Abstract
Purpose of Review
Cardiomyopathies due to genetic mutations are a heterogeneous group of disorders that comprise diseases of contractility, myocardial relaxation, and arrhythmias. Our goal here is to discuss a limited list of genetically inherited cardiomyopathies and the specific therapeutic strategies used to treat them.
Recent Findings
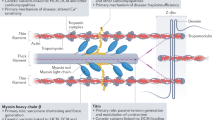
Research into the molecular pathophysiology of the development of these cardiomyopathies is leading to the development of novel treatment approaches. Therapies targeting these specific mutations with gene therapy vectors are on the horizon, while other therapies which indirectly affect the physiologic derangements of the mutations are currently being studied and used clinically. Many of these therapies are older medications being given new roles such as mexiletine for Brugada syndrome and diflunisal for transthyretin amyloid cardiomyopathy. A newer targeted therapy, the inhibitor of myosin ATPase MYK-461, has been shown to suppress the development of ventricular hypertrophy, fibrosis, and myocyte disarray and is being studied as a potential therapy in patients with hypertrophic cardiomyopathy.
Summary
While this field is too large to be completely contained in a single review, we present a large cross section of recent developments in the field of therapeutics for inherited cardiomyopathies. New therapies are on the horizon, and their development will likely result in improved outcomes for patients inflicted by these conditions.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010;460:223–37.
Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, et al. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293:447–54.
Brugada P, Brugada J. Right bundle branch block, persistent st segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–6.
Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–6.
Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, et al. Scn5a mutations associated with an inherited cardiac arrhythmia, long qt syndrome. Cell. 1995;80:805–11.
Baroudi G, Acharfi S, Larouche C, Chahine M. Expression and intracellular localization of an scn5a double mutant r1232w/t1620m implicated in brugada syndrome. Circ Res. 2002;90:E11–6.
Valdivia CR, Ackerman MJ, Tester DJ, Wada T, McCormack J, Ye B, et al. A novel scn5a arrhythmia mutation, m1766l, with expression defect rescued by mexiletine. Cardiovasc Res. 2002;55:279–89.
Valdivia CR, Tester DJ, Rok BA, Porter CB, Munger TM, Jahangir A, et al. A trafficking defective, Brugada syndrome-causing scn5a mutation rescued by drugs. Cardiovasc Res. 2004;62:53–62.
Tan BH, Valdivia CR, Song C, Makielski JC. Partial expression defect for the scn5a missense mutation g1406r depends on splice variant background q1077 and rescue by mexiletine. Am J Physiol Heart Circ Physiol. 2006;291:H1822–8.
Pfahnl AE, Viswanathan PC, Weiss R, Shang LL, Sanyal S, Shusterman V, et al. A sodium channel pore mutation causing Brugada syndrome. Heart Rhythm. 2007;4:46–53.
Ruan Y, Denegri M, Liu N, Bachetti T, Seregni M, Morotti S, et al. Trafficking defects and gating abnormalities of a novel scn5a mutation question gene-specific therapy in long qt syndrome type 3. Circ Res. 2010;106:1374–83.
• Mazzanti A, Maragna R, Faragli A, Monteforte N, Bloise R, Memmi M, et al. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long qt syndrome type 3. J Am Coll Cardiol. 2016;67:1053–8. In this retrospective cohort study, patients with LQT3 syndrome treated with mexiletine had shortening of their QTc interval and a reduction in arrhythmic events.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 esc guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (esc). Endorsed by: Association for european paediatric and congenital cardiology (aepc). Eur Heart J. 2015;36:2793–867.
Fisher JD, Krikler D, Hallidie-Smith KA. Familial polymorphic ventricular arrhythmias: a quarter century of successful medical treatment based on serial exercise-pharmacologic testing. J Am Coll Cardiol. 1999;34:2015–22.
Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, et al. Mutations in the cardiac ryanodine receptor gene (hryr2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200.
Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, et al. Mutations of the cardiac ryanodine receptor (ryr2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–90.
Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91:e21–6.
Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–67.
Nyegaard M, Overgaard MT, Sondergaard MT, Vranas M, Behr ER, Hildebrandt LL, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–12.
Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat Rev Cardiol. 2012;9:561–75.
Tester DJ, Arya P, Will M, Haglund CM, Farley AL, Makielski JC, et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm. 2006;3:800–5.
Watanabe H, Knollmann BC. Mechanism underlying catecholaminergic polymorphic ventricular tachycardia and approaches to therapy. J Electrocardiol. 2011;44:650–5.
van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2012;14:175–83.
Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the ryr2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner’s cases. Mayo Clin Proc. 2004;79:1380–4.
Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, et al. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98:1151–8.
Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–34.
Leren IS, Saberniak J, Majid E, Haland TF, Edvardsen T, Haugaa KH. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with beta1-selective beta-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2016;13:433–40.
Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–3.
Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, et al. Flecainide inhibits arrhythmogenic ca2+ waves by open state block of ryanodine receptor ca2+ release channels and reduction of ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301.
• van der Werf C, Kannankeril PJ, Sacher F, Krahn AD, Viskin S, Leenhardt A, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–54. In this retrospective analysis, patients with genotype positive CPVT previously uncontrolled by conventional drug therapy started on flexainide had a reduction in exercise-induced ventricular arrhymias.
Watanabe H, van der Werf C, Roses-Noguer F, Adler A, Sumitomo N, Veltmann C, et al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10:542–7.
Pott C, Dechering DG, Reinke F, Muszynski A, Zellerhoff S, Bittner A, et al. Successful treatment of catecholaminergic polymorphic ventricular tachycardia with flecainide: a case report and review of the current literature. Europace. 2011;13:897–901.
Hong RA, Rivera KK, Jittirat A, Choi JJ. Flecainide suppresses defibrillator-induced storming in catecholaminergic polymorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2012;35:794–7.
Hwang HS, Hasdemir C, Laver D, Mehra D, Turhan K, Faggioni M, et al. Inhibition of cardiac ca2+ release channels (ryr2) determines efficacy of class i antiarrhythmic drugs in catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol. 2011;4:128–35.
Smith GL, MacQuaide N. The direct actions of flecainide on the human cardiac ryanodine receptor: keeping open the debate on the mechanism of action of local anesthetics in cpvt. Circ Res. 2015;116:1284–6.
Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, et al. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011;109:291–5.
Bannister ML, Thomas NL, Sikkel MB, Mukherjee S, Maxwell C, MacLeod KT, et al. The mechanism of flecainide action in cpvt does not involve a direct effect on ryr2. Circ Res. 2015;116:1324–35.
Khoury A, Marai I, Suleiman M, Blich M, Lorber A, Gepstein L, et al. Flecainide therapy suppresses exercise-induced ventricular arrhythmias in patients with casq2-associated catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2013;10:1671–5.
Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–9.
Schneider HE, Steinmetz M, Krause U, Kriebel T, Ruschewski W, Paul T. Left cardiac sympathetic denervation for the management of life-threatening ventricular tachyarrhythmias in young patients with catecholaminergic polymorphic ventricular tachycardia and long qt syndrome. Clin Res Cardiol. 2013;102:33–42.
Hofferberth SC, Cecchin F, Loberman D, Fynn-Thompson F. Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J Thorac Cardiovasc Surg. 2014;147:404–9.
McNamara C, Cullen P, Rackauskas M, Kelly R, O'Sullivan KE, Galvin J, et al. Left cardiac sympathetic denervation: case series and technical report. Ir J Med Sci. 2017;
Costello JP, Wilson JK, Louis C, Peer SM, Zurakowski D, Nadler EP, et al. Surgical cardiac denervation therapy for treatment of congenital ion channelopathies in pediatric patients: a contemporary, single institutional experience. World J Pediatr Congenit Heart Surg. 2015;6:33–8.
Atallah J, Fynn-Thompson F, Cecchin F, DiBardino DJ, Walsh EP, Berul CI. Video-assisted thoracoscopic cardiac denervation: a potential novel therapeutic option for children with intractable ventricular arrhythmias. Ann Thorac Surg. 2008;86:1620–5.
Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–14.
Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, et al. Na+-dependent sr ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human cpvt mutation. Cardiovasc Res. 2010;87:50–9.
Li N, Wang Q, Sibrian-Vazquez M, Klipp RC, Reynolds JO, Word TA, et al. Treatment of catecholaminergic polymorphic ventricular tachycardia in mice using novel ryr2-modifying drugs. Int J Cardiol. 2017;227:668–73.
Chakraborty R, Muchtar E, Gertz MA. Newer therapies for amyloid cardiomyopathy. Curr Heart Fail Rep. 2016;13:237–46.
Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7:398–408.
Connors LH, Prokaeva T, Lim A, Theberge R, Falk RH, Doros G, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin v122i amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158:607–14.
Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, et al. Prospective evaluation of the morbidity and mortality of wild-type and v122i mutant transthyretin amyloid cardiomyopathy: The Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164:222–8. e221
Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:9629–34.
Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92.
Maurer MS, Grogan DR, Judge DP, Mundayat R, Packman J, Lombardo I, et al. Tafamidis in transthyretin amyloid cardiomyopathy: effects on transthyretin stabilization and clinical outcomes. Circ Heart Fail. 2015;8:519–26.
Damy T, Judge DP, Kristen AV, Berthet K, Li H, Aarts J. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-val30met and non-val122ile hereditary transthyretin amyloidosis. J Cardiovasc Transl Res. 2015;8:117–27.
Berk JL, Dyck PJ, Obici L, Zeldenrust SR, Sekijima Y, Yamashita T, et al. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid. 2011;18(Suppl 1):196–7.
Merlini G, Plante-Bordeneuve V, Judge DP, Schmidt H, Obici L, Perlini S, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-val30met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6:1011–20.
Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J Med Chem. 2004;47:355–74.
Castano A, Helmke S, Alvarez J, Delisle S, Maurer MS. Diflunisal for attr cardiac amyloidosis. Congest Heart Fail. 2012;18:315–9.
Obici L, Cortese A, Lozza A, Lucchetti J, Gobbi M, Palladini G, et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase ii study. Amyloid. 2012;19(Suppl 1):34–6.
• Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. In this non-randomised heathy volunteer controlled study, patients with transthyreting amyloidosis were treated with RNA interference which suppressed the production of transthyretin. This proved the concept that targeting messenger RNA was feasible in reducing the disease-causing mutant protein.
Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac involvement in hemochromatosis. Cardiol Rev. 2014;22:56–68.
Dabestani A, Child JS, Henze E, Perloff JK, Schon H, Figueroa WG, et al. Primary hemochromatosis: anatomic and physiologic characteristics of the cardiac ventricles and their response to phlebotomy. Am J Cardiol. 1984;54:153–9.
Cecchetti G, Binda A, Piperno A, Nador F, Fargion S, Fiorelli G. Cardiac alterations in 36 consecutive patients with idiopathic haemochromatosis: polygraphic and echocardiographic evaluation. Eur Heart J. 1991;12:224–30.
Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73.
Davis BA, O'Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–9.
Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using t2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–55.
Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56:1001–12.
Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;5:30.
Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, et al. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24:2102–11.
Schiffmann R, Murray GJ, Treco D, Daniel P, Sellos-Moura M, Myers M, et al. Infusion of alpha-galactosidase a reduces tissue globotriaosylceramide storage in patients with fabry disease. Proc Natl Acad Sci U S A. 2000;97:365–70.
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human alpha-galactosidase a--replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16.
Eng CM, Banikazemi M, Gordon RE, Goldman M, Phelps R, Kim L, et al. A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet. 2001;68:711–22.
Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, et al. Improvement of cardiac function during enzyme replacement therapy in patients with fabry disease: a prospective strain rate imaging study. Circulation. 2003;108:1299–301.
Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86.
• Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet. 2009;374:1986–96. In this registry study of the 5-year outcomes of enzyme replacement therapy for patients with Fabry’s disease, quality of life improved significantly. A reduction in left ventricular mass for those with baseline left ventricular hypertrophy was also observed.
Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S, et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–9.
Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, et al. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet. 2015;52:353–8.
Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, et al. Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry Outcome Survey. J Med Genet. 2009;46:548–52.
Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201–11.
Alves ML, Gaffin RD, Wolska BM. Rescue of familial cardiomyopathies by modifications at the level of sarcomere and ca2+ fluxes. J Mol Cell Cardiol. 2010;48:834–42.
Hwang PM, Sykes BD. Targeting the sarcomere to correct muscle function. Nat Rev Drug Discov. 2015;14:313–28.
Yamazaki T, Suzuki J, Shimamoto R, Tsuji T, Ohmoto-Sekine Y, Ohtomo K, et al. A new therapeutic strategy for hypertrophic nonobstructive cardiomyopathy in humans. A randomized and prospective study with an angiotensin ii receptor blocker. Int Heart J. 2007;48:715–24.
Shimada YJ, Passeri JJ, Baggish AL, O'Callaghan C, Lowry PA, Yannekis G, et al. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1:480–7.
Penicka M, Gregor P, Kerekes R, Marek D, Curila K, Krupicka J. The effects of candesartan on left ventricular hypertrophy and function in nonobstructive hypertrophic cardiomyopathy: a pilot, randomized study. J Mol Diagn. 2009;11:35–41.
Axelsson A, Iversen K, Vejlstrup N, Ho C, Norsk J, Langhoff L, et al. Efficacy and safety of the angiotensin ii receptor blocker losartan for hypertrophic cardiomyopathy: the inherit randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015;3:123–31.
Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. 2016;18:1106–18.
Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation. 2004;109:1284–91.
Pena JR, Szkudlarek AC, Warren CM, Heinrich LS, Gaffin RD, Jagatheesan G, et al. Neonatal gene transfer of serca2a delays onset of hypertrophic remodeling and improves function in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2010;49:993–1002.
Gaffin RD, Pena JR, Alves MS, Dias FA, Chowdhury SA, Heinrich LS, et al. Long-term rescue of a familial hypertrophic cardiomyopathy caused by a mutation in the thin filament protein, tropomyosin, via modulation of a calcium cycling protein. J Mol Cell Cardiol. 2011;51:812–20.
Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, et al. The l-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–20.
Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, et al. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–21.
McTaggart DR. Diltiazem reverses tissue doppler velocity abnormalities in pre-clinical hypertrophic cardiomyopathy. Heart Lung Circ. 2004;13:39–40.
Ho CY, Lakdawala NK, Cirino AL, Lipshultz SE, Sparks E, Abbasi SA, et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail. 2015;3:180–8.
Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol. 2007;293:C1148–53.
Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–903.
Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–41.
Rodriguez HM, Whitman-Cox S, Kawas R, Song Y, Sran A, Oslob J. Modulation of the cardiac sarcomere by a small molecule agent myk0000461: a potential therapeutic for the treatment of genetic hypertrophic cardiomyopathies. Biophys J. 2015;106
• Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351:617–21. In this study, the small molecule MYK-461, which reduces contractility by inhibiting myosin ATPase, suppressed the development of the typical phenotypic markers of hypertrophic cardiomyopathy in mice carrying human mutations in the myosin heavy chain.
Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, et al. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–83.
Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127:575–84.
Flenner F, Friedrich FW, Ungeheuer N, Christ T, Geertz B, Reischmann S, Wagner S, Stathopoulou K, Sohren KD, Weinberger F, Schwedhelm E, Cuello F, Maier LS, Eschenhagen T, Carrier L. Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc Res 2016;109(1):90–102.
Gentry JL 3rd, Mentz RJ, Hurdle M, Wang A. Ranolazine for treatment of angina or dyspnea in hypertrophic cardiomyopathy patients (rhyme). J Am Coll Cardiol. 2016;68:1815–7.
Olivotto I, Hellawell JL, Farzaneh-Far R, Blair C, Coppini R, Myers J, et al. Novel approach targeting the complex pathophysiology of hypertrophic cardiomyopathy: the impact of late sodium current inhibition on exercise capacity in subjects with symptomatic hypertrophic cardiomyopathy (LIBERTY-HCM) trial. Circ Heart Fail. 2016;9:e002764.
Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant n-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827–34.
Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, et al. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation. 2009;119:1398–407.
Wilder T, Ryba DM, Wieczorek DF, Wolska BM, Solaro RJ. N-acetylcysteine reverses diastolic dysfunction and hypertrophy in familial hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;309:H1720–30.
Graham RM, Owens WA. Pathogenesis of inherited forms of dilated cardiomyopathy. N Engl J Med. 1999;341:1759–62.
Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, et al. Missense mutations in the rod domain of the lamin a/c gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–24.
Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, et al. Mutations in the gene encoding lamin a/c cause autosomal dominant emery-dreifuss muscular dystrophy. Nat Genet. 1999;21:285–8.
Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, et al. Natural history of dilated cardiomyopathy due to lamin a/c gene mutations. J Am Coll Cardiol. 2003;41:771–80.
Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, et al. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin a/c gene mutation. Hum Mol Genet. 2012;21:4325–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kenneth Varian declares no conflict of interest.
W. H. Wilson Tang is supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, P20HL113452, R01DK106000, R01HL126827).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Therapy
Rights and permissions
About this article
Cite this article
Varian, K., Tang, W.H.W. Therapeutic Strategies Targeting Inherited Cardiomyopathies. Curr Heart Fail Rep 14, 321–330 (2017). https://doi.org/10.1007/s11897-017-0346-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-017-0346-8