidence suggests that this newly defined HF category should be approached separately from other HF classes of (near-) normal LVEF associated with ongoing evidence of the clinical HF syndrome. The TRED-HF trial confirmed that these patients are often in remission while the path to complete recovery is less clear. It is possible that not all drug components of contemporary GDMT are required to maintain remission and reduce the risk of cardiac events, but separate patient profiles may benefit from distinct and individualized medical regimens. This will, hopefully, be elucidated with ongoing clinical investigations.
Abbreviations
- ACC:
-
American College of Cardiology
- ACEi:
-
Angiotensin-converting enzyme inhibitor
- AHA:
-
American Heart Association
- ARB:
-
Angiotensin receptor blocker
- ARNi:
-
Angiotensin-receptor neprilysin inhibitor
- CMR:
-
Cardiac magnetic resonance
- CPET:
-
Cardiopulmonary exercise test
- CRT:
-
Cardiac resynchronization therapy
- DCM:
-
Dilated cardiomyopathy
- ESC:
-
European Society of Cardiology
- GDMT:
-
Guideline-directed medical therapy
- GLS:
-
Global longitudinal strain
- HFA:
-
Heart Failure Association
- HF:
-
Heart failure
- HFimpEF:
-
Heart failure with improved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- HFrEFrem:
-
Heart failure with reduced ejection fraction in remission
- HFSA:
-
Heart Failure Society of America
- ICD:
-
Implantable cardioverter defibrillator
- LVEDVi:
-
LV end-diastolic volume index
- LVEF:
-
Left ventricular ejection fraction
- LVESVi:
-
Left ventricular end-systolic volume index
- LVRR:
-
Left ventricular reverse remodeling
- MRA:
-
Mineralocorticoid receptor antagonist
- NT-proBNP:
-
N-terminal pro-b-type natriuretic peptide
- NYHA:
-
New York Heart Association
- RAS:
-
Renin-angiotensin system
- SGTLT2i:
-
Sodium-glucose cotransporter 2 inhibitor
- TTN:
-
Titin
- TTNtv:
-
Truncating variant in titin
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145:e895-1032. https://doi.org/10.1161/CIR.0000000000001063.
Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction. J Am Coll Cardiol. 2020;76:719–34. https://doi.org/10.1016/j.jacc.2020.05.075.
Ragavan A, Hogan J, Halliday BP. The spectrum of heart failure with improved ejection fraction: persistent congestion, to heart failure remission and perhaps recovery? Eur J Heart Fail. 2022;24:1180–2. https://doi.org/10.1002/ejhf.2571.
Halliday BP, Cleland JGF. Maintaining success for patients with dilated cardiomyopathy and remission of heart failure. JACC Basic Transl Sci. 2022;7:500–3. https://doi.org/10.1016/j.jacbts.2022.03.008.
Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet Lond Engl. 2019;393:61–73. https://doi.org/10.1016/S0140-6736(18)32484-X.
Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC Guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J. 2023;ehad194. https://doi.org/10.1093/eurheartj/ehad194.
Boulet J, Mehra MR. Left ventricular reverse remodeling in heart failure: remission to recovery. Struct Heart. 2021;5:466–81. https://doi.org/10.1080/24748706.2021.1954275.
DeVore AD, Hellkamp AS, Thomas L, Albert NM, Butler J, Patterson JH, et al. Improvement in left ventricular ejection fraction in outpatients with heart failure with reduced ejection fraction. Circ Heart Fail. 2020;13:e006833. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006833.
Ghimire A, Fine N, Ezekowitz JA, Howlett J, Youngson E, McAlister FA. Frequency, predictors, and prognosis of ejection fraction improvement in heart failure: an echocardiogram-based registry study. Eur Heart J. 2019;40:2110–7. https://doi.org/10.1093/eurheartj/ehz233.
Tayal U, Wage R, Newsome S, Manivarmane R, Izgi C, Muthumala A, et al. Predictors of left ventricular remodelling in patients with dilated cardiomyopathy - a cardiovascular magnetic resonance study. Eur J Heart Fail. 2020;22:1160–70. https://doi.org/10.1002/ejhf.1734.
Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380–7. https://doi.org/10.1161/CIRCULATIONAHA.113.006855.
Savarese G, Vedin O, D’Amario D, Uijl A, Dahlström U, Rosano G, et al. Prevalence and prognostic implications of longitudinal ejection fraction change in heart failure. JACC Heart Fail. 2019;7:306–17. https://doi.org/10.1016/j.jchf.2018.11.019. This study investigated LVEF trajectories in a large registry and reported on associated variables.
Lupón J, Díez-López C, de Antonio M, Domingo M, Zamora E, Moliner P, et al. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail. 2017;19:1615–23. https://doi.org/10.1002/ejhf.824.
Manca P, Stolfo D, Merlo M, Gregorio C, Cannatà A, Ramani F, et al. Transient versus persistent improved ejection fraction in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2022;24:1171–9. https://doi.org/10.1002/ejhf.2512. This study examined the clinical course of transient versus persistent improvement in LVEF in patients with non-ischemic cardiomyopathy.
Moon J, Ko Y-G, Chung N, Ha J-W, Kang S-M, Choi E-Y, et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25:e147–50. https://doi.org/10.1016/S0828-282X(09)70497-0.
Merlo M, Stolfo D, Anzini M, Negri F, Pinamonti B, Barbati G, et al. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: does real healing exist? J Am Heart Assoc. 2015;4:e001504. https://doi.org/10.1161/JAHA.114.000570.
Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1:510–8. https://doi.org/10.1001/jamacardio.2016.1325.
Nadruz W, West E, Santos M, Skali H, Groarke JD, Forman DE, et al. Heart failure and midrange ejection fraction. Circ Heart Fail. 2016;9:e002826. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002826.
Halliday BP, Vazir A, Owen R, Gregson J, Wassall R, Lota AS, et al. Heart rate as a marker of relapse during withdrawal of therapy in recovered dilated cardiomyopathy. JACC Heart Fail. 2021;9:509–17. https://doi.org/10.1016/j.jchf.2021.03.010. An analysis of the TRED-HF trial identified the association of heart rate following withdrawal of therapy with myocardial relapse.
Halliday BP, Owen R, Gregson J, Vazir A, Wassall R, Khalique Z, et al. Changes in clinical and imaging variables during withdrawal of heart failure therapy in recovered dilated cardiomyopathy. ESC Heart Fail. 2022;9:1616–24. https://doi.org/10.1002/ehf2.13872.
Halliday BP, Owen R, Gregson J, S Vassiliou V, Chen X, Wage R, et al. Myocardial remodelling after withdrawing therapy for heart failure in patients with recovered dilated cardiomyopathy: insights from TRED-HF. Eur J Heart Fail. 2021;23:293–301. https://doi.org/10.1002/ejhf.2063.
Lota AS, Hazebroek MR, Theotokis P, Wassall R, Salmi S, Halliday BP, et al. Genetic architecture of acute myocarditis and the overlap with inherited cardiomyopathy. Circulation. 2022;146:1123–34. https://doi.org/10.1161/CIRCULATIONAHA.121.058457. An analysis of patients with acute myocarditis showed a high prevalence of dilated and arrhythmogenic cardiomyopathy-associated genes.
Ware JS, Amor-Salamanca A, Tayal U, Govind R, Serrano I, Salazar-Mendiguchía J, et al. Genetic etiology for alcohol-induced cardiac toxicity. J Am Coll Cardiol. 2018;71:2293–302. https://doi.org/10.1016/j.jacc.2018.03.462.
Pirruccello JP, Bick A, Wang M, Chaffin M, Friedman S, Yao J, et al. Analysis of cardiac magnetic resonance imaging in 36,000 individuals yields genetic insights into dilated cardiomyopathy. Nat Commun. 2020;11:2254. https://doi.org/10.1038/s41467-020-15823-7.
Bhatia S. Genetics of anthracycline cardiomyopathy in cancer survivors. JACC CardioOncology. 2020;2:539–52. https://doi.org/10.1016/j.jaccao.2020.09.006.
Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. Genetic variants associated with cancer therapy–induced cardiomyopathy. Circulation. 2019;140:31–41. https://doi.org/10.1161/CIRCULATIONAHA.118.037934.
Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:207–21. https://doi.org/10.1016/j.jacc.2019.11.014.
Koerber D, Khan S, Kirubarajan A, Spivak A, Wine R, Matelski J, et al. Meta-analysis of long-term (> 1 year) cardiac outcomes of peripartum cardiomyopathy. Am J Cardiol. 2023;194:71–7. https://doi.org/10.1016/j.amjcard.2023.01.043.
Davis MB, Sliwa K. To infinity and beyond. JACC Heart Fail. 2023;S2213177923004079. https://doi.org/10.1016/j.jchf.2023.06.035.
Lovell JP, Bermea K, Yu J, Rousseau S, Cohen CD, Bhalodia A, et al. Serum proteomic analysis of peripartum cardiomyopathy reveals distinctive dysregulation of inflammatory and cholesterol metabolism pathways. JACC Heart Fail. 2023. https://doi.org/10.1016/j.jchf.2023.05.031.
Ramaccini D, Montoya-Uribe V, Aan FJ, Modesti L, Potes Y, Wieckowski MR, et al. Mitochondrial function and dysfunction in dilated cardiomyopathy. Front Cell Dev Biol. 2021;8:624216. https://doi.org/10.3389/fcell.2020.624216.
Goli R, Li J, Brandimarto J, Levine LD, Riis V, McAfee Q, et al. Genetic and phenotypic landscape of peripartum cardiomyopathy. Circulation. 2021;143:1852–62. https://doi.org/10.1161/CIRCULATIONAHA.120.052395.
Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374:233–41. https://doi.org/10.1056/NEJMoa1505517.
Bertero E, Fracasso G, Eustachi V, Coviello D, Cecconi M, Giovinazzo S, et al. Diagnostic yield and predictive value on left ventricular remodelling of genetic testing in dilated cardiomyopathy. ESC Heart Fail. 2023;10:2745–50. https://doi.org/10.1002/ehf2.14395.
Dal Ferro M, Stolfo D, Altinier A, Gigli M, Perrieri M, Ramani F, et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart Br Card Soc. 2017;103:1704–10. https://doi.org/10.1136/heartjnl-2016-311017.
Escobar-Lopez L, Ochoa JP, Mirelis JG, Espinosa MÁ, Navarro M, Gallego-Delgado M, et al. Association of genetic variants with outcomes in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2021;78:1682–99. https://doi.org/10.1016/j.jacc.2021.08.039. A retrospective study of genotyped patients with DCM that provided information on prognosis by genetic profile.
Tayal U, Ware JS, Lakdawala NK, Heymans S, Prasad SK. Understanding the genetics of adult-onset dilated cardiomyopathy: what a clinician needs to know. Eur Heart J. 2021;42:2384–96. https://doi.org/10.1093/eurheartj/ehab286.
Jansweijer JA, Nieuwhof K, Russo F, Hoorntje ET, Jongbloed JDH, Lekanne Deprez RH, et al. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail. 2017;19:512–21. https://doi.org/10.1002/ejhf.673.
Verdonschot JAJ, Hazebroek MR, Wang P, Sanders-van Wijk S, Merken JJ, Adriaansen YA, et al. Clinical phenotype and genotype associations with improvement in left ventricular function in dilated cardiomyopathy. Circ Heart Fail. 2018;11:e005220. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005220.
Luk K, Bakhsh A, Giannetti N, Elstein E, Lathrop M, Thanassoulis G, et al. Recovery in patients with dilated cardiomyopathy with loss-of-function mutations in the titin gene. JAMA Cardiol. 2017;2:700–2. https://doi.org/10.1001/jamacardio.2017.0763.
Henkens MTHM, Stroeks SLVM, Raafs AG, Sikking MA, Tromp J, Ouwerkerk W, et al. Dynamic ejection fraction trajectory in patients with dilated cardiomyopathy with a truncating titin variant. Circ Heart Fail. 2022;15:e009352. https://doi.org/10.1161/CIRCHEARTFAILURE.121.009352.
Akhtar MM, Lorenzini M, Cicerchia M, Ochoa JP, Hey TM, Sabater Molina M, et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN gene. Circ Heart Fail. 2020;13:e006832. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006832.
Verdonschot JAJ, Hazebroek MR, Derks KWJ, Barandiarán Aizpurua A, Merken JJ, Wang P, et al. Titin cardiomyopathy leads to altered mitochondrial energetics, increased fibrosis and long-term life-threatening arrhythmias. Eur Heart J. 2018;39:864–73. https://doi.org/10.1093/eurheartj/ehx808.
Fatkin D, Huttner IG, Kovacic JC, Seidman JG, Seidman CE. Precision medicine in the management of dilated cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:2921–38. https://doi.org/10.1016/j.jacc.2019.10.011.
Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ Heart Fail. 2017;10:e003788. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003788.
Merlo M, Masè M, Perry A, Franca EL, Deych E, Ajello L, et al. Prognostic significance of longitudinal strain in dilated cardiomyopathy with recovered ejection fraction. Heart. 2022;108:710–6. https://doi.org/10.1136/heartjnl-2021-319504. This study examined the association of global longitudinal strain with outcomes in patients with normalized LVEF.
Raafs AG, Boscutti A, Henkens MTHM, van den Broek WWA, Verdonschot JAJ, Weerts J, et al. Global longitudinal strain is incremental to left ventricular ejection fraction for the prediction of outcome in optimally treated dilated cardiomyopathy patients. J Am Heart Assoc. 2022;11:e024505. https://doi.org/10.1161/JAHA.121.024505.
Janwanishstaporn S, Cho JY, Feng S, Brann A, Seo J-S, Narezkina A, et al. Prognostic value of global longitudinal strain in patients with heart failure with improved ejection fraction. JACC Heart Fail. 2022;10:27–37. https://doi.org/10.1016/j.jchf.2021.08.007.
Merken J, Brunner-La Rocca H-P, Weerts J, Verdonschot J, Hazebroek M, Schummers G, et al. Heart failure with recovered ejection fraction. J Am Coll Cardiol. 2018;72:1557–8. https://doi.org/10.1016/j.jacc.2018.06.070.
Sun Y, Chen X, Zhang Y, Yu Y, Zhang X, Si J, et al. Reverse atrial remodeling in heart failure with recovered ejection fraction. J Am Heart Assoc. 2023;12:e026891. https://doi.org/10.1161/JAHA.122.026891.
Takada T, Matsuura K, Minami Y, Abe T, Yoshida A, Kishihara M, et al. Prognosis and diastolic dysfunction predictors in patients with heart failure and recovered ejection fraction. Sci Rep. 2022;12:8768. https://doi.org/10.1038/s41598-022-12823-z.
Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging. 2019;12:1645–55. https://doi.org/10.1016/j.jcmg.2018.07.015.
Prasad SK, Halliday BP. Myocardial fibrosis in dilated cardiomyopathy: moving from stratifying risk to improving outcomes∗. JACC Cardiovasc Imaging. 2021;14:1351–3. https://doi.org/10.1016/j.jcmg.2021.03.015.
Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging. 2018;11:1274–84. https://doi.org/10.1016/j.jcmg.2018.03.006.
Nabeta T, Ishii S, Ikeda Y, Maemura K, Oki T, Yazaki M, et al. Late gadolinium enhancement for re-worsening left ventricular ejection fraction in patients with dilated cardiomyopathy. ESC Heart Fail. 2021;8:615–24. https://doi.org/10.1002/ehf2.13133.
Saraste A, Knuuti J, Bengel F. Phenotyping heart failure by nuclear imaging of myocardial perfusion, metabolism, and molecular targets. Eur Heart J - Cardiovasc Imaging. 2023;jead128. https://doi.org/10.1093/ehjci/jead128.
Wang R-S, Maron BA, Loscalzo J. Multiomics network medicine approaches to precision medicine and therapeutics in cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2023;43:493–503. https://doi.org/10.1161/ATVBAHA.122.318731.
Joshi A, Rienks M, Theofilatos K, Mayr M. Systems biology in cardiovascular disease: a multiomics approach. Nat Rev Cardiol. 2021;18:313–30. https://doi.org/10.1038/s41569-020-00477-1. A state-of-the-art review that discusses the utility of different omics data for the study of cardiovascular disease.
Hansen KB, Sörensen J, Hansson NH, Nielsen R, Larsen AH, Frøkiær J, et al. Myocardial efficiency in patients with different aetiologies and stages of heart failure. Eur Heart J Cardiovasc Imaging. 2022;23:328–37. https://doi.org/10.1093/ehjci/jeab227.
Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–8. https://doi.org/10.1161/01.cir.86.6.1810.
Barton AK, Tzolos E, Bing R, Singh T, Weber W, Schwaiger M, et al. Emerging molecular imaging targets and tools for myocardial fibrosis detection. Eur Heart J Cardiovasc Imaging. 2022;24:261–75. https://doi.org/10.1093/ehjci/jeac242.
Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13:e010628. https://doi.org/10.1161/CIRCIMAGING.120.010628.
Rurik JG, Tombácz I, Yadegari A, Méndez Fernández PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–6. https://doi.org/10.1126/science.abm0594.
Sun J, Guo H, Wang W, Wang X, Ding J, He K, et al. Identifying novel subgroups in heart failure patients with unsupervised machine learning: a scoping review. Front Cardiovasc Med. 2022;9:895836. https://doi.org/10.3389/fcvm.2022.895836.
Tayal U, Verdonschot JAJ, Hazebroek MR, Howard J, Gregson J, Newsome S, et al. Precision phenotyping of dilated cardiomyopathy using multidimensional data. J Am Coll Cardiol. 2022;79:2219–32. https://doi.org/10.1016/j.jacc.2022.03.375. This trial utilized machine learning on multiparametric data to identify mechanistically distinct DCM subtypes.
Verdonschot JAJ, Merlo M, Dominguez F, Wang P, Henkens MTHM, Adriaens ME, et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur Heart J. 2021;42:162–74. https://doi.org/10.1093/eurheartj/ehaa841.
Perry A, Loh F, Adamo L, Zhang KW, Deych E, Foraker R, et al. Unsupervised cluster analysis of patients with recovered left ventricular ejection fraction identifies unique clinical phenotypes. PLOS ONE. 2021;16:e0248317. https://doi.org/10.1371/journal.pone.0248317.
Nuzzi V, Cannatà A, Pellicori P, Manca P, Stolfo D, Gregorio C, et al. Diuretic dose trajectories in dilated cardiomyopathy: prognostic implications. Clin Res Cardiol. 2023;112:419–30. https://doi.org/10.1007/s00392-022-02126-8.
Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simões MV, et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J. 2019;40:3605–12. https://doi.org/10.1093/eurheartj/ehz554.
Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Adverse effects of beta-blockade withdrawal in patients with congestive cardiomyopathy. Heart. 1980;44:134–42. https://doi.org/10.1136/hrt.44.2.134.
Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A. Long-term beta-blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation. 1989;80:551–63. https://doi.org/10.1161/01.cir.80.3.551.
Morimoto S, Shimizu K, Yamada K, Hiramitsu S, Hishida H. Can beta-blocker therapy be withdrawn from patients with dilated cardiomyopathy? Am Heart J. 1999;138:456–9. https://doi.org/10.1016/s0002-8703(99)70147-x.
Enzan N, Matsushima S, Ide T, Kaku H, Tohyama T, Funakoshi K, et al. Beta-blocker use is associated with prevention of left ventricular remodeling in recovered dilated cardiomyopathy. J Am Heart Assoc. 2021;10:e019240. https://doi.org/10.1161/JAHA.120.019240.
Pflugfelder PW, Baird MG, Tonkon MJ, DiBianco R, Pitt B. Clinical consequences of angiotensin-converting enzyme inhibitor withdrawal in chronic heart failure: a double-blind, placebo-controlled study of quinapril. The Quinapril heart failure trial investigators. J Am Coll Cardiol. 1993;22:1557–63. https://doi.org/10.1016/0735-1097(93)90578-o.
Solomon SD, Vaduganathan M, L Claggett B, Packer M, Zile M, Swedberg K, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–61. https://doi.org/10.1161/CIRCULATIONAHA.119.044586.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. https://doi.org/10.1056/NEJMoa1908655.
Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:347–53. https://doi.org/10.1161/CIRCHEARTFAILURE.109.906909.
Chen Y, Qiu Z, Jiang J, Su X, Huang F, Tang J, et al. Outcomes of spironolactone withdrawal in dilated cardiomyopathy with improved ejection fraction. Front Cardiovasc Med. 2021;8:725399. https://doi.org/10.3389/fcvm.2021.725399.
Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–64. https://doi.org/10.1038/s41591-022-01971-4.
Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98. https://doi.org/10.1056/NEJMoa2206286.
Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, et al. Baseline characteristics of patients with hf with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10:184–97. https://doi.org/10.1016/j.jchf.2021.11.006.
Vardeny O, Fang JC, Desai AS, Jhund PS, Claggett B, Vaduganathan M, et al. Dapagliflozin in heart failure with improved ejection fraction: a prespecified analysis of the DELIVER trial. Nat Med. 2022;28:2504–11. https://doi.org/10.1038/s41591-022-02102-9. A pre-specified analysis of the DELIVER trial showing the benefits of SGLT2i in patients with HFimpEF.
Zelniker TA, Morrow DA, Mosenzon O, Goodrich EL, Jarolim P, Murphy SA, et al. Relationship between baseline cardiac biomarkers and cardiovascular death or hospitalization for heart failure with and without sodium-glucose co-transporter 2 inhibitor therapy in DECLARE-TIMI 58. Eur J Heart Fail. 2021;23:1026–36. https://doi.org/10.1002/ejhf.2073.
Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5:632–44. https://doi.org/10.1016/j.jacbts.2020.02.004.
Yafasova A, Butt JH, Elming MB, Nielsen JC, Haarbo J, Videbæk L, et al. Long-term follow-up of DANISH (the Danish study to assess the efficacy of icds in patients with nonischemic systolic heart failure on mortality). Circulation. 2022;145:427–36. https://doi.org/10.1161/CIRCULATIONAHA.121.056072.
Gracia E, Hamid A, Butler J. Timely management of new-onset heart failure. Circulation. 2019;140:621–3. https://doi.org/10.1161/CIRCULATIONAHA.118.035452.
Pastore MC, Mandoli GE, Giannoni A, Benfari G, Dini FL, Pugliese NR, et al. Sacubitril/valsartan reduces indications for arrhythmic primary prevention in heart failure with reduced ejection fraction: insights from DISCOVER-ARNI, a multicenter Italian register. Eur Heart J Open. 2022;2:oeab046. https://doi.org/10.1093/ehjopen/oeab046.
Thomas IC, Wang Y, See VY, Minges KE, Curtis JP, Hsu JC. Outcomes following implantable cardioverter-defibrillator generator replacement in patients with recovered left ventricular systolic function: the National Cardiovascular Data Registry. Heart Rhythm. 2019;16:733–40. https://doi.org/10.1016/j.hrthm.2018.11.005.
Smer A, Saurav A, Azzouz MS, Salih M, Ayan M, Abuzaid A, et al. Meta-analysis of Risk of ventricular arrhythmias after improvement in left ventricular ejection fraction during follow-up in patients with primary prevention implantable cardioverter defibrillators. Am J Cardiol. 2017;120:279–86. https://doi.org/10.1016/j.amjcard.2017.04.020.
Adabag S, Patton KK, Buxton AE, Rector TS, Ensrud KE, Vakil K, et al. Association of implantable cardioverter defibrillators with survival in patients with and without improved ejection fraction: secondary analysis of the sudden cardiac death in heart failure Trial. JAMA Cardiol. 2017;2:767–74. https://doi.org/10.1001/jamacardio.2017.1413.
Masarone D, Limongelli G, Ammendola E, Verrengia M, Gravino R, Pacileo G. Risk stratification of sudden cardiac death in patients with heart failure: an update. J Clin Med. 2018;7:436. https://doi.org/10.3390/jcm7110436.
Ahmad T, Fiuzat M, Neely B, Neely ML, Pencina MJ, Kraus WE, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart Fail. 2014;2:260–8. https://doi.org/10.1016/j.jchf.2013.12.004.
Montembeau SC, Merchant FM, Speight C, Kramer DB, Matlock DD, Horný M, et al. Patients’ perspectives regarding generator exchanges of implantable cardioverter defibrillators. Circ Cardiovasc Qual Outcomes. 2023;16:509–18. https://doi.org/10.1161/CIRCOUTCOMES.122.009827.
Naqvi SY, Jawaid A, Vermilye K, Biering-Sørensen T, Goldenberg I, Zareba W, et al. Left ventricular reverse remodeling in cardiac resynchronization therapy and long-term outcomes. JACC Clin Electrophysiol. 2019;5:1001–10. https://doi.org/10.1016/j.jacep.2019.07.012.
Ypenburg C, Van Bommel RJ, Marsan NA, Delgado V, Bleeker GB, van der Wall EE, et al. Effects of interruption of long-term cardiac resynchronization therapy on left ventricular function and dyssynchrony. Am J Cardiol. 2008;102:718–21. https://doi.org/10.1016/j.amjcard.2008.05.009.
Nijst P, Martens P, Dauw J, Tang WHW, Bertrand PB, Penders J, et al. Withdrawal of neurohumoral blockade after cardiac resynchronization therapy. J Am Coll Cardiol. 2020;75:1426–38. https://doi.org/10.1016/j.jacc.2020.01.040. This study examined whether withdrawing neurohormonal blockade in patients with normalized LVEF following CRT is feasible.
Kraai IH, Vermeulen KM, Luttik MLA, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail. 2013;15:1113–21. https://doi.org/10.1093/eurjhf/hft071.
Rasmussen AA, Wiggers H, Jensen M, Berg SK, Rasmussen TB, Borregaard B, et al. Patient-reported outcomes and medication adherence in patients with heart failure. Eur Heart J - Cardiovasc Pharmacother. 2021;7:287–95. https://doi.org/10.1093/ehjcvp/pvaa097.
Jarab AS, Al-Qerem WA, Hamam HW, Alzoubi KH, Abu Heshmeh SR, Mukattash TL, et al. Medication adherence and its associated factors among outpatients with heart failure. Patient Prefer Adherence. 2023;17:1209–20. https://doi.org/10.2147/PPA.S410371.
Li P, Luo X, Hou C, Wu S, Wang L, Sun N, et al. Maintenance of recovered dilated cardiomyopathy patients with half-dose neurohumoral blockades (MED-CHARM): a protocol for an open-label, pilot, randomized trial. Front Cardiovasc Med. 2022;9:966537. https://doi.org/10.3389/fcvm.2022.966537.
Funding
BPH is supported by a British Heart Foundation Intermediate Fellowship (FS/ICRF/20/26019) and the Rosetrees Trust.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the manuscript. AK drafted the manuscript, figures and tables. All authors critically reviewed the manuscript for intellectual content. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kasiakogias, A., Ragavan, A. & Halliday, B.P. Your Heart Function Has Normalized—What Next After TRED-HF?. Curr Heart Fail Rep 20, 542–554 (2023). https://doi.org/10.1007/s11897-023-00636-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00636-8