Abstract
Fabry disease (FD) is a progressive, X-linked inherited disorder of glycosphingolipid metabolism due to deficient or absent lysosomal α-galactosidase A activity. FD is pan-ethnic and the reported annual incidence of 1 in 100,000 may underestimate the true prevalence of the disease. Classically affected hemizygous males, with no residual α-galactosidase A activity may display all the characteristic neurological (pain), cutaneous (angiokeratoma), renal (proteinuria, kidney failure), cardiovascular (cardiomyopathy, arrhythmia), cochleo-vestibular and cerebrovascular (transient ischemic attacks, strokes) signs of the disease while heterozygous females have symptoms ranging from very mild to severe. Deficient activity of lysosomal α-galactosidase A results in progressive accumulation of globotriaosylceramide within lysosomes, believed to trigger a cascade of cellular events. Demonstration of marked α-galactosidase A deficiency is the definitive method for the diagnosis of hemizygous males. Enzyme analysis may occasionnally help to detect heterozygotes but is often inconclusive due to random X-chromosomal inactivation so that molecular testing (genotyping) of females is mandatory. In childhood, other possible causes of pain such as rheumatoid arthritis and 'growing pains' must be ruled out. In adulthood, multiple sclerosis is sometimes considered. Prenatal diagnosis, available by determination of enzyme activity or DNA testing in chorionic villi or cultured amniotic cells is, for ethical reasons, only considered in male fetuses. Pre-implantation diagnosis is possible. The existence of atypical variants and the availability of a specific therapy singularly complicate genetic counseling. A disease-specific therapeutic option - enzyme replacement therapy using recombinant human α-galactosidase A - has been recently introduced and its long term outcome is currently still being investigated. Conventional management consists of pain relief with analgesic drugs, nephroprotection (angiotensin converting enzyme inhibitors and angiotensin receptors blockers) and antiarrhythmic agents, whereas dialysis or renal transplantation are available for patients experiencing end-stage renal failure. With age, progressive damage to vital organ systems develops and at some point, organs may start to fail in functioning. End-stage renal disease and life-threatening cardiovascular or cerebrovascular complications limit life-expectancy of untreated males and females with reductions of 20 and 10 years, respectively, as compared to the general population. While there is increasing evidence that long-term enzyme therapy can halt disease progression, the importance of adjunctive therapies should be emphasized and the possibility of developing an oral therapy drives research forward into active site specific chaperones.
Similar content being viewed by others
Review
I - Disease name and synonyms
Fabry disease
-
Fabry's disease
-
Anderson-Fabry disease
-
Alpha-galactosidase A deficiency
-
Angiokeratoma corporis diffusum
-
Ceramide trihexosidosis
-
Ruiter-Pompen-Wyers syndrome
-
Sweeley-Klionsky disease
II - Definition
Fabry disease (FD, OMIM 301500) [1, 2] is a devastating, progressive inborn error of metabolism with, particularly in the early stages, important roles being played by cellular dysfunction and microvascular pathology induced by lysosomal glycosphingolipid deposition [3]. Absent or deficient activity of lysosomal exoglycohydrolase α-galactosidase A (α-D-galactoside galactohydrolase, EC 3.2.1.22; α-gal A) [4, 5] results in progressive accumulation of globotriaosylceramide (Gb3 or GL-3; also known as ceramidetrihexoside or CTH) and related glycosphingolipids (galabiosylceramide) within lysosomes which are ubiquitous subcellular organelles [6], in a variety of cell types, including capillary endothelial cells, renal (podocytes, tubular cells, glomerular endothelial, mesangial and intersticial cells), cardiac (cardiomyocytes and fibroblasts) and nerve cells [7]. The primary disease process starts in infancy, or even as early as in the fetal stage of development [8, 9]. However, in contrast to many other lysosomal storage diseases [10, 11], most patients remain clinically asymptomatic during the very first years of life. In FD, lysosomal storage and cellular dysfunction are believed to trigger a cascade of events including cellular death, compromised energy metabolism [12–14], small vessel injury [15], K(Ca)3.1 channel dysfunction in endothelial cells [16], oxidative stress [17], impaired autophagosome maturation [18], tissue ischemia and, importantly, development of irreversible cardiac [19–21] and renal [22] tissue fibrosis. The first clinical symptoms interfering with the child's well-being and performance arise in childhood, typically between the ages of 3 and 10 years, and generally a few years later in girls than in boys [23, 24]. With age, progressive damage to vital organ systems develops in both genders [24] leading to organ failure. End-stage renal disease and life-threatening cardiovascular or cerebrovascular complications limit life-expectancy [25–27].
FD has long been regarded as an adult disease with most, if not all, affected males developing a "classic" phenotype. Later on, the sub-classifications "cardiac variant" [28, 29] and "renal variant" [30] were introduced for patients with predominant or exclusive cardiac or renal involvement, respectively. Female heterozygotes were erroneously described as "carriers of the defective gene" more or less safeguarded against developing disease manifestations and symptoms. However, evolving knowledge about the natural course of disease suggests that it is more appropriate to describe FD as a disease with a wide spectrum of heterogeneously progressive clinical phenotypes. This spectrum ranges from the "classic" severe phenotype in males to a seemingly asymptomatic disease course occasionally observed in females, with a variety of clinical presentations inbetween. Indeed, most female heterozygotes develop symptoms due to yet undetermined mechanisms [24, 31, 32] and a high percentage of females develop vital organ involvement including the kidneys, heart and/or brain about a decade later than males [24].
III - Epidemiology
FD belongs to a group of at least 50 genetically distinct, biochemically related lysosomal storage disorders. Each disorder is caused by an inborn error of metabolism due to a monogenetic defect specifically resulting in the deficiency of lysosomal enzyme(s). FD is pan-ethnic, but due to its rarity, determining an accurate disease frequency is difficult. Reported incidences, ranging from 1 in 476,000 [33] to 1 in 117,000 [34] in the general population, may largely underestimate the true prevalence (Table 1). Newborn screening initiatives have found an unexpectedly high prevalence of the disease, as high as 1 in ~3,100 newborns in Italy [35] and have identified a surprisingly high frequency of newborn males with FD (approximately 1 in 1,500) in Taiwan, 86% having the IVS4+919G > A cryptic splice mutation previously found in later-onset cardiac phenotype patients [36] (Table 1). The intronic IVS4+919G > A mutation was also found in a number of Taiwan Chinese adult patients with idiopathic hypertrophic cardiomyopathy [37].
IV - Clinical description
A. Early signs and symptoms: Fabry disease at the pediatric age
Early neural damage primarily involves small nerve fibers of the peripheral somatic [38] and autonomic nerve systems [39] with onset of related symptoms generally occurring at an earlier age in boys than in girls [23, 40–42]. Pain is experienced by 60-80% of classically affected boys and girls [23, 43] and is one of the earliest symptoms of FD. Two types of pain have been described: episodic crises ("Fabry crises") characterized by agonizing burning pain originating in the extremities and radiating inwards to the limbs and other parts of the body, and chronic pain characterized by burning and tingling paraesthesias [44]. Fabry crises may be precipitated by fever, exercise, fatigue, stress, and rapid changes in temperature [45]. When the crises are triggered or accompanied by fever, patients usually also have an elevated erythrocyte sedimentation rate. As a result of their pain, patients with FD have a greatly diminished quality of life [46, 47]. Other possible causes of pain that must be ruled out are rheumatoid arthritis, rheumatic fever, Raynaud's disease, systemic lupus erythematosus (SLE) and 'growing pains' (a frequent misdiagnosis in children with FD) (Table 2). Pain may wane in adulthood and it is important to search for a medical history of acroparesthesia in childhood during the first examination of a newly diagnosed adult patient [48].
Other early-onset signs appearing in childhood will usually remain present during adulthood and, among them, gastrointestinal involvement is a common, but under-appreciated, manifestation of FD [49]. Patients may complain of abdominal pain (often after eating), diarrhea, nausea, and vomiting, which are a significant cause of anorexia [50]. These gastrointestinal symptoms may be related to the deposition of Gb3 in the autonomic ganglia of the bowel and mesenteric blood vessels [51]. Diarrhea-predominant irritable bowel syndrome (IBS) is a differential diagnosis [50].
Absence of sweating (anhidrosis) [52] or a decreased ability to sweat (hypohidrosis) [53] with decreased skin impedance [54] is a significant problem for patients and can cause heat [55] and exercise intolerance [51, 56].
The most visible early clinical feature of FD is angiokeratoma (skin lesions) and clusters of small reddish purple, raised skin lesions (Figure 1) are typically found on the buttocks, groin, umbilicus and upper thighs, but also sometimes on mucosal areas, such as the mouth. Histologically, the skin lesions are small superficial angiomas caused by cumulative damage of the vascular endothelial cells of the skin with vessel dilatation in the dermis (Figure 2) that increase in number and size with age and can occur singly or in groups [53, 56, 57]. Telangiectasia [53, 55] and subcutaneous edema [58] have also been reported.
Angiokeratoma: the angiokeratoma are small, raised, dark-red spots that increase in number and size with age and can occur singly or in clusters. They are typically found on the lower back (A), buttocks (C), groin, flanks (D) and upper thighs but their distribution may be restricted to a limited area, such as the umbilicus (B).
Corneal changes ("cornea verticillata"), rarely of visual significance and readily detectable by slit lamp examination, are frequently encountered. Retinal vessel tortuosity may be observed.
Tinnitus may be an early symptom and hearing loss has been reported in children [59].
Chronic fatigue and difficulty gaining weight may also frequently occur, particularly during adolescence. High-flow priapism can also be observed in young boys affected with FD.
Despite the absence of major organ dysfunction, these symptoms, individually or in combination, may cause significant morbidity limiting the child's physical, school and social performances [60]. Early signs and symptoms of FD are presented in Table 2.
Early signs of cardiac and cerebrovascular abnormalities may be present during adolescence in both genders. Signs of involvement of the sinus node and conduction system (e.g. shortened PR interval, arrhythmias, impaired heart rate variability, and mild valvular insufficiency) have been demonstrated [61]. Although rare, evidence of microvascular ischemic brain involvement on magnetic resonance imaging (MRI) may be detectable at young ages [62].
The natural course of Fabry nephropathy in children or adolescent patients is still largely not understood. Signs indicative of early, insidiously progressing renal damage include microalbuminuria and proteinuria developing as early as in the second decade of life [63–65]. Histologic, potentially irreversible changes to glomeruli, interstitial tubules and vascular structures before the first appearance of microalbuminuria can be observed in renal biopsy specimens from children [65]. Podocyte foot process effacement has been reported and indicates focal segmental glomerulosclerosis. A decline in glomerular filtration rate (GFR) is uncommon at pediatric ages but may be seen as early as adolescence [56, 66]. Studies on renal function in children with FD have mainly been done using estimated creatinine-based GFR. The widely used original Schwartz formula [67] substantially overestimates GFR with a low accuracy, whereas the new abbreviated Schwartz formula [68] shows relatively good performances with a mean GFR overestimation of 5.3 ml/min/1.73 m2, being only slightly superior to the Counahan-Barratt formula [69]. The new abbreviated Schwartz formula should replace the original Schwartz formula in the routine follow-up of children with FD [70]. The current creatinine-based GFR formulas are all hampered by low accuracy in the "creatinine-blind" GFR range. Supplemental measured GFR is, therefore, recommended in patients where changes in GFR have potential impact on important treatment regimens [70].
B. Kidney involvement
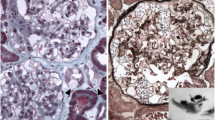
Like most aspects of the disease, renal pathology increases in severity with age. In classically affected Fabry patients, renal lesions result from Gb3 deposition in the glomerular endothelial, mesangial, intersticial cells and in podocytes (Figures 3 and 4), which are terminally-differenciated epithelial cells that accumulate numerous myelin-like inclusions in their lysosomes (Figure 5). Podocyte foot process effacement has been described. Glycosphingolipid storage also occur in the epithelium of the loop of Henle and the distal tubules (Figure 6), and in the endothelial and smooth muscle cells of the renal arterioles (Figure 7) [63, 71].
Renal impairment often begins with microalbuminuria and proteinuria in the 2nd to 3rd decade of life which, like in diabetic nephropathy, are believed to directly contribute to the progression of the Fabry nephropathy. With advancing age, proteinuria worsens [72]. Isosthenuria accompanied by alterations in tubular reabsorption, secretion and excretion develop. Initially, glomerular compensation (hyperfiltration) may mask impairment of renal function but, once a critical number of nephrons have been damaged, renal function will progressively decline. Gradual deterioration of renal function and development of azotemia usually occur in the third to fifth decades of life [73]. At this stage, fibrosis, sclerosis, and tubular atrophy dominate the disease activity portending end-stage renal disease that generally occurs in males in the 4th to 5th decade of life [25, 74]. The nephrological aspects of FD are major contributors to the morbidity and mortality associated with the disorder. Progression to end-stage renal failure is the primary cause of death in male patients with untreated FD and death most often results from uremia, unless chronic hemodialysis or renal transplantation is undertaken [25].
The evaluations of kidney function that should be carried out in every patient include serum creatinin, cystatin C, estimates of GFR, total protein, (micro) albumin excretion and urinary sodium excretion. In early stages of kidney involvement, quantitative estimates of GFR are necessary [75]. The utility of "spot" urine protein/creatinine ratios and estimated GFR with the modification of diet with renal disease (MDRD) equation has been established. Assessment of proteinuria and GFR can be used for the staging of chronic kidney disease (CKD), as described in the Kidney Disease Outcomes Quality Initiative (K/DOQI CKD) guidelines [51]. Kidney biopsies may be useful as a baseline assessment and in patients with atypical presentations, including a repeat kidney biopsy when the disease is progressing despite therapy [71].
Urinary protein excretion is strongly associated with renal disease progression in men and women with Fabry disease [76, 77].
C. Cardiac involvement
Cardiac symptoms including left ventricular hypertrophy, arrhythmia, angina and dyspnea are reported in approximately 40-60% of patients with FD [25, 78–81]. Arrhythmias and impaired heart rate variability arise from involvement of the sinus node, conduction system and imbalance between sympathetic and parasympathetic tone. Diastolic dysfunction and concentric left ventricular hypertrophy, which is typically non-obstructive, are important features, with men generally more severely affected than women. Myocardial ischemia and infarction may result from compromised function of the coronary vascular bed [82]. With age, progressive myocardial fibrosis develops with both intersticial and replacement fibrosis [21, 83]. Replacement fibrosis almost always starts in the posterior-lateral wall and in the mid-myocardium. In end-stage patients, transmural replacement fibrosis gradually reduces cardiac function to the stage of congestive heart failure [19, 84–86]. Malignant arrhythmias are responsible for a number of cardiac deaths in patients affected with FD [81, 86, 87].
Left ventricular structural changes
Left ventricular (LV) structural abnormalities are frequent in patients with FD and can be demonstrated using echocardiography (Figures 8 and 9) or cardiac MRI (Figure 10) [19, 78–80]. It is particularly important to measure the septum thickness since the posterior wall may become thinner with age due to replacement fibrosis. Concentric hypertrophy has been reported as the most common structural change [78]. Despite these structural changes, however, systolic function appears to be largely preserved when assessed with conventional measurements [19, 78–80, 84, 88]. The cardiomyopathy of FD is characterized by reduced myocardial contraction and relaxation tissue doppler velocities (Figures 11 and 12), sometimes detectable even before development of left ventricular hypertrophy (LVH). Tissue Doppler Imaging (TDI) can provide a preclinical diagnosis of Fabry cardiomyopathy [89, 90] and myocardial function can be quantified by ultrasonic strain rate imaging to assess radial and longitudinal myocardial deformation (Figures 11 and 12) [91].
Cardiac MRI for the assessment of left ventricular hypertrophy and fibrosis: A: Left ventricular hypertrophy in a 51-year-old male patient with cerebrovascular involvement and end stage renal disease (dialysis). B: Hypertrophic cardiomyopathy in a 56-year-old male patient with arrythmya, leukoareiosis and kidney transplant. C: Late enhancement after gadolinium in a 63-year-old female patient with end stage renal disease (dialysis).
Right ventricular structural changes
Right ventricular hypertrophy (RVH) with normal chamber size and preserved systolic but impaired diastolic function represents the typical right ventricular (RV) structural change in FD. When a detailed echocardiographic examination was performed in 58 patients with FD (mean age 40 ± 16 years), RVH was present in 40% of affected subjects with similar prevalence in both genders [92]. Two thirds of patients with LVH also exhibited RVH. RV dilatation was not present in any subject. RV diastolic dysfunction was present in 47% of 45 subjects in whom RV filling was assessed. RV diastolic dysfunction was associated with the presence of RVH. A significant correlation between RV wall thickness and age and left ventricular mass index was noted [92]. In another study, the degree of right ventricular involvement in FD was also related to the left ventricular cardiomyopathy stage [93]. RV involvement is common in FD and ultimately progresses to severe diastolic RV dysfunction. These findings might explain why patients with preserved left ventricule (LV) function can develop clinical features such as reduced exercise capacity, organomegaly and lymphoedema [94].
Electrocardiographic abnormalities
Electrocardiographic (ECG) changes in patients with FD are frequent and include voltage criteria and repolarization changes related to LVH and/or remodeling, ST segment depression and T-wave inversions [95]. Other abnormalities include a short PR interval (< 0.12 msec) [96] due to a short P wave, enlarged QRS complex and prolonged QTC intervals, intermittent supraventricular tachycardia [97], AV node blocks [98], bundle branch blocks [99] and arrhythmias (Figure 13) [19, 78–81]. 24-hour-ECG holter is therefore useful and recommended at baseline and during follow-up of enzyme replacement therapy (ERT) (Figure 14). The cardiac manifestations observed in patients with classic FD are also observed in patients with the cardiac variant of FD [28, 100].
Valvular involvement
Although previous work reported a high prevalence of mitral valve prolapse in Fabry patients [101], this finding was not confirmed by recent studies [80, 102].
Coronary involvement
The myocardial perfusion reserve was found to be significantly reduced in patients affected with FD [103]. Patients with FD have abnormal coronary microvascular function [82].
Exercise capacity
Exercise capacity is reduced in patients with FD compared with that predicted from normative population data [104, 105].
Autonomic dysfunction
Fabry patients have autonomic dysfunction but usually do not present clinically overt signs of orthostatic dysregulation [106].
Aortic root dilatation
FD is associated to an increased risk of developing aortic root dilatation in male patients [107]. Aortic root dilation was detected in 24% of 71 hemizygous male patients and was statistically associated with the presence of a dolicho-ectatic basilar artery (p = 0.008) (Germain DP, unpublished data) (Figures 15 and 16) [107].
D. Cerebrovascular lesions
The early peripheral neuropathic hallmarks of FD [38, 108, 109] are often followed by cerebrovascular complications and autonomic dysfunction in adulthood. Some of the most devastating neurological features of FD are caused by cerebrovascular lesions - the result of multifocal involvement of small blood vessels [110, 111]. Cerebrovascular involvement can lead to a wide variety of signs and symptoms, ranging from mild to severe, including headache, vertigo/dizziness, transient ischemic attacks, ischemic strokes (Figure 17) [111–113] and more rarely vascular dementia [114, 115]. Using data from the Fabry Registry®, the prevalence of strokes in FD was estimated to be 6.9% in males and 4.3% in females, much higher than in the general population. Median age at first stroke was 39 in men and 46 years in women and stroke may be the first manifestation of the disease [111]. There is a high prevalence of hypertension, cardiac disease and renal disease in patients who have had a stroke in the context of FD [111]. Data from both the Fabry Registry®[111] and the Fabry Outcome Survey® (FOS®) [110] have shown that the majority of strokes in FD are due to small vessel events. A dilative arteriopathy of the vertebrobasilar circulation has also been documented (Figure 18) [112, 116]. Thrombus formation may be enhanced in FD due to the adhesion of neutrophils and monocytes to endothelial cell walls [117] or to changes in the regional cerebral hyperperfusion [118–120]. Serum myeloperoxidase level has been found to predict the risk of a vasculopathy-related event in males affected with FD [121].
Imaging modalities that can be used to explore cerebrovascular involvement in Fabry patients include MRI [116], trans-cranial Doppler (TCD) [122], proton MR spectroscopy (MRS), positron emission tomography (PET) and diffusion tensor imaging [123]. White matter lesions may be single, multiple or confluent on MRI (Figure 19) [124, 125]. In addition, diffuse neuronal involvement, extending beyond the areas of MRI-visible cerebrovascular abnormalities has been found, and in such cases, 1H-MRS may be the preferred modality [126]. Cerebral MRI can reveal periventricular white-matter lesions, microbleeds (Figure 19), cortical grey-matter infarcts and deep lacunar infarcts in both grey and white matter [111, 127–130]. Some patients affected with FD have an aseptic meningitis [113, 131, 132]. Hyperintensity in the pulvinar on T1-weighted images is a common finding in FD, likely reflecting the presence of calcification [133, 134]. Recent findings suggest that the pulvinar sign is a highly specific sign, distinctively characteristic of FD [135], more frequent in male patients with cardiomyopathy and severe kidney involvement (Colas F, Carlier RY and Germain DP, unpublished data) (Figure 20).
Cerebral white matter hyperintensities, lacuna and microbleeds: A. Fluid-attenuated inversion recovery (FLAIR)-weighted axial MRI section showing multiple white matter lesions in the cerebral hemispheres in a 53-year-old male patient who had a Fazekas score of 9. B. Lacuna and microbleeds in the same patient. Courtesy: Dr Robert CARLIER and Dr Frédéric COLAS, CHU Raymond Poincaré, Garches, France.
The pulvinar sign: T1-weighted sagital (A) and axial (B) MRI sections showing the pulvinar sign in a 66 year-old male patient. T1-weighted sagital (C) and axial (D) MRI section showing symmetrical high signals in the pulvinar region in a 42-year-old male patient. Courtesy: Dr Robert CARLIER and Dr Frédéric COLAS, CHU Raymond Poincaré, Garches, France.
In a pilot study, head MRI was performed in a cohort of 44 consecutive hemizygous male patients and 7 heterozygous females affected with FD. Chiari type I malformation was identified in 6 individuals (3 males and 3 females) [136]. Whether the association is coincidental or not, does need further studies but Chiari malformation may explain the episodes of headache frequently encountered in FD and should be ruled out in all Fabry patients [136].
Comprehensive neurological evaluation is essential before the institution of ERT, to assess disease extent and severity. Frequency and severity of pain should be assessed using tools such as the Brief Pain Inventory (BPI) or the McGill Pain Inventory. Clinical investigations include brain imaging by MRI with T1, T2 and FLAIR-weighted images and magnetic resonance angiography (MRA) may be indicated to exclude cerebral vasculopathy. Laboratory evaluation of comorbid stroke risk factors may identify patients with significantly elevated homocysteine, with a vitamin deficiency state, or with other genetic prothrombotic risk factors [51].
E. Auditory and vestibular abnormalities
Auditory and vestibular abnormalities are frequent deficits observed in FD, resulting in a range of symptoms, such as hearing loss [137, 138], tinnitus and vertigo [137, 139]. The high incidence of both progressive hearing loss and sudden deafness in male patients affected with classic FD has been demonstrated (Figure 21) [137]. A correlation of neuropathic and vascular damage with hearing loss was found in males in whom residual α-galactosidase A activity appears to have a protective effect against hearing loss [140]. Progressive vestibular loss was found in 80% of males and 77% of females when assessed with head impulse testing [141].
Hypoacousia in patients affected with Fabry disease: A. Hypoacousia in a 39-year-old male with hypertrophic cardiomyopathy, cerebral lacuna and kidney transplant. B. Sudden deafness of the left ear and bilateral hypoacousia in a 54-year-old male patient with tinnitus, vertigo, vertebro-basilar TIA, hypertrophic cardiomyopathy and kidney transplant. Courtesy: Dr Philippe AUBERT and Dr Karelle BENISTAN, CHU Raymond Poincaré, Garches, France.
F. Ocular manifestations
Corneal opacities (visible by slit-lamp microscopy) are the most common and early of ocular signs, occurring in almost all hemizygous males (Figure 22) [142–144]. It should be noted, however, that treatment with amiodarone or chloroquine can produce similar ophthalmological signs [145]. Mild to marked tortuosity of the conjunctival and retinal vessels is also observed in patients with FD [142, 143]. Neither corneal dystrophy nor retinal/conjunctival lesions impair visual acuity; however, acute visual loss caused by unilateral occlusion of the central retinal artery has been reported [146]. Anterior and posterior subcapsular cataracts are also observed, the latter also being termed the 'Fabry cataract' in that it represents a pathognomonic ocular sign of FD. More recently, an enlargement of the blind spot (Figure 23) was reported in 38.7% (n = 27) of patients, although this was not associated with any defects in colour vision [142].
G. Respiratory involvement
Respiratory involvement, manifesting as dyspnea with exercise, chronic cough and wheezing, is frequent in both genders with FD [147, 148]. A recent study has found the prevalence of airway obstruction in FD to be 26% in women and 61% in men [149, 150]. A clinically relevant age- and gender-dependent progressive pulmonary involvement in FD patients has been demonstrated [150] and the effects of ERT on pulmonary involvement are currently being investigated. Recently, ERT was shown to stabilize obstructive pulmonary FD associated with respiratory Gb3 storage in one heterozygous female [151].
In another study, 39 patients with a diagnosis of FD underwent pulmonary function testing (spirometry), and a non-invasive cardiopulmonary exercise test. A control group was selected for comparison. Eighteen of the 39 Fabry patients (46%) exhibited a significant decrease in diastolic blood pressure (DBP) during exercise. The drop in DBP was evident in 9 of the 24 female patients (38%). None of the control patients had a significant drop in DBP during exercise. The finding of a significant decrease in DBP in patients with FD may explain deficits in exercise tolerance [104].
H. Skeletal involvement
In a recent study, bone mineral density of the lumbar spine and the femoral neck was assessed by dual-energy X-ray absorptiometry (DEXA) in 23 hemizygous male patients with a mean age of 31 years (range: 16-60 years) affected with classic FD. Using the World Health Organization classification, 20 of the 23 patients (88%) with FD had either osteopenia (n =11) or osteoporosis (n = 9) at one or both sites (Figure 24) [152, 153]. Skeletal involvement has been subsequently confirmed in a larger cohort of 53 patients in which osteopenia was present in approximately 50% of cases [154]. Cases of severe osteoporosis with spontaneous lumbar fractures have recently been described (Figure 25) [155]. Patients suffering from Fabry disease should follow current recommendations regarding identification and treatment of vitamin D deficiency.
Dual-energy X-ray absorptiometry (DEXA) assessment of bone mineral density of the femoral neck (A) and the lumbar spine (B): T scores of - 4.2 and - 4.3 were found at the hip (A) and lumbar spine (B), respectively in a 53 year-old male patient affected with Fabry disease. Courtesy: Dr Caroline LEBRETON, CHU Raymond Poincaré, Garches, France.
Bone magnetic resonance imaging in a Fabry patient with severe osteoporosis: A (STIR, sagittal view) and B (T1, sagital median): several vertebral body fractures are seen, without signal anomaly in T1 or T2 in favor of ancient fractures. A mild spondylolisthesis of L5 on S1 can be observed. C (T2, axial view): fracture of the right pedicula of L5 (arrow) in a 72-year-old patient with severe osteoporosis. Courtesy: Dr Robert CARLIER, CHU Raymond Poincaré, Garches, France.
I. Depression and quality of life
Depression is a frequent and under-reported problem in patients with FD [156]. As many as 46% and 28% of patients may have depression and severe clinical depression, respectively [47]. Most patients identified in recent surveys were undiagnosed for depression, which underscores the need for the correct assessment of depressive symptoms in patients with FD. Since this is an under-recognized problem, the benefits of treatment are unknown. Depression can seriously impact quality of life in patients with FD. Reduction in quality of life has been demonstrated using a variety of questionnaires including the SF-36, EuroQoL and MMPI-2 [46, 157–159]. Psychiatric and neuro-psychological evaluations have been recommended in the assessment of patients with FD [160, 161].
J. Miscellaneous
Anemia
Data from the FOS® and the Fabry Registry® show that mild peripheral blood cytopenias, particularly anemia [162], are prevalent among patients with FD [163].
Arterial remodeling and intima-media thickening
Large artery phenotype (arterial wall structure and function) was non-invasively investigated in 21 hemizygous patients with FD and 24 age-matched male controls. Common carotid and radial artery diameter, intima-media thickness (IMT) and distensibility were determined with high-definition echotracking systems and aplanation tonometry. Patients with FD had a significant twofold increase in radial artery IMT and distensibility, independent of body surface area, age and mean blood pressure. Radial artery IMT increased significantly with age in each group. However, the slope was 2.3-fold higher in FD patients than in controls (p < 0.001). Common carotid artery (CCA) IMT was mildly but significantly increased in patients with FD (+18%), whereas distensibility was unchanged [164, 165].
Another study presented evidence of a major increase in CCA IMT, both in hemizygous and heterozygous patients with FD, in the absence of focal atherosclerotic plaques [166]. The authors examined the possible correlation between left ventricular hypertrophy and IMT of the common carotid artery. Thirty male and 38 female patients were enrolled. LVH was found in 60% of men and 39% of women. Increased CCA IMT was equally present in males and females. LVH and CCA IMT occurred concomitantly in FD suggesting common pathogenesis. The underlying cause may be a circulating growth-promoting factor whose presence has been confirmed in vitro[167].
Azoospermia
Testicular biopsies performed in two infertile men suffering from FD with azoospermia revealed characteristic aspects of trihexoside ceramide (Gb3) deposits in Leydig cells by optical and electronic microscopic analysis [168].
Facial dysmorphism
Although facial dysmorphism is not a prominent sign in FD, minor facial abnormalities have been previously reported. By analysing three-dimensional images of faces, facial dysmorphology was quantified in a cohort of both males and females affected with FD. Morphometric analysis of different regions of the face revealed significant differences in face shape in male patients and to a lesser extent in female patients. In male patients, the most prominent abnormalities were located in the peri-orbital region. Pattern recognition techniques achieved a discrimination accuracy of up to 85% for male patients compared with healthy controls. The discrimination accuracy in female patients only reached 67% [169].
Hypothyroidism
In a small study, subclinical hypothyroidism (normal serum free thyroxine concentrations along with elevated serum TSH levels) was found in 4 of 11 patients (36.4%) who were investigated [170]. An endocrine work-up should be recommended in all patients suffering from FD [171].
Lymphoedema
Lymphoedema, already mentionned in one of the original papers on FD [1], has since been observed in a number of patients [58] and linked to structural and functional changes of the lymphatic microvessels of the skin [172].
Parapelvic kidney cysts
Twenty-four patients who were enrolled in an enzyme replacement trial underwent prospective renal imaging evaluation with kidney MRI and computed tomography (CT). Nineteen age-matched healthy controls were concurrently enrolled in this cross-sectionnal, case-control study. The presence and localization of kidney cysts as well as the ratio of the signal intensity between medulla and cortex were determined. Fifty percent of FD patients had renal sinus cysts, compared to one individual (7%) in the control group. The cause of such cysts in FD remains to be elaborated [173] but they may contribute to earlier recognition of the disease [174].
Priapism
Cases of priapism have been observed in young boys affected with FD. Conventional treatment with cavernovenous shunting was only partly successful in one case, and percutaneous gelfoam embolization of the internal pudendal artery may prove a better option [175]. Additional cases of priapism associated with FD were identified through a search of the literature [176].
K. Heterozygous females
Traditionally, it was considered that heterozygotes did not develop symptoms and heterozygous females were erroneously described as "carriers of the defective gene" who were more or less safeguarded against developing disease symptoms. However, an increasing number of publications and evolving knowledge about the natural course of disease indicate that the term X-linked recessive should probably be discontinued and FD simply described as following "X-linked inheritance" [177, 178].
Clinical signs and symptoms vary widely in heterozygous females. This phenotypic heterogeneity is thought to be partly due to lyonization [179], a process whereby one copy of the X-chromosome is randomly inactivated in all cells of the female embryo, so that heterozygous females are essentially a 'mosaic' of normal and mutant cells in varying proportions. In X-linked diseases, heterozygous females may be symptomatic, probably as a consequence of skewed X-chromosome inactivation, which results in a higher percentage of the × chromosome bearing the mutant gene being expressed in the particular tissue of importance. Such variability in symptom severity is characteristic of X-linked heterozygotes [180] and should be kept in mind when assessing and diagnosing potential patients.
The clinical spectrum in females ranges from a seemingly asymptomatic disease course occasionally observed to the "classic" severe phenotype observed in males, with a variety of clinical presentations in between [24, 26, 181–183]. Heterozygotes may display all symptoms of the disease including pain [184], orthostatic hypotension [185], angiokeratoma [53], ocular abnormalities [186], cochleovestibular involvement [51, 139], gastrointestinal symptoms [50] and respiratory involvement [150]. A high percentage of females develop vital organ damage involving the heart [26, 78, 79, 96, 187, 188], brain [111, 129, 189–191] and, more rarely, kidneys [26, 32, 73, 76, 186] about a decade later than males [24, 184]. Of the 1077 enrolled females in the Fabry Registry®, 69.4% had symptoms and signs of FD. The median age at symptom onset among females was 13 years, and twenty percent experienced major cerebrovascular, cardiac, or renal events, at a median age of 46 years [24].
In a retrospective chart review of 279 affected males and 168 females suffering from Fabry disease, the mean rate of estimated glomerular filtration rate (eGFR) decline for patients was -1.02 ml/min/1.73 m2/year for females as compared to -2.93 ml/min/1.73 m2/year for males and advanced Fabry nephropathy was less prevalent and occurred later among females than males [25].
Altogether, females with FD have a significant risk for major organ involvement and decreased quality of life [158], and should be regularly monitored for signs and symptoms of FD [24, 51].
L. Atypical variants
FD has long been regarded as a full-blown multisystemic disease with most, if not all, affected males developing a "classic" phenotype. Later on, the sub-classifications "cardiac variant" [29] and "renal variant" [30] were introduced for patients with predominant cardiac or renal involvement, respectively. In high-risk adult populations, screening efforts have been shown to be effective in diagnosing Fabry patients among individuals with end-stage renal disease [30, 192, 193], unexplained cardiac hypertrophy [194–196] or strokes in young people with no apparent predisposing factors [197–200]. Screening of patients with atherosclerosis [201] or ophthalmological screening [202] may be of less value.
Atypical variants have few or none of the hallmark symptoms of classical FD, but have manifestations confined predominantly to one organ system [28, 100]. Presenting much later in life (fourth to sixth decades) than patients with classical disease, they are often identified serendipitously. In contrast to their classically affected counterparts, atypical variants have residual α-galactosidase A activity that varies between 2 and 20% of normal [35, 203, 204].
Cardiac variant
The cardiac variant - the most widely reported atypical variant - presents with cardiac manifestations in the absence of overt systemic involvement [28, 29, 100]. Manifestations include cardiomegaly, electrocardiographic abnormalities consistent with cardiomyopathy, non-obstructive hypertrophic cardiomyopathy and myocardial infarctions; mild proteinuria may also be detected.
The cardiac variant was initially thought to be rare, but a Japanese study of 1603 males undergoing routine echocardiography found that 7 (3%) of 230 patients with left ventricular hypertrophy had clinically unsuspected FD [29]. Furthermore, recent reports suggested that FD should also be considered in all cases of unexplained homogeneous hypertrophic cardiomyopathy [194–196]. In a British study, 6 of 153 males (4%) consecutively referred with hypertrophic cardiomyopathy were found to have α-galactosidase A levels diagnostic of FD [194]. In a Spanish study, 0.9% of males and 1.1% of females with hypertrophic cardiomyopathy were diagnosed with FD [195].
Renal variant
There are also reports of hemizygous males with disease manifestations confined to the kidney. Renal variants have been identified among Japanese chronic dialysis patients whose end-stage renal disease had been misdiagnosed as chronic glomerulonephritis [30]. The patients had absent or low α-galactosidase A activity, and were, subsequently, found to have GLA gene mutations [30]. These findings suggest that cases of FD may be underdiagnosed among renal dialysis [192] and transplant [205] patients. Their early detection is important since these patients may later develop vascular disease of the heart or brain. However, a much lower prevalence of FD (0.22%) was found in both a Dutch [206] and another Japanese [193] study performed in similar high-risk groups of hemodialyzed patients.
Intermediate variant
Presentation and clinical course can vary within the aforementioned phenotypes, and an intermediate phenotype has been described in which patients, in the absence of cardinal signs of FD in childhood, presented with a cardiac variant with hypertrophic cardiomyopathy and arrhythmia around age 40 but subsequently progressed to end-stage kidney failure [207].
V - Etiology
A. Genetics
FD is transmitted as an X-linked trait. Contrary to the misconception that females will be marginally affected given the X-chromosome linked inheritance pattern, many heterozygotes will develop early symptoms and, later on, vital organ involvement [24, 26, 182]. The use of the term X-linked 'recessive' is therefore misleading and should be discontinued and FD described as following X-linked inheritance [177, 208].
B. Gene location
Lysosomal α-galactosidase A (EC 3.2.1.22) is coded by a unique gene, GLA, whose locus is situated on the long arm of chromosome X, in position Xq22. The GLA gene consists of seven exons distributed over 12,436 base pairs (bp). There is extensive allelic heterogeneity, but no genetic locus heterogeneity.
C. Molecular pathology
FD can be caused by a variety of missense or nonsense point mutations, splicing mutations, small deletions or insertions [203, 204, 209–236], and large deletions [237, 238]. The defects in the GLA gene encoding α-galactosidase A are heterogeneous with over 585 mutations recorded [239, 240]; the majority of these mutations render the enzyme non-functional [239]. Most families have unique mutations potentially explaining the marked variability in the residual enzyme activity but only in part the natural course of the disease since intra-familial variability does exist. Novel α-galactosidase A mutations have been recently identified by our research group [e.g. p.Met42Arg (c.125T > G) (Figure 26), p.Gly43Ser (c.127G > A), p.Gly132Glu (c.395G > A), p.Lys168Asn (c.504A > C), p.Gln212Stop (c.634C > T), p.Phe295Cys (c.884T > G) (Figure 26), p.Leu300Pro (c.899T > C), and p.Gly328Glu (c.983G > A), D.P. Germain, unpublished data]. Non pathological single nucleotide polymorphisms such as c.-30G > A, c.-12G > A, and c.-10C > T in the 5' untranslated region (5'UTR), p.Asp313Tyr in exon 6 [241] and other sequence variations (VNTR) have been described [239, 242, 243]. Whether some published sequence changes, such as p.Arg112His, are true mutations or polymorphisms is still a matter of debate [244].
Genotyping of the GLA gene in heterozygous females: A. Patient CB, a 17-year-old girl, was shown to carry a T to G transversion in exon 6 at position 884 in the cDNA sequence. This nucleotide substitution alters the codon (TT C) for phenylalanine to the codon (TG C) for cysteine at position 295 of the α-galactosidase A protein (p.Phe295Cys). B. Patient ZB, a 46-year-old woman, was shown to carry a T to G transversion in exon 1 at position 125 in the cDNA sequence. This nucleotide substitution alters the codon (ATG) for methionine to the codon (AGG) for arginine at position 42 of the α-galactosidase A protein (p.Met42Arg). C. Patient NL, a 63-year-old woman was shown to carry a G to T transversion in exon 6 at position 982 in the cDNA sequence. This nucleotide substitution alters the codon (GGG) for glycine to the codon (TGG) for tryptophan at position 328 of the α-galactosidase A protein (p.Gly328Trp). Despite scanning of the rest of the gene, no other sequence abnormality was found. Courtesy: Pr Xavier JEUNEMAITRE and Dr Anne-Laure FAURET, HEGP, Paris, France.
D. Structure of human α-Galactosidase A
The three-dimensional structure of human α-galactosidase A was determined by x-ray crystallography. The crystal structure showed a homodimeric molecule with each monomer containing two domains. The N-terminal domain is a classic (β/α)8 barrel, and the C-terminal domain contains eight antiparallel β strands packed into a β sandwich. Residues 32-328 comprise the N-terminal domain, and residues 329-421 fold into the C-terminal antiparallel domain. The N-terminal domain contains the active site, which is located at the C-terminal end of β strands β 1-β 7, near the center of the β barrel. Three N-linked carbohydrates are found on the surface of the molecule, away from the location of the active site and away from the dimer interface. The carbohydrate residues attach to aspartic acid residues N139, N192 and N215 and extend from the surface of the molecule [245]. The enzyme folds into a three dimensional fold that gathers 15 residues into an active site configuration specific for α-galactosides. The active site is formed from side chain residues of W47, D92, D93, Y134, C142, K168, D170, C172, E203, L206, Y207, R227, D231, D266, and M267. Residues C142 and C172 make a disulfide bond. The two active sites in the dimer are separated by approximately 50 Å [245]. The α-galactosidase A enzyme uses a double displacement reaction mechanism, where two consecutive nucleophilic attacks on the anomeric carbon of the substrate lead to breakage of the glycosidic linkage with overall retention of the anomer of the product. In human α-galactosidase A, the catalytic nucleophile is D170 and the catalytic acid/base is D231 [246].
VI - Diagnosis
Early onset of FD signs and symptoms warrant prompt diagnosis, particularly because ERT is available. However, recognizing the early manifestations in clinical practice may be challenging due to a variety of reasons. The disease presentation is generally heterogeneous, symptoms may resemble more common diseases, and major renal or cardiac dysfunction is uncommon in pediatric patients. Nowadays, diagnostic delays may still be considerable and patients often have to visit several medical specialists before a correct diagnosis is made. Recent data showed that the overall diagnostic delays were ~15 years for both genders [24]. If clinical examination raises a suspicion of FD, appropriate biochemical and/or genetic confirmation is needed [247].
A. Biochemical diagnosis
Enzymatic assay
The demonstration of a deficient activity of α-galactosidase activity in plasma or leukocytes is the reference laboratory method which should systematically be used to confirm the clinical diagnosis of FD in males in whom the result will be conclusive [248]. Plasma assay may occasionally lead to false diagnosis and should be confirmed by a leukocyte assay [249]. In contrast, affected girls and adult females may have their enzyme activity falling within the normal range [250]. Therefore, all females should have their status determined by genotyping (analysis of the GLA gene mutation) [208].
A fluorimetric method that uses filter paper cards containing dried blood spots instead of the leukocyte pellet as the enzyme source was recently introduced for enzymatic diagnosis, allowing storage of the samples for up to 6 months due to stability of the enzyme [251–255].
Globotriaosylceramide measurement
Plasma Gb3 has also been proposed and used in the biochemical diagnosis of FD, but this method is time-consuming and, in females, plasma Gb3 levels are generally lower than in males and usually in the normal range [256].
Urinary Gb3 is a more reliable marker allowing diagnosis in the majority of both male and female patients [257–260]. However urinary Gb3 is not elevated in some patients with late-onset variants and/or particular mutations in the GLA gene (p.Asn215Ser) [261–263].
The analysis of tissue glycolipid composition [264] and the use of atmospheric pressure photoionization mass spectrometry (APPI-MS) for the analysis of Gb3 molecular species [265] and MALDI-TOF imaging of biomarkers [266] are not done routinely and are confined to research laboratories.
B. Genotyping
In female heterozygotes, a-galactosidase activity may be within the normal range [250, 252] and therefore, the definitive diagnostic confirmation should be made by genetic analysis in suspected cases (Figure 26). The publication of the complementary (cDNA) [267] and genomic DNA [268] sequences of the GLA gene (Genbank X14448) has paved the way towards understanding of the molecular basis of FD. Direct molecular analysis is easy because of the small size of the gene and allows the precise characterization of the mutation of the GLA gene. A method that uses filter paper cards containing dried blood spots instead of the leukocytes pellet as the source of DNA was recently developed for sequencing, allowing genotyping from a dried blood spot on filter paper to confirm enzymatic diagnosis (Figure 27) [196].
Sequencing of PCR products obtained from amplification of DNA directly eluted from a 3-mm punch of dried blood spot (DBS) on filter paper: a 60-year-old man with left ventricular hypertrophy of unknown origin was enrolled in a screening protocol for FD. Markedly decreased α-galactosidase activity was found on DBS. Using a second DBS, the patient was subsequently shown to carry a T to C transition in exon 2 at position 337 in the cDNA sequence of the GLA gene (c.337T > C). This nucleotide substitution alters the codon (T TT) for phenylalanine to the codon (C TT) for leucine at position 113 of the α-galactosidase A protein (p.Phe113Leu). Pr Dominique GERMAIN, University of Versailles - St Quentin en Yvelines (UVSQ), Versailles, France
Denaturing high-performance liquid chromatography (DHPLC) has been shown to be useful as a screening method [269]. Since direct sequencing limited to exons may miss deletions, the use of Multiplex Ligation-dependent Probe Amplification (MLPA) has been recommended in cases where a decreased enzyme activity is not associated with the identification of a pathogenic point mutation [270].
C. Screening
Screening individuals with a family history of FD or newborn screening programs are the only practical ways of identifying patients before the development of symptoms. Moreover, screening of patients in high risk groups who may be exhibiting late-onset symptoms of FD but who have not been diagnosed may be key in optimizing the management of disease in these patients.
Any screening requires a reliable and preferably rapid and low-cost method. Measurement of the accumulated urinary Gb3 has been proposed [258], but its reliability as a biomarker of FD, particularly in females, is unproven [262, 271]. Screening of at-risk groups is often conducted by measuring plasma a-galactosidase A activity, but clinicians should be aware that this can fail to detect all cases of FD [272]. Identification of the deficient enzyme activity in dried blood spots (DBS) may be a more reliable method of screening for FD and this approach has been validated in males [198, 250–252, 273, 274] but fails to detect about one third of heterozygous females [250, 252, 253].
D. Histology
Light microscopy
The observation of biopsies with light microscopy does not usually contribute a great deal to diagnosis but lipid staining of kidney biopsies can reveal storage cells within glomeruli and, when electron microscopy (EM) is not being done or not available, semi-thin sections stained with toluidine blue or Masson's trichrome can allow diagnosis (Figures 3 and 4). However, given the number of false negatives and the non specificity of the results, this invasive procedure should not be used for diagnostic purpose.
Electron microscopy
Ultrastructural studies of endomyocardial and kidney biopsies can reveal lysosomal storage in cardiomyocytes or in a variety of kidney cellular types, respectively. The ultrastructural appearance of the inclusions is of whorled layers of alternating dense and pale material ('zebra bodies' or myelin figures) (Figures 5, 6 and 7). However, due to the invasive nature of the procedure and the availability of reliable biochemical or molecular methods, these procedures should be considered only in the rare instances where there is residual α-galactosidase A activity in males or doubts on the causality of a DNA sequence change in females. Skin biopsy observed by EM may be a useful additional diagnostic test when carefully interpreted by an expert pathologist [275]. However, acquired metabolic disorders, such as the one induced by chloroquine therapy, may result in storage of ultrastructurally similar inclusions in many of the same cells as FD, leading to erroneous interpretation [276]. In addition, skin biopsies are often normal in heterozygous females and therefore not of great utility.
E. Ancillary markers
Although laboratory tests are usually normal, anemia [162], hyperhomocysteinemia [277], raised HDL cholesterol [278] and elevated Lp(a) (Germain DP, unpublished data) have been reported in a number of patients with FD. Urinary sediment examination can reveal casts, erythrocytes and cells containing accumulated Gb3. Elevated serum levels of B natriuretic peptide (BNP) and troponin IC have been found in patients with advanced left ventricular hypertrophy (Germain DP, unpublished data). 25(OH) vitamin D levels should be investigated in all patients suffering from FD since vitamin D deficiency is found in about 40% of them in France (Germain DP, unpublished data).
F. Biomarkers
One of the most urgent research needs is for (a) reliable and validated biomarker(s) with which to assess disease progression and treatment response. Ideally, measurement of such (a) surrogate marker(s) would involve non-invasive testing. Although various imaging techniques have shown promising results, the clinical relevance of what they reveal in patients with FD has yet to be evaluated for its correlation with clinical endpoints. There is currently no proper plasma or urinary biomarker for FD.
Mildly elevated plasma chitotriosidase levels have been reported in male patients but not in heterozygous females [279].
Globotriaosylsphingosine or lyso-Gb3 has been reported to be elevated in FD patients. This analyte is elevated in the plasma of hemizygous males and to a lesser extent in that of adult females with classical FD and lyso-Gb3 appears interesting to monitor enzyme replacement therapy [244, 280]. Lyso-Gb3 was shown to be an independent risk factor for the development of cerebrovascular white matter lesions in male patients with FD while, in females, plasma lyso-Gb3 concentration correlated with overall disease severity [281].
Lyso-Gb3 could be a potential biomarker since plasma lyso-Gb3 level in Fabry patients who had received ERT was shown to be elevated at baseline and to fall more dramatically on ERT than that of Gb3[282]. Urinary lyso-Gb3 may also prove a potential biomarker [283]. Lyso-Gb3 may have a role in glomerular injury in FD by promoting the release of secondary mediators of glomerular injury (Transforming growth factor-beta1 (TGF-β 1) and the macrophage inhibitory factor receptor CD74) common to diabetic nephropathy [284].
Sphingosine-1-phosphate (S1P) was recently identified as a biologically active growth-promoting factor involved in cardiovascular remodelling in both males and females with FD [285]. Male patients had significantly higher plasma S1P levels compared with healthy controls. Moreover, there was a strong correlation between plasma S1P levels and LVM index, and increased common carotide artery IMT in patients with FD [285]. Sphingosine-1 phosphate has been shown to induce in vitro vascular smooth muscle cells proliferation by a variety of signal transduction pathways [285].
In the interest of future research, biobanking of plasma, serum and urine samples remains highly recommended in all patients affected with FD prior to initiation of ERT.
VII - Differential diagnosis
In childhood, other possible causes of pain such as rheumatoid arthritis [286], rheumatic fever, systemic lupus erythematosus, Raynaud's disease, and 'growing pains' (a frequent misdiagnosis in children with FD) must be ruled out. In adulthood, celiac disease and multiple sclerosis [287] are the most often-cited differential diagnoses particularly in females. Similarly, when no mutation of the GLA gene has been identified, the possiblity of a phenocopy mimicking FD, should be considered [288].
Finally, whether a combination of several single nucleotide polymorphisms (SNPs) in the GLA gene leading to decreased but residual α-galactosidase activity may be a risk factor and predispose to hypertrophic cardiomyopathy and/or ischemic stroke, when combined with additional environmental or genetic factors, is unknown and warrants further studies.
VIII - Genetic counseling
In contrast to the vast majority of lysosomal storage disorders, which are inherited in an autosomal recessive manner, FD, together with mucopolysaccharidosis type II (Hunter syndrome) and Danon disease (LAMP2 deficiency), is inherited as an X-linked trait [208]. Consequently, there is no male-to-male transmission of FD, but affected fathers will pass the defective gene to all their daughters, while heterozygous females have a 50% risk with each conception of transmitting the gene; sons who inherit the mutant gene from their mother will have the disease, while daughters will be heterozygotes who may or may not develop disease manifestations.
Once the diagnosis has been confirmed, the opinion of a geneticist should be sought and family screening carried out [289]. Pedigree analysis and effective screening of the family of a diagnosed (adult) patient is likely to result in identification of several previously unrecognized affected family members, including young relatives at a relatively early stage of their disease [208, 290]. This provides the opportunity to offer genetic counseling and timely therapeutic intervention [290]. Appropriate family support should be provided which may be achieved through the help of patients' associations (Appendix).
IX - Prenatal diagnosis
Biochemical or molecular prenatal diagnosis of FD is technically feasible by determination of α-gal A activity in direct and/or cultured chorionic villi at 10 weeks of pregnancy or in cultured amniotic cells at about 14 weeks of pregnancy, respectively. Determination of fetal sex using maternal blood at 9-11 weeks of pregnancy is occasionnaly used. Genetic counseling prior to prenatal diagnosis should be provided to discuss the options and risks since intra-familial phenotype variations, existence of atypical late-onset variants and recent availability of a specific therapy have singularly complicated genetic counseling and prenatal diagnosis. For ethical reasons, prenatal diagnosis of FD has always been controversial for female fetuses and has now become questionable even for males fetuses since the advent of ERT. There is limited experience with preimplantation diagnosis of FD, but the diagnosis has been performed successfully (no reports in the literature) [291].
X - Management
FD is a paradigm of a multi-system condition and symptoms express themselves in many organs [25, 51, 292, 293]. Maximal, comprehensive therapy for FD includes ERT [294–298], conventional medical treatment [51] and adjunctive therapies [181, 299, 300].
A. Conventional medical treatment and adjunctive therapies for Fabry disease related morbidities
Supportive care is important. The effective management of FD requires a multidisciplinary approach [301]. Symptom management in patients may consist of lifestyle modifications and prophylactic medications [51, 299].
Pain
Patients with neuropathic pain may benefit from avoidance of circumstances triggering acute pain attacks, e.g. significant physical exertion and temperature changes. The neuropathic pain associated with FD can be managed with analgesics, but nonsteroidal anti-inflammatory drugs are generally ineffective (and potentially harmful for kidney function) while narcotic analgesics should be avoided [292] although this has been debated [302]. Carbamazepine [303, 304], oxcarbazepin, gabapentin [299, 305], pregabalin and phenytoin [306] are classically used to manage pain in FD (Table 3) [51, 299]. Some patients use illicit drugs, particularly marijuana for pain control and GI manifestations, especially if their symptoms have been overlooked by doctors.
Gastrointestinal symptoms
Gastrointestinal problems resulting from delayed gastric emptying and slow bowel movements may respond to metoclopramide [307] and changes in eating habits, e.g. small and frequent meals. Some success has been achieved by managing dyspepsia with H-2 blockers [51].
Skin symptoms
Laser methods to treat angiokeratomas have not shown good results in FD and are not able to prevent the formation of new lesions [57].
Cochleo-vestibular symptoms
Moderate hearing loss can be managed with hearing aids while profound deafness requires cochlear implants [51, 137]. Vertigo-related nausea can be addressed with trimethobenzamide or prochlorperazine [51].
Renal function
FD is often associated with proteinuric chronic kidney disease, and it appears that the treatment paradigms that have proven to be effective in diabetes mellitus and other forms of proteinuric renal disease are also effective in FD [308]. The use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) is useful in patients with proteinuria (Table 3) [299]. Furthermore, these agents may help to control hypertension when present. Indeed, severe proteinuria does not respond to ERT alone [309], but carefully titrated ACEi/ARB therapy may be effective in lowering proteinuria [299, 310, 311]. In a pilot study, sustained reductions in proteinuria with stabilization of kidney function were achieved in a small number of patients with severe Fabry nephropathy receiving a combination of agalsidase beta at 1 mg/kg every other week (EOW) and ACEi/ARB therapy [312].
Although FD represents an interesting example of progressive proteinuric renal disease in which the usual blood pressure is lower than in other renal diseases, hypertension can occur and, if present [313], should be treated appropriately. Many patients with FD and renal involvement will require dialysis [314] and/or renal transplant [315, 316]. Transplanted kidneys remain free of Gb3 accumulation and 5-year organ survival is above average for renal transplants [315–318].
Cerebrovascular involvement
The use of enteric coated aspirin for prophylaxis to minimize the risk of stroke is recommended in guidelines proposed by clinical experts [51]. Clopidrogel will be considered if aspirin is not tolerated and a combination of both drugs may be proposed in case of stroke or transient ischemic attack. Coumadin is often given to patients who have had stroke on aspirin and clopidrogel. Adequate intake of vitamins B12, B9, and B6 should be promoted [51] especially in case of hyperhomocysteinemia [277]. Statins may have potential beneficial effects [319].
Cardiac involvement
In the case of exertion chest pain, conventional anti-anginal therapy should be administered (calcium channel blockers that do not limit heart rate may be preferred to β-blockers as the later can aggravate both sinus bradycardia and the fact that some patients have a propensity to develop atrioventricular [AV] block). Beta-blockers are not necessarily contraindicated but should be used cautiously. Aspirin can be prescribed in case of isolated left atrial enlargement and warfarin treatment should be offered to any patient affected with FD and atrial fibrillation. Cardiac pacing or implantation of cardioverter defibrillator (ICD) devices is increasingly used in patients with FD with AV block or to prevent sudden cardiac death due to sustained ventricular tachycardia and malignant arrhythmia [81]. Amiodarone interferes with lysosome metabolism and should therefore probably be avoided during enzyme replacement. If there is evidence of heart failure, ACEi, ARBs or diuretics should be preferred to β-blockers because of the aforementioned caveats [88]. In patients with advanced congestive heart failure, heart transplantation is an option [207, 320]. Vitamin D levels and lipid profil should be controlled and, if anormal, normalized, using both diet and statins for the later (Table 3).
Respiratory involvement
Cessation of smoking should be encouraged [299].
Endocrine dysfunction
Adequate monitoring of endocrine glands and hormonal therapy, when required, have to be performed in cases of subclinical endocrine dysfunction [171].
Bone involvement
Although no data exist, the use of biphosphonate therapy is currently being investigated. Vitamin D insufficiency or deficiency should also be corrected.
Psychological aspects
Psychological support should be provided. Anxiety and depression should be treated [159, 161].
B. Prophylactic measures
Patients should be advised to carry with them a letter and/or an emergency healthcare card (Figure 28) indicating the nature of their illness, the complications to which they are at risk, their current medication and the contact details of a medical practitioner. Intense physical activity and excessive sun exposure are inadvisable. Various medications such as chloroquine or amiodarone interfere with lysosome metabolism and their prescription is contraindicated in the license of recombinant α-galactosidase A (agalsidase alfa and agalsidase beta), and should therefore be avoided during enzyme replacement.
Emergency healthcare card from the French Ministry of Health: an emergency healthcare card was created by the Ministry of Health, the center of excellence for Fabry disease and patients' associations for Fabry disease or lysosomal storage diseases. The card is made of two parts: one of which contains general data on FD while the second one includes the personal medical history and medications of the patient in order to provide useful information for emergency care situations.
XI - Enzyme replacement therapy
Conventional treatment does not address the underlying defect of FD and the year 2001 witnessed the introduction of ERT using recombinant human α-galactosidase A. Since then, long term safety and efficacy of replacement therapy have been investigated and ERT has been validated as a disease-specific therapeutic agent for patients affected with FD but with this, has come the realization that numerous aspects have yet to be explored and understood. As an example, current guidelines for starting ERT in patients vary from one country to another and remain a matter of debate especially in heterozygous females and children. Current expert recommendations [51] are presented in Table 4, but may evolve in the future. In Europe, there are currently two commercially available enzyme preparations for FD [321, 322]: agalsidase alfa (Replagal®; Shire, Cambridge, MA, USA), produced using cultured human skin fibroblasts and registered for use at a dose of 0.2 mg/kg biweekly, and agalsidase beta (Fabrazyme®; Genzyme Corp, Cambridge, MA, USA), produced by the expression of human α-galactosidase cDNA in Chinese Hamster Ovary (CHO) cells and registered for a use at 1.0 mg/kg biweekly. The safety and efficacy of both enzymes have been assessed in randomized, double-blind, placebo-controlled trials [323–326] and their extension studies for agalsidase alfa [327, 328] and agalsidase beta [309, 329], studies originating from industry-sponsored registries [330–333] and investigator-sponsored studies independent from the industry [334–338]. Hereunder, we review the clinical efficacy data currently available for each drug since their marketing authorization within the European Union [339, 340].
A. Efficacy and safety data of agalsidase alfa treatment
Agalsidase alfa (Replagal®; Shire, Cambridge, MA, USA) is an enzyme replacement therapy for FD. Agalsidase alfa first received marketing authorization in the European Union in August 2001, and is approved for the treatment of FD in 45 countries. Agalsidase alfa is purified from a stably transfected human cell line and is infused at a dose of 0.2 mg/kg of body weight over a period of 40 minutes every 14 days [294, 341]. Double-blind, randomized clinical trials of ERT with agalsidase alfa in FD involved relatively small numbers of patients [324, 326] and most of the data presented here originates from industry-sponsored FOS® or open-label clinical trials.
Amelioration of early clinical symptoms
In two pediatric clinical trials of ERT with agalsidase alfa, including 37 children [342, 343], boys demonstrated reductions in plasma Gb3 levels, and both boys and girls reported reductions in neuropathic pain and in the use of neuropathic pain medications. Heart rate variability, which is classically reduced in boys with FD, was statistically significantly improved with 6 months of agalsidase alfa treatment [342–344]. With the possible exception of clearance in younger patients, agalsidase alpha appears to have comparable pharmacokinetic and pharmacodynamic profiles in pediatric and adult Fabry patients of both genders [345]. In the 3.5-year extension study of one of the pediatric clinical trials, there were sustained, statistically significant improvements in the clinical features of FD, including reduced plasma Gb3 levels, reduced pain severity assessed by the brief pain inventory (BPI) questionnaire, and improved heart rate variability. Mean urine Gb3 levels were reduced to normal range. Kidney function and left ventricular mass indexed to height remained stable throughout the study [346].
In a small open-label study, improvements in acroparesthesia and anhidrosis were associated with a normalization of sympathetic skin responses after 2 years on agalsidase alfa [347].
In a larger cohort of patients from the FOS® observational database, pain severity was significantly reduced in 81 patients on agalsidase alfa for 2 years and in 62 patients on agalsidase alfa for 3 years, and all dimensions of pain perception were improved [43]. Improvements in health-related quality of life (QoL) paralleled improvements in pain and were maintained after 24 months of ERT [330, 331].
In an analysis of agalsidase alfa replacement therapy in patients with FD who were enrolled in the FOS®, a clinically significant reduction of pain (defined as improvement of >1 point on the BPI) was recorded for average and worst pain (60.4% and 53.1% of patients, respectively) after 5 years of treatment [333]. Before initiation of ERT, QoL was worse in patients with FD than in the general population. Mean QoL deviation score from normal EuroQol values improved significantly compared with baseline after 5 years of treatment [from - 0.24 (0.30) to - 0.17 (0.28) p = 0.0483] [333].
There are several reports suggesting that ERT with agalsidase alfa may ameliorate the abdominal pain and diarrhea associated with FD. A reduction in the incidence of GI pain in 62 patients was shown after 12 months of ERT (from 49% to 39% of patients) and in 58 patients after 24 months of ERT [50]. The prevalence of diarrhea was also reduced after 12 and 24 months of ERT compared with baseline, the absence of a control group being a limitation of this study [50].
Renal function
In several studies, the estimated glomerular filtration rate (eGFR) and creatinine clearance remained stable after 1-2 years of ERT [330, 332, 348]. However, in a study aiming to determine the effects of ERT with agalsidase alpha on renal function in patients with Fabry nephropathy, eGFR declined in males with stage 1 and 2 kidney disease treated by agalsidase alfa at 0.2 mg/kg during 3 years, although proteinuria was under 1 g/24 h in all patients enrolled in this open-label study [349].
In patients whose renal function continues to decline while receiving agalsidase alfa at 0.2 mg/kg (eGFR decline of ≥ 5 mL/min/1.73 m2/year), there may be benefits from doubling the dose through weekly infusions rather than infusions every 2 weeks (mean rate of change in eGFR improved from - 8.0 ml/min/1.73 m2/year to - 3.3 ml/min/1.73 m2/year; p = 0.01) [328].
A recent meta-analysis showed that ERT with agalsidase alfa appears to slow down the decline in GFR in patients with mild to moderate nephropathy and baseline proteinuria under 1 g per day [350]. Patients with more advanced nephropathy and/or overt proteinuria did not respond as well to agalsidase alfa alone. No histological data was shown with respect to clearance of Gb3 from podocytes or other renal cell types. Treatment with agalsidase alfa did not improve proteinuria [350].
Cardiac morphology and function
In an open-label study, significant reductions in left ventricular mass (LVM) were observed in heterozygous women after 27 weeks on agalsidase alfa [351]. Mean ventricular wall thickness and LVM were reduced in a larger cohort of patients from the FOS® after 1 and 2 years of ERT [330]. Of note, the largest decreases in LVM were observed in patients with the greatest degree of hypertrophy at baseline [330], a result that contrasts with those from a number of studies with agalsidase beta [20, 91, 338, 352]. In a double-blind randomized clinical trial on a small number of patients with FD and cardiac involvement, ERT resulted in a progressive decrease in LVM measured by MRI (p = 0.041) after 6 months on agalsidase alfa at 0.2 mg/kg every other week [326]. Cardiomyocyte Gb3 clearance which was the primary efficacy endpoint did not reach statistical significance [326].
In an analysis of agalsidase alfa replacement therapy in patients with FD who were enrolled in the FOS®, treatment resulted in a sustained reduction in LVM index from 71.4 g/m2.7 (SD 22.5) to 64.1 g/m2.7 (SD 18.7) after 5 years (p = 0.0111) and a significant increase in midwall fractional shortening from 14.3% (SD = 2.3) to 16.0% (SD = 3.8) after 3 years (p = 0.02) [333]. Sentinel clinical cardiac and cerebrovascular events occurred in a greater proportion of patients with LVH than without LVH after 5 years of treatment [333].
Cerebrovascular events
Initial results on the effect of agalsidase alfa (0.2 mg/kg every other week) on CNS involvement in FD showed progression of white matter lesions in 2 out of 7 patients [128]. This study involved a small number of patients with a limited follow-up for 1 year [128] and, to date, it is not known if agalsidase alfa therapy can reduce or prevent the cerebrovascular complications and hearing loss associated with FD [353]. During the 4.5 year follow-up study of the original phase III pivotal trial, four out of the 25 patients (16%) suffered a cerebrovascular accident or a transient ischemic attack [327].
Severity score and causes of death
S core index of FD severity, such as the Mainz Severity Score Index (MSSI) [354], have shown a general reduction in disease severity after one year of ERT with agalsidase alfa [355, 356].
Data on causes of death in a cohort of 1453 patients (699 male and 754 female) from 19 countries worldwide enrolled in the FOS® were analysed, while causes of death of their affected relatives were analysed separately. The principal causes of death among 181 affected relatives of patients in FOS®, most of who had died before 2001, were renal failure in males (42%) and cerebrovascular disease in females (25%). In contrast, of the 42 patients enrolled in the FOS® whose deaths were reported between 2001 and 2007, cardiac disease was the main cause of death in both male (34%) and female (57%) patients [87].
B. Efficacy and safety data of agalsidase beta treatment
Agalsidase beta (Fabrazyme®, Genzyme Corporation, Cambridge, MA, USA) is indicated for long-term ERT in patients with a confirmed diagnosis of FD. It is intended to replace deficient endogenous α-galactosidase A in these patients. World-wide, agalsidase beta is currently approved in 55 countries, including the USA. In February 2008, the European Medicine Agency's Committee for Medicinal Products for Human Use (CHMP) granted full marketing authorization to Fabrazyme® superseding its approval under exceptional circumstances [Fabrazyme® Summary of Product Characteristics (SPC)] [339].
Clearance of Gb3 from renal cells, urine and cardiac cells
Renal capillary endothelial cells were (nearly) completely cleared of Gb3 after 20 weeks of agalsidase beta at 1 mg/kg EOW in 98% of the patients in the original multicenter, randomized, placebo-controlled, double blind phase III clinical trial [323]. Complete clearance of Gb3 was also observed in mesangial and interstitial cells in the majority of patients [357]. All improvements were maintained with sustained treatment over 4.5 years, and signs of contained improvement in clearance from epithelial cells (podocytes, distal tubular epithelial cells) were noted, although Gb3 was never completely cleared from podocytes [309]. Reductions were less complete in non-capillary smooth muscle cells. The capacity of agalsidase beta at 1 mg/kg EOW to normalize Gb3 content of renal capillary endothelial cells after 20 weeks of treatment was confirmed in a bridging study in 13 Japanese male patients [358]. Urinary Gb3 excretion was reduced in both studies after 20 weeks of therapy [323, 358].
In the heart, 5 months of agalsidase beta treatment in the phase III clinical trial [323] resulted in complete clearance of Gb3 from the microvasculature in 72% of treated patients compared with only 3% of placebo-treated patients (p < 0.001) [359]. The placebo group achieved similar results after 6 months of treatment in the open-label extension study [359]. In addition, the capillary endothelium remained free of Gb3 for up to 60 months [309] in 6 of 8 patients who consented to an end-of-study cardiac biopsy [359]. No clearance of Gb3 was observed in the cardiomyocytes during the trial [359].
Of note, repeated infusions with agalsidase beta over a prolonged period did not appreciably clear storage material in cells other than vascular endothelial cells in two case reports [360, 361]. In the samples from the heart and some other tissues biopsied from two male patients after several months of ERT with agalsidase beta, only the endothelial cells were free of Gb3 and persistent storage was found in cardiomyocytes, smooth muscle cells, fibroblasts and sweat glands [361]. Similarly, extensive glycolipid storage deposits were seen in all organ systems with the exception of vascular endothelial cells in the autopsy study of a 47-year-old male patient who died after 2.5 years of ERT with agalsidase beta [360].
Amelioration of early clinical symptoms
Fourteen boys and 2 girls, 8 to 16 years old, were treated in an open-label pediatric clinical trial. A 12-week-observation period to collect baseline data preceeded the 48-week-treatment period when agalsidase beta (1 mg/kg) was infused intravenously at 1 mg/kg EOW. No primary efficacy endpoint was specified [362]. Before treatment, results of skin biopsies from 12 male patients showed moderate or severe Gb3 accumulation in superficial dermal capillary endothelial cells; with treatment, these cells were completely cleared of Gb3 in week-24 biopsies from all 12 male patients and in all available week-48 biopsies. Agalsidase beta was generally well tolerated; most treatment-related adverse events were mild or moderate with infusion-associated reactions involving rigors, fever, or rhinitis. Children treated with agalsidase beta experienced less pain and gastrointestinal problems, and were reported to have more energy and improved school attendance as documented by patients' diaries [362]. No overall significant change in serum creatinine, mild proteinuria and eGFR was found in pediatric patients after 48 weeks of treatment [362].
In the extension study of the original phase III trial in adult patients, pain scores as measured by the McGill Pain Questionnaire improved over time with sustained agalsidase beta treatment at 1 mg/kg EOW for those who reported pain at baseline and use of pain medications was reduced in some patients [309, 329]. For most SF-36 components, patients experienced a mean improvement after long-term treatment with agalsidase beta (Fabrazyme®). The mean changes from pretreatment through month 54 for the components of Physical Functioning, Role Emotional, Body Pain, and Standardized Physical Component Scale (for patients with score <100 at first measurement before treatment) were statistically significant (p < 0.015, 0.031, 0.003, and 0.006, respectively) [309].
Pain reduction was also found in 2 other studies after ~20 months of agalsidase beta therapy at 1 mg/kg EOW [334, 363]. One of these studies evaluated nerve fiber function in 22 males with Fabry neuropathy and reported subclass-dependent improvements in small nerve fiber function [363]. Such improvements were not seen in patients with severe thermal perception dysfunction at baseline [363].
Health-related quality of life was also measured using the SF-36® health survey in 71 men and 59 women enrolled in the Fabry Registry® who were treated with agalsidase beta and who had baseline and at least 2 yearly post-treatment health-related quality of life measurements. Long-term treatment with agalsidase beta resulted in substantial improvements in health-related quality of life in both men and women [364].
Renal function
It has been shown that renal function in adult patients can be preserved with sustained treatment with agalsidase beta at 1 mg/kg EOW [309]. Estimated glomerular filtration rate, proteinuria and serum creatinine remained stable and normal in the vast majority of patients treated for 4.5 years (54 months) [309]. The 6 patients showing a rise in serum creatinine shared a common profile at baseline including age >40 (n = 4/6), high proteinuria levels (> 2 g/24 h, n = 4/6) and significant glomerulosclerosis (> 50%, n = 4/4). This profile predisposed them to progression of renal disease, even under agalsidase beta therapy. The mean rate of eGFR decline for the remaining patients (n = 52) as a group was 0.4 ml/min per 1.73 m2/yr and not significantly different from 0 (p = 0.6785). Subgroup analyses were performed to examine the impact of baseline proteinuria or glomerulosclerosis on renal function during the study period. The mean yearly decline in eGFR in patients (n = 42) with low (< 1 g/24 h) proteinuria at baseline was minimal [mean eGFR slope = -1.0 ml/min per 1.73 m2/yr (1.0; p = 0.3052)] (Figure 29) [309], and not statistically different from normal yearly reduction of GFR [365]. Progressive Gb3 clearance from podocytes was observed on kidney biopsies (n = 8) after 54 months of ERT (Figure 30) [309].
Median estimated glomerular filtration rate (eGFR; ml/min per 1.73 m2) over time in 44 patients treated with agalsidase beta for 54 months: Patients in the “as treated” population maintained a stable median eGFR during the 54-month treatment. Subgroup analyses of patients who were stratified by baseline proteinuria (>1 g/24 h versus <1 g/24 h) showed differences in the rate of eGFR decline during the 54-mo treatment period [309]. High (>1 g/24 h) baseline proteinuria was associated with higher rate of eGFR decline and increased probability of renal events.
Long-term agalsidase beta therapy decreases Gb 3 accumulation in podocytes: A) Kidney biopsy which was obtained prior to agalsidase beta therapy shows dark-staining granules in podocytes. B) By month 54, fewer Gb3 inclusions are evident from a specimen which was obtained from the same patient. Methylene blue/azure II stain, magnification × 400 [309].
Favorable renal outcomes in patients with less impaired renal function have also been reported [334]. Patients with normal kidney function (GFR > 90 ml/min/1.73 m2) at baseline treated for a mean of 23 months showed stabilization of kidney function, whereas patients with GFR < 90 ml/min/1.73 m2 had a significant decrease in mean eGFR (from 71 to 60 ml/min/1.73 m2) [334].
Cardiac morphology and function
Several research groups have found reductions in LVH and amelioration of LV stiffness and regional myocardial function in patients with FD treated with agalsidase beta at 1 mg/kg EOW. Significant improvement in LV hypertrophy and function (both radial and longitudinal LV function) were found in a strain rate imaging study in 16 adult patients (mean age 42) treated for 1 year [91].
In another study, the effects of agalsidase beta (1 mg/kg EOW) on cardiac morphology, function, and late gadolinium enhancement were studied during 12 months of ERT. Only patients without late enhancement (LE) at baseline had significant reductions in LVM during ERT. No patients without late enhancement at baseline developed LE during ERT. Echocardiography revealed an improvement of regional myocardial function in patients without LE. In contrast, in patients with LE at baseline, the amount of LE significantly increased and the follow-up examinations showed neither regression of LVM nor improvement in regional myocardial function [20].
In an open-label study, stabilization of mean LVM has been demonstrated after 1 year of agalsidase beta treatment at 1 mg/kg EOW in patients aged >30 years who had significant degrees of baseline myocardial hypertrophy [335]. In contrast, neither resting or dipyridamole-stimulated myocardial perfusion nor myocardial perfusion reserve changed during ERT [335]. Self-estimated cardiovascular condition, QoL, diastolic function, exercise capacity, ECG parameters, ejection fraction and ventricular mass did not change in an open-label prospective follow-up study of 24-month ERT with agalsidase beta at 1 mg/kg EOW in 5 male and 4 female patients. ERT had only minimal effect on symptoms and cardiovascular morphology and function [366].
In an open-label study on 11 patients (8 males and 3 females) a significant reduction in myocardial T2 relaxation times was noted in all myocardial regions on MRI, (interventricular septum, apex, and lateral wall) after a mean treatment duration of 45 months with agalsidase beta at 1 mg/kg EOW [367].
Progression to major renal, cardiac, or cerebrovascular events, or death
A multicentric, double-blind, randomized, placebo-controlled phase IV study has shown that agalsidase beta at 1 mg/kg EOW can slow the progression of the serious, life-threatening complications of FD, even in patients who already have overt kidney dysfunction [325]. This study enrolled 82 patients (72 males, 10 females; aged 20-72 years) who were followed for 18.5 months (median). The group of 51 patients randomized to receive agalsidase beta treatment, overall, had pronounced renal disease with a mean eGFR of 53 mL/min/1.73 m2 at baseline. A significant 61% reduction of the risk of progression to major renal, cardiac, or cerebrovascular events, or death, was found in treated patients as compared to placebo-treated patients in the per-protocol analysis that adjusted for an imbalance in baseline proteinuria [hazard ratio, 0.39 (CI, 0.16 to 0.93); p = 0.034]. Greater and highly significant treatment effects were seen in patients that had less severe renal impairment at baseline (eGFR > 55 ml/min/1.73 m2) [325].
To date, it is not known if agalsidase beta therapy can reduce or prevent the cerebrovascular complications and hearing loss associated with FD [309, 329]. In the long-term extension study of the original pivotal trial, five of 58 patients (9%) experienced symptomatic stroke or transient ischemic attacks as an adverse event [309].
Whether a lower dose could maintain the Gb3 clearance achieved with 1.0 mg/kg was explored in a study where 21 adult male patients were treated with agalsidase beta for 6 months at 1.0 mg/kg EOW followed by 18 months at 0.3 mg/kg/2 weeks. A lower dose of agalsidase beta was sufficient in some, but not all, patients to maintain the cellular Gb3 clearance achieved with 1.0 mg/kg/2 weeks. Long-term clinical effects of transitioning to the lower dose have not been evaluated [368].
C. Comparison between agalsidase alfa and agalsidase beta treatments
Randomized controlled trials
The results of the published randomized controlled clinical trials and their extension studies, together with the pediatric trials for the two enzyme preparations, agalsidase alfa [324, 326, 327, 342] and agalsidase beta [309, 323, 329, 359, 362] are shown in Table 5.
Head to head clinical trials
The efficacy of and tolerability towards the two agalsidase preparations administered at identical protein dose (0.2 mg/kg/14 days) were compared in a randomized controlled open-label trial. The study revealed no difference in reduction of LVM or other disease parameters after 12 and 24 months of treatment with either agalsidase alfa (Replagal®) or beta (Fabrazyme®) at a dose of 0.2 mg/kg biweekly. Treatment failure occurred frequently in both groups and seemed related to age and severe pre-treatment disease [336]. In another comparative study, the occurrence of α-galactosidase A antibodies and their effect on urinary and plasma Gb3, chitotriosidase and clinical outcome were assessed in 52 patients after 12 months of treatment with either 0.2 mg/kg agalsidase alpha (10 males, 8 females) or beta (8 males, 5 females) or 1.0 mg/kg agalsidase beta (10 males, 11 females) [337]. Alpha-galactosidase A antibodies frequently developped in male patients (18/28) and interfered with urinary Gb3 excretion. From urinary Gb3 studies, it appears that persistence of antibodies impairs ERT at a dose of 0.2 mg/kg EOW. Infusion of a dose of 1.0 mg/kg resulted in a more robust decline in Gb3, less impact of antibodies, stable renal function and reduction of LVM [337]. Some concerns have been expressed about the methodological design and data interpretation of the later study [369].
An independent study of patients with FD in Canada, aiming to compare the effects of agalsidase alfa, 0.2 mg/kg/14 days, versus agalsidase beta, 1.0 mg/kg/14 days on clinical outcomes is currently ongoing [370]. However, interpretation of the study results will be meaningful only if any imbalance between the agalsidase alfa and agalsidase beta treated groups in terms of baseline parameters is corrected, and proper analysis of the data accounts for confounding factors such as gender of patients and nature of clinical events.
The supply of agalsidase beta has been reduced, since June 2009, due to production problems. The supply shortage of agalsidase beta resulted in some patients either being switched to receiving agalsidase alfa or to having a reduced dose of agalsidase beta. The potential impact of IgG antibodies on the response to enzyme replacement therapy in these patients remains unresolved [327, 337, 368, 371, 372]. Patients in whom the dose or formulation of ERT has been amended will require careful monitoring in order to assess impact on safety and clinical efficacy [27] and help decision making when supply of agalsidase beta is restored.
Kidney function
The goal for treatment of Fabry nephropathy is reduction in the rate of loss of GFR to <-1.0 mL/min/1.73 m2/year [77, 373].
After 5 years of treatment with agalsidase alfa at the dose recommended by the manufacturer (0.2 mg/kg EOW), data reported in the FOS® database showed that mean yearly fall in estimated GFR was - 2.83 mL/min/1.73 m2 for male patients with chronic kidney disease (CKD) stage 1 at baseline [333], statistically different (p = 0.0001) from the normal yearly reduction of - 0.9 mL/min/1.73 m2[365]. The mean yearly loss of eGFR for men with stage 2 disease at baseline was - 2.17 mL/min/1.73 m2 (p = 0.0004). In male patients with stage 3 CKD at baseline, the mean yearly fall in eGFR after 5 years was - 3.0 ml/min/1.73 m2 (p = 0.006) [333, 374]. In contrast, corresponding values for men with CKD stage 1 or stage 2 disease and proteinuria < 1 g/day at baseline treated for 5 years with agalsidase beta at 1 mg/kg EOW (n = 42) were - 1.005 mL/min/1.73 m2/year [309], not statistically different (p= 0.3052) from normal yearly reduction rate [365].
D. Practical considerations of ERT for Fabry disease
Infusion management
During the pivotal, double-blind trials of agalsidase alfa (Replagal®) [324] and agalsidase beta (Fabrazyme®) [323], 57% (8/14) and 59% (34/58) of patients experienced mild-to-moderate infusion-related reactions, respectively, the incidence peaking around the fifth to eighth infusion. Fevers, chills and rigors were the only treatment-related adverse events occurring significantly more frequently in the treatment group than in the placebo group; all were transient, mild-to-moderate in severity and were managed conservatively [323]. In the follow-up study with agalsidase alfa, 13 of 25 patients experienced an infusion-reaction during or shortly after one or more infusions. These reactions typically consisted of facial flushing and rigors [327]. After 3 to 5 years of treatment, the number of patients treated with agalsidase beta experiencing infusion reactions fell to between 10 and 20%, suggesting that patients develop tolerance to the infusions over time [309, 329].
The precise cause of the infusion-associated reactions is unknown, but may be related to IgG antibodies specific to the infused enzyme (IgG seroconversion occurred in 24% of agalsidase alfa treated-patients [339, 340] and in 51 of the 58 (88%) who received agalsidase beta during double-blind [323] or open-label treatment [329]), or to complement activation.
Experience gained in our center [375] suggests that infusion-associated reactions (IAR) tend to occur during the first 6 months of treatment - usually after 20-40 minutes of the infusion - and last for approximately 10-30 minutes. The risk of events tends to increase with increasing infusion rates. Based on these observations, it is recommended that, at the first occurrence of an infusion reaction, the patient's temperature and vital signs should be assessed and the infusion rate temporarily slowed or stopped. In the case of a severe reaction, the infusion must be stopped and the administration of antihistamines and/or corticosteroids should be considered. The infusion can be continued in the case of mild reactions, with close supervision. After cessation of or a decrease in symptoms, the infusion may be re-started and the infusion rate gradually increased to the original rate. Subsequent infusions should be started at a lower infusion rate and increased every 30 minutes. Pre-medication with an antihistamine, paracetamol and/or dexamethasone (1 hour before infusion) may also be considered [295].
A few of the approximately 3000 patients treated to date with agalsidase beta have developed plasma IgE antibodies and a few others have had a positive prick-test together with urticaria or skin rash (Figure 31). Most patients have successfully undergone a rechallenge protocol [376]. No IgE antibodies have been detected during agalsidase alfa treatment [327]. Whether seroconversion affects treatment efficacy is currently unknown but neutralizing antibodies to both agalsidase alfa and agalsidase beta have been demonstrated [371] and shown to lead to a relapse in urinary [327, 336, 377] and cutaneous [372] Gb3. This warrants further studies since in Gaucher disease [378], another lysosomal storage disorder due to the deficient activity of acid β-glucosidase [379], neutralizing antibodies have been shown to block the catalytic activity of the exogeneous enzyme and lead to a deterioration of clinical course in the very rare instances where they occur [380]. In these cases, the potential use of immunosuppressive therapy in combination with ERT should be investigated [381].
Skin rash during infusion of recombinant α-galactosidase A in a patient with positive IgE antibodies to agalsidase beta: In year 2002, a 39-year-old male Fabry patient (GLA mutation p.Ala121Pro) was initially treated with agalsidase beta (1 mg/kg EOW). ERT was changed to agalsidase alfa (0.2 mg/kg EOW) after 18 months due to poor tolerance (mild laryngeal edema, urticaria and chills during infusions). Two years later, a rash appeared on both arms during agalsidase alfa infusions. In 2007, concomittant deterioration of kidney function on agalsidase alfa (mGFR decreased from 85 to 70 mL/min/1.73 m2) led to switch ERT back to agalsidase beta. No data was obtained with respect to antibodies (IgG or IgE) to agalsidase alfa. After 1 year of agalsidase beta therapy, extensive skin rash and bronchospasm appeared during the infusions despite premedication (hydroxyzine, paracetamol and oral steroids) and minimal infusion rates (0.05 - 0.2 mg/min) and kidney function kept on deteriorating (mGFR = 54 mL/min/1.73 m2). The patient tested positive for IgE to agalsidase beta and ERT was discontinued. Mutation p.Ala121Pro is not responsive to the ASSC deoxygalactonojirymicin [424]. Both rechallenge protocol and concomitant use of immunosuppressive therapy and ERT are currently being considered.
Infusion during dialysis and post transplant
Many physicians involved in treating patients with FD using ERT have queried whether dialysis influences the pharmacokinetics of the recombinant enzyme. Although experience of infusing the enzyme during dialysis is currently limited, no problems have been encountered to date. Virtually no difference in the plasma activity of agalsidase beta was found regardless of whether or not the infusion was given during hemodialysis [382]. The procedure used a low-flux polysulphone filter, with which there was no loss of enzyme. Theoretically, enzyme adsorption to the filter could occur. A recommendation, therefore, is to begin enzyme infusion approximately 15 minutes after the start of dialysis, by which time the membrane's surface will be covered by plasma proteins such as fibrinogen or albumin, reducing the likelihood of enzyme adsorption to the membrane and to the tubing system. The feasibility of infusing ERT during dialysis confers a considerable practical advantage to patients requiring dialysis [382].
Pregnancy
Although ERT is theoretically contra-indicated during pregnancy and lactation, both agalsidase alfa [383, 384] and agalsidase beta [385, 386] have been used in a limited number of cases. No adverse event was reported and both recombinant enzymes appear safe. However, few data are available and the decision to initiate or maintain ERT during pregnancy should be made on an individual basis and carefully monitored [385].
Home therapy
Home therapy can help to alleviate the burden of intravenous infusions every 14 days for stable patients who tolerate the infusions and have a suitable home environment [387]. Several reports suggest that patients appreciate home treatment and, if implemented successfully, ERT can be administered in the home setting in a safe and reliable manner [310, 327, 388]. This should however not lead to decreased medical care and patients should be referred to a tertiary center of excellence every 6 to 12 months.
XII - Prognosis
With age, progressive damage to vital organ systems develops and at some point, organs may start to fail in functioning. End-stage renal disease and life-threatening cardiovascular or cerebrovascular complications limit life-expectancy of untreated males and females to approximately 50 and 70 years, representing reductions of 20 and 10 years, respectively, as compared to the general population [25, 26]. While it is hoped that long-term enzyme therapy can halt disease progression, the importance of adjunctive therapies should be noted and the possibility of developing an oral therapy drives forward research into active site specific chaperones.
XIII - Current research
A. Basic research: cellular model of Fabry disease
In a recent study, a cell model of FD was established [389]. The expression of α-galactosidase A was transiently silenced by RNA interference in HK2 and primary human renal epithelial cells and stably silenced in HK2 cells by retroviral transfection with small hairpin RNA (shRNA). All of the silenced cells had reduced viability, significant accumulation of intracellular Gb3, and a modest but significant increase in membranous Gb3 (CD77) expression compared to non-silenced cells. When silenced HK2 cells were reconstituted with agalsidase alfa, they decreased their membranous CD77 expression to levels indistinguishable from those of non-silenced cells. These data suggest that membranous CD77 levels may mirror Gb3 tissue load and that CD77 expression levels may be used to monitor the efficacy of ERT [390].
B. Basic research: animal models
Genetically authentic animal models of human lysosomal diseases occur spontaneously in many mammalian species. However, most are among larger domestic or farm animals with only few well-defined genetic lysosomal diseases known among rodents. This status changed dramatically with the advent of the combined homologous recombination and embryonic stem cell technology, which allows directed generation of mouse models that are genetically equivalent to human diseases [391]. This technology has allowed generation of knock-out mice for FD [392, 393] as well as transgenic mice [394, 395]. These animal models have played an important role in studies of the pathogenesis [15, 396, 397] and treatments (including bone marrow transplant [398], substrate deprivation [399], enzyme replacement therapy [322, 377, 400], active site specific chaperones [401] and gene therapy [402–407]) for FD. While the utility of these mouse models is obvious, species differences in metabolic pathways must always be remembered, if the ultimate goal of the study is application to human patients.
C. Clinical research: registries and outcome surveys
The Fabry Registry®[408] and the Fabry Outcome Survey® (FOS®) [409] are ongoing, observational databases that compile clinical and laboratory data on patients with FD. As of March 2010, the Fabry Registry® and FOS® included 3200 and 1700 patients respectively. All patients with FD are eligible for enrollment in the Fabry Registry®, regardless of age, gender, symptoms, or whether they are receiving ERT while enrollment in FOS® is limited to patients treated with agalsidase alfa or naïve to ERT. Patient and physician participation is voluntary. All patients provide informed consent through local institutional review boards/ethics committees and may decline to participate or withdraw consent at any time. Treating physicians determine the actual frequency of assessments according to patients' individualized needs. A schedule of recommended clinical assessments is available in the Fabry Registry®[408]. Given the voluntary nature of reporting data, patients' ages at clinical assessments and time intervals between assessments are variable. Due to the rarity of the condition, clinical trials of ERT in FD generally involved relatively small numbers of patients and much of the available data on the natural history of the disease and the long term safety and efficacy of the recombinant enzymes available in the literature stems from the FOS®[40, 43, 53, 84, 87, 144, 293, 330, 331, 410] or the Fabry Registry®[23, 24, 51, 76, 111, 184, 411, 412].
XIV - Future perspectives
A. Use of a modified alpha-N-acetylgalactosaminidase in the development of enzyme replacement therapy for Fabry disease
The human lysosomal enzymes alpha-galactosidase (α-gal A, EC 3.2.1.22) and alpha-N-acetylgalactosaminidase (α-NAGAL, EC 3.2.1.49) share 46% amino acid sequence identity and have similar folds. The active sites of the two enzymes share 11 of 13 amino acids, differing only where they interact with the 2-position of the substrates. Using a rational protein engineering approach, the enzymatic specificity of α-galactosidase A and α-NAGAL were interconverted. The engineered α-NAGAL [or α-NAGAL(EL)] retains the antigenicity of α-NAGAL but has acquired the enzymatic specificity of the α-galactosidase A enzyme. Comparison of the crystal structures of the designed enzyme to the wild-type enzymes shows that active sites of α-galactosidase A and α-NAGAL superimpose well, indicating success of the rational design. The designed enzymes might be useful as non-immunogenic alternatives in ERT for treatment of FD [413].
In another experiment, a modified alpha-N-acetylgalactosaminidase (NAGA or α-NAGAL, EC 3.2.1.49) with α-galactosidase A-like substrate specificity was designed on the basis of structural studies and was produced in CHO cells. The enzyme acquired the ability to catalyze the degradation of 4-MU-alpha-D-galactopyranoside. There was no immunological cross-reactivity between the modified NAGA and α-galactosidase A, and the modified NAGA did not react to serum from a patient with FD treated with recombinant α-galactosidase A. The enzyme cleaved Gb3 accumulated in cultured fibroblasts from a patient with FD. Furthermore, like recombinant agalsidases currently used for ERT for FD, the enzyme injected intravenously into FD model mice prevented Gb3 storage in the liver, kidneys, and heart and improved the pathological changes in these organs. Because the modified NAGA is not expected to cause an allergic reaction in patients with FD, it is promising as a new and safe enzyme for ERT [414].
B. Active site specific chaperones
In FD, a significant number of disease-causing mutations are missense mutations, which cause the newly synthesized lysosomal protein to be unstable, but still catalytically competent [231, 415]. Despite the fact that unstable mutant α-galactosidases are catalytically comparable to their wild type counterpart in their purified forms [204], the newly synthesized enzymes are unable to undergo trafficking to their appropriate location within the cell - the lysosomal compartment (Figure 32). Studies of trafficking and degradation of various mutant forms of α-galactosidase A indicate that the mutant enzymes are retained in the endoplasmic reticulum (ER) and degraded by ER-associated degradation (ERAD) because of their misfolded conformations [204]. This provides a rationale for a therapeutic intervention using active-site-specific chaperones to stabilize the conformation or reduce misfolding of the mutant protein in order to prevent the premature degradation by ERAD (Figure 32) [416–419].
Proposed mechanism of action of active site-specific chaperones (ASSCs): A: During synthesis of a wild-type lysosomal enzyme, cells assemble amino acids in a correctly folded tertiary structure. Molecular chaperones are naturally occurring molecules that assist in protein folding. B: In contrast, mutant misfolded lysosomal enzymes are unstable and retained in the endoplasmic reticulum (ER) where they may not meet quality control and are prone to endoplasmic reticulum-associated degradation (ERAD). C: ASSCs are designed to stabilize and rescue misfolded lysosomal enzymes, leading to reduced ER retention or accumulation, and enhanced trafficking to the Golgi apparatus and the lysosome where they dissociate from the enzyme.
Enzyme inhibitors from the imino-sugars family were shown to be effective active-site-specific chaperones, causing an increase in residual enzyme activity and stabilizing enzyme activity in cultured lymphoblasts and transfected COS-1 cells [420, 421]. Subsequently, galactose, a weak inhibitor of α-galactosidase A, was intravenously infused to a male patient with the cardiac variant of FD at a dose of 1 g/kg body weight every other day. After 3 months of galactose infusions, the myocardial fibres, which initially appeared severely hypertrophic and extensively vacuolated were smaller, and vacuolization was decreased [422].
The imino sugars are monosaccharide mimetics, characterized by having a nitrogen atom in place of the ring oxygen present in monosaccharides and often are potent inhibitors of glycosidases. As the imino sugars have a high affinity for the active site of the target enzyme, they can also act as active-site-specific chaperones, assisting protein folding or stabilizing misfolded enzymes [423]. 1-Deoxygalactonojirymicin (DGJ), currently under investigation by the trade name of Amigal™ (migalastat hydrochloride; Amicus Therapeutics, Cranbury, NJ, USA), is a small imino sugar which mimics the α-galactose of Gb3, the substrate for α-galactosidase A, when it binds to the active site of the enzyme. The firm binding between DGJ and the mutant enzyme shifts the folding and stability of the enzyme in favor of the appropriate and proper conformation, potentially permitting a smooth escape from the ER for further maturation and trafficking to the lysosomal compartment [419] (Figure 32). DGJ may be effective only in patients with specific, "responsive" GLA mutations coding for a mutant α-galactosidase with enhancable residual enzyme activity [419, 424]. DGJ is an orally active, small molecule drug which could provide additional advantages of convenience and cost savings. However, since DGJ is primarily an inhibitor of α-galactosidase A activity, finding the right dosing and regimen for chaperoning is a key issue of this novel therapeutic approach [419]. Phase II extension and phase III clinical trials are ongoing.
Appendix
I - Organizations that provide support and information for patients with Fabry disease and their families
Fabry International Network (FIN)
http://www.fabryintnetwork.com
Fabry Support and Information Group (FSIG)
French Center of Excellence for Fabry disease
II - Web sites with medical, technical, and bibliographic information about Fabry disease and/or the GLA gene
Orphanet
The portal for rare diseases and orphan drugs
The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff
http://www.hgmd.cf.ac.uk/ac/index.php
Online Mendelian Inheritance in Man (OMIM)
A catalog of human genes and genetic disorders. The data base contains textual information, pictures, reference information, and links to NCBI's Entrez database of MEDLINE articles.
http://www.ncbi.nlm.nih.gov/omim
ClinicalTrials.gov
is a registry of federally and privately supported clinical trials conducted in the United States and around the world. ClinicalTrials.gov gives you information about purpose of a trial, who may participate, locations, and phone numbers for more details.
http://clinicaltrials.gov/ct2/results?term=fabry
III - Fabry Disease Registries
Fabry Registry ®
https://www.lsdregistry.net/fabryregistry/
Fabry Outcome Survey® (FOS®)
Abbreviations
- 5' UTR:
-
5' untranslated region
- α-gal A:
-
alpha-galactosidase A
- α-NAGAL (NAGA):
-
alpha-N-acetylgalactosaminidase
- ACE:
-
angiotensin-converting enzyme
- ACEi:
-
angiotensin-converting enzyme inhibitors
- ARBs:
-
angiotensin receptor blockers
- ASSC:
-
active site specific chaperone
- BPI:
-
Brief Pain Inventory
- CCA:
-
common carotid artery
- CHO:
-
Chinese hamster ovary
- CKD:
-
chronic kidney disease
- CNS:
-
central nervous system
- CT:
-
computed tomography
- DGJ:
-
deoxygalactonojirymicin
- CHMP:
-
European Medicines Agency's Committee for Medicinal Products for Human Use
- eGFR:
-
estimated glomerular filtration rate
- EMA:
-
European Medicines Agency
- EOW:
-
every other week
- ER:
-
endoplasmic reticulum
- ERAD:
-
Endoplasmic Reticulum associated degradation
- ERT:
-
enzyme replacement therapy
- FD:
-
Fabry disease
- FDA:
-
Food and Drug Administration
- FOS®:
-
Fabry Outcome Survey®
- Gb3:
-
globotriaosylceramide
- GFR:
-
glomerular filtration rate
- GI:
-
gastro-intestinal
- IAR:
-
infusion-associated reaction
- ICD:
-
implantation of cardioverter defibrillator
- IMT:
-
intima-media thickness
- IRB:
-
Institutional Review Board
- LE:
-
late enhancement
- LSD:
-
lysosomal storage diseases
- LVH:
-
left ventricular hypertrophy
- LVM:
-
left ventricular mass
- MRI:
-
magnetic resonance imaging
- MSSI:
-
Mainz Severity Score Index
- NSAID:
-
non-steroidal anti-inflammatory drug
- QoL:
-
quality of life
- RRT:
-
renal replacement therapy
- SD:
-
standard deviation
- SF-36:
-
Short form with 36 items
- SPC:
-
Summary of Product Characteristics
- TIA:
-
transient ischemic attack.
References
Anderson W: A case of "Angeio-keratoma". Br J Dermatol. 1898, 10: 113-117. 10.1111/j.1365-2133.1898.tb16317.x.
Fabry J: Ein Beitrag zur Kenntnis der Purpura haemorragica nodularis (Purpura papulosa hemorrhagica Hebrae). Arch Dermatol Syphilol. 1898, 43: 187-200. 10.1007/BF01986897.
Sweeley CC, Klionsky B: Fabry's disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. J Biol Chem. 1963, 238: 3148-3150.
Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L: Enzymatic defect in Fabry's disease: ceramide-trihexosidase deficiency. N Engl J Med. 1967, 276: 1163-1167. 10.1056/NEJM196705252762101.
Kint JA: The enzyme defect in Fabry's disease. Nature. 1970, 227: 1173-10.1038/2271173b0.
De Duve C: Exploring cells with a centrifuge. Science. 1975, 189: 186-194. 10.1126/science.1138375.
Desnick RJ, Ioannou YA, Eng CM: Alpha-galactosidase A deficiency: Fabry disease. The metabolic and molecular bases of inherited disease. Edited by: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B. New York: McGraw Hill, 2001, 3733-3774.
Popli S, Leehey DJ, Molnar ZV, Nawab ZM, Ing TS: Demonstration of Fabry's disease deposits in placenta. Am J Obstet Gynecol. 1990, 162: 464-465.
Vedder AC, Strijland A, vd Bergh Weerman MA, Florquin S, Aerts JM, Hollak CE: Manifestations of Fabry disease in placental tissue. J Inherit Metab Dis. 2006, 29: 106-111. 10.1007/s10545-006-0196-0.
Hers HG: Inborn Lysosomal Diseases. Gastroenterology. 1965, 48: 625-633.
Neufeld EF: Lysosomal storage diseases. Annu Rev Biochem. 1991, 60: 257-280. 10.1146/annurev.bi.60.070191.001353.
Lucke T, Hoppner W, Schmidt E, Illsinger S, Das AM: Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab. 2004, 82: 93-97. 10.1016/j.ymgme.2004.01.011.
Palecek T, Bultas J, Hajek M, Karetova D, Kuchynka P, Kautzner J, Elleder M, Linhart A: Association between cardiac energy metabolism and gain of left ventricular mass in Fabry disease. Int J Cardiol. 2009, 144: 337-339. 10.1016/j.ijcard.2009.03.045.
Das AM, Naim HY: Biochemical basis of Fabry disease with emphasis on mitochondrial function and protein trafficking. Adv Clin Chem. 2009, 49: 57-71. full_text.
Park JL, Shu L, Shayman JA: Differential involvement of COX1 and COX2 in the vasculopathy associated with the {alpha}-galactosidase A-knockout mouse. Am J Physiol Heart Circ Physiol. 2009, 296: 1133-1140. 10.1152/ajpheart.00929.2008.
Park S, Kim JA, Joo KY, Choi S, Choi EN, Shin JA, Han KH, Jung SC, Suh SH: Globotriaosylceramide leads to KCa3.1 channel dysfunction: A new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res. 2010,
Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, Kaneski CR: Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab. 2008, 95: 163-168. 10.1016/j.ymgme.2008.06.016.
Chevrier M, Brakch N, Lesueur C, Genty D, Ramdani Y, Moll S, Djavaheri-Mergny M, Brasse-Lagnel C, Laquerriere A, Barbey F, Bekri S: Autophagosome maturation is impaired in Fabry disease. Autophagy. 2010,
Weidemann F, Breunig F, Beer M, Sandstede J, Stork S, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM: The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J. 2005, 26: 1221-1227. 10.1093/eurheartj/ehi143.
Beer M, Weidemann F, Breunig F, Knoll A, Koeppe S, Machann W, Hahn D, Wanner C, Strotmann J, Sandstede J: Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in Fabry's cardiomyopathy. Am J Cardiol. 2006, 97: 1515-1518. 10.1016/j.amjcard.2005.11.087.
Moon JC, Sheppard M, Reed E, Lee P, Elliott PM, Pennell DJ: The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J Cardiovasc Magn Reson. 2006, 8: 479-482. 10.1080/10976640600605002.
Torra R: Renal manifestations in Fabry disease and therapeutic options. Kidney Int Suppl. 2008, S29-32. 10.1038/ki.2008.522.
Hopkin RJ, Bissler J, Banikazemi M, Clarke L, Eng CM, Germain DP, Lemay R, Tylki-Szymanska A, Wilcox WR: Characterization of Fabry Disease in 352 Pediatric Patients in the Fabry Registry. Pediatr Res. 2008, 64: 550-555. 10.1203/PDR.0b013e318183f132.
Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunig F, Wanner C, Warnock DG, Lemay RM, Germain DP: Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008, 93: 112-128. 10.1016/j.ymgme.2007.09.013.
Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ: Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009, 24: 2102-2111. 10.1093/ndt/gfp031.
MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001, 38: 769-775. 10.1136/jmg.38.11.769.
Mehta A, Beck M, Eyskens F, Feliciani C, I Kantola I, Ramaswami U, Rolfs A, Rivera A, Waldek S, Germain DP: Fabry disease: a review of current management strategies. QJM. 2010, 103: 641-659. 10.1093/qjmed/hcq117.
Elleder M, Bradova V, Smid F, Budesinsky M, Harzer K, Kustermann-Kuhn B, Ledvinova J, Belohlavek X, Kral V, Dorazilova V: Cardiocyte storage and hypertrophy as a sole manifestation of Fabry's disease. Virchows Arch Pathol Anat Histopathol. 1990, 417: 449-455. 10.1007/BF01606034.
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, Tanaka H: An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995, 333: 288-293. 10.1056/NEJM199508033330504.
Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ: Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int. 2003, 64: 801-807. 10.1046/j.1523-1755.2003.00160.x.
Maier EM, Osterrieder S, Whybra C, Ries M, Gal A, Beck M, Roscher AA, Muntau AC: Disease manifestations and × inactivation in heterozygous females with Fabry disease. Acta Paediatr Suppl. 2006, 95: 30-38. 10.1080/08035320600618809.
Migeon BR: X inactivation, female mosaicism, and sex differences in renal diseases. J Am Soc Nephrol. 2008, 19: 2052-2059. 10.1681/ASN.2008020198.
Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP: The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999, 105: 151-156.
Meikle PJ, Hopwood JJ, Clague AE, Carrey WF: Prevalence of lysosomal storage disorders. JAMA. 1999, 281: 249-254. 10.1001/jama.281.3.249.
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, Ponzone A, Desnick RJ: High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006, 79: 31-40. 10.1086/504601.
Hwu WL, Chien YH, Lee NC, Chiang SC, Dobrovolny R, Huang AC, Yeh HY, Chao MC, Lin SJ, Kitagawa T, Desnick RJ, Hsu LW: Newborn screening for Fabry disease in Taiwan reveals a high incidence of the later-onset GLA mutation c.936+919G > A (IVS4+919G > A). Hum Mutat. 2009, 30: 1397-1405. 10.1002/humu.21074.
Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, Lin SJ, Chen CH, Chiang CC, Ho HJ, Lee PC, Kao CH, Cheng KH, Hsueh C, Niu DM: High Incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009, 2: 450-456. 10.1161/CIRCGENETICS.109.862920.
Dutsch M, Marthol H, Stemper B, Brys M, Haendl T, Hilz MJ: Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol. 2002, 19: 575-586. 10.1097/00004691-200212000-00011.
Cable WJ, Kolodny EH, Adams RD: Fabry disease: impaired autonomic function. Neurology. 1982, 32: 498-502.
Ramaswami U, Whybra C, Parini R, Pintos-Morell G, Mehta A, Sunder-Plassmann G, Widmer U, Beck M: Clinical manifestations of Fabry disease in children: data from the Fabry Outcome Survey. Acta Paediatr. 2006, 95: 86-92. 10.1080/08035250500275022.
Desnick RJ, Brady RO: Fabry disease in childhood. J Pediatr. 2004, 144: S20-26. 10.1016/j.jpeds.2004.01.051.
Zarate YA, Hopkin RJ: Fabry's disease. Lancet. 2008, 372: 1427-1435. 10.1016/S0140-6736(08)61589-5.
Hoffmann B, Beck M, Sunder-Plassmann G, Borsini W, Ricci R, Mehta A: Nature and prevalence of pain in Fabry disease and its response to enzyme replacement therapy--a retrospective analysis from the Fabry Outcome Survey. Clin J Pain. 2007, 23: 535-542. 10.1097/AJP.0b013e318074c986.
Charrow J: A 14-year-old boy with pain in hands and feet. Pediatr Ann. 2009, 38: 190-192. 10.3928/00904481-20090401-01.
Hilz MJ, Stemper B, Kolodny EH: Lower limb cold exposure induces pain and prolonged small fiber dysfunction in Fabry patients. Pain. 2000, 84: 361-365. 10.1016/S0304-3959(99)00236-5.
Miners AH, Holmes A, Sherr L, Jenkinson C, MacDermot KD: Assessment of health-related quality-of-life in males with Anderson Fabry Disease before therapeutic intervention. Qual Life Res. 2002, 11: 127-133. 10.1023/A:1015009210639.
Cole AL, Lee PJ, Hughes DA, Deegan PB, Waldek S, Lachmann RH: Depression in adults with Fabry disease: a common and under-diagnosed problem. J Inherit Metab Dis. 2007, 30: 943-951. 10.1007/s10545-007-0708-6.
Naleschinski D, Arning K, Baron R: Fabry disease - Pain doctors have to find the missing ones. Pain. 2009, 145: 10-11. 10.1016/j.pain.2009.07.025.
Sheth KJ, Werlin SL, Freeman ME, Hodach AE: Gastrointestinal structure and function in Fabry's disease. Am J Gastroenterol. 1981, 76: 246-251.
Hoffmann B, Schwarz M, Mehta A, Keshav S: Gastrointestinal symptoms in 342 patients with Fabry disease: prevalence and response to enzyme replacement therapy. Clin Gastroenterol Hepatol. 2007, 5: 1447-1453. 10.1016/j.cgh.2007.08.012.
Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ, Bultas J, Lee P, Sims K, Brodie SE, Pastores GM, Strotmann JM, Wilcox WR: Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006, 8: 539-548. 10.1097/01.gim.0000237866.70357.c6.
Kang WH, Chun SI, Lee S: Generalized anhidrosis associated with Fabry's disease. J Am Acad Dermatol. 1987, 17: 883-887. 10.1016/S0190-9622(87)70274-6.
Orteu CH, Jansen T, Lidove O, Jaussaud R, Hughes DA, Pintos-Morell G, Ramaswami U, Parini R, Sunder-Plassman G, Beck M, Mehta AB: Fabry disease and the skin: data from FOS, the Fabry Outcome Survey. Br J Dermatol. 2007, 157: 331-337. 10.1111/j.1365-2133.2007.08002.x.
Gupta SN, Ries M, Murray GJ, Quirk JM, Brady RO, Lidicker JR, Schiffmann R, Moore DF: Skin-impedance in Fabry Disease: a prospective, controlled, non-randomized clinical study. BMC Neurol. 2008, 8: 41-10.1186/1471-2377-8-41.
Shelley ED, Shelley WB, Kurczynski TW: Painful fingers, heat intolerance, and telangiectases of the ear: easily ignored childhood signs of Fabry disease. Pediatr Dermatol. 1995, 12: 215-219. 10.1111/j.1525-1470.1995.tb00161.x.
Germain DP: [Fabry's disease (alpha-galactosidase-A deficiency): physiopathology, clinical signs, and genetic aspects]. J Soc Biol. 2002, 196: 161-173.
Mohrenschlager M, Braun-Falco M, Ring J, Abeck D: Fabry disease: recognition and management of cutaneous manifestations. Am J Clin Dermatol. 2003, 4: 189-196. 10.2165/00128071-200304030-00005.
Wattanasirichaigoon D, Svasti J, Cairns JR, Tangnararatchakit K, Visudtibhan A, Keeratichamroen S, Ngiwsara L, Khowsathit P, Onkoksoong T, Lekskul A, Mongkolsiri D, Jariengprasert C, Thawil C, Ruencharoen S: Clinical and molecular characterization of an extended family with Fabry disease. J Med Assoc Thai. 2006, 89: 1528-1535.
Keilmann A, Hajioff D, Ramaswami U: Ear symptoms in children with Fabry disease: data from the Fabry Outcome Survey. J Inherit Metab Dis. 2009, 32: 739-744. 10.1007/s10545-009-1290-x.
Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, Rosing DR, Robinson C, Schaefer E, Gal A, Dambrosia JM, Garman SC, Brady RO, Schiffmann R: Pediatric Fabry disease. Pediatrics. 2005, 115: e344-355. 10.1542/peds.2004-1678.
Kampmann C, Wiethoff CM, Whybra C, Baehner FA, Mengel E, Beck M: Cardiac manifestations of Anderson-Fabry disease in children and adolescents. Acta Paediatr. 2008, 97: 463-469. 10.1111/j.1651-2227.2008.00700.x.
Cabrera-Salazar MA, O'Rourke E, Charria-Ortiz G, Barranger JA: Radiological evidence of early cerebral microvascular disease in young children with Fabry disease. J Pediatr. 2005, 147: 102-105. 10.1016/j.jpeds.2005.03.004.
Gubler MC, Lenoir G, Grunfeld JP, Ulmann A, Droz D, Habib R: Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978, 13: 223-235. 10.1038/ki.1978.32.
Sessa A, Meroni M, Battini G, Maglio A, Brambilla PL, Bertella M, Nebuloni M, Pallotti F, Giordano F, Bertagnolio B, Tosoni A: Renal pathological changes in Fabry disease. J Inherit Metab Dis. 2001, 24: 66-70. 10.1023/A:1012423924648.
Tondel C, Bostad L, Hirth A, Svarstad E: Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008, 51: 767-776. 10.1053/j.ajkd.2007.12.032.
Ramaswami U, Najafian B, Schieppati A, Mauer M, Bichet DG: Assessment of renal pathology and dysfunction in children with Fabry disease. Clin J Am Soc Nephrol. 2010, 5: 365-370. 10.2215/CJN.08091109.
Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976, 58: 259-263.
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009, 20: 629-637. 10.1681/ASN.2008030287.
Counahan R, Chantler C, Ghazali S, Kirkwood B, Rose F, Barratt TM: Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child. 1976, 51: 875-878. 10.1136/adc.51.11.875.
Tondel C, Ramaswami U, Aakre KM, Wijburg F, Bouwman M, Svarstad E: Monitoring renal function in children with Fabry disease: comparisons of measured and creatinine-based estimated glomerular filtration rate. Nephrol Dial Transplant. 2010, 25: 1507-1513. 10.1093/ndt/gfp658.
Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noel LH, Grunfeld JP, Valbuena C, Oliveira JP, Muller J, Breunig F, Zhang X, Warnock DG: Scoring system for renal pathology in Fabry disease: report of the international study group of fabry nephropathy (ISGFN). Nephrol Dial Transplant. 2010, 25: 2168-2177. 10.1093/ndt/gfp528.
Fervenza FC, Torra R, Lager DJ: Fabry disease: an underrecognized cause of proteinuria. Kidney Int. 2008, 73: 1193-1199. 10.1038/sj.ki.5002677.
Ortiz A, Oliveira JP, Waldek S, Warnock DG, Cianciaruso B, Wanner C: Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant. 2008, 23: 1600-1607. 10.1093/ndt/gfm848.
Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB: Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore). 2002, 81: 122-138. 10.1097/00005792-200203000-00003.
Froissart M, Benistan K, Germain DP: [Functional renal investigation in Fabry disease]. Presse Med. 2007, 36: S36-42.
Wanner C, Oliveira JP, Ortiz A, Mauer M, Germain DP, Linthorst GE, Serra AL, Marodi L, Mignani R, Cianciaruso B, Vujkovac B, Lemay R, Beitner-Johnson D, Waldek S, Warnock DG: Prognostic indicators of renal disease progression in adults with Fabry disease: Natural history data from the Fabry Registry. Clin J Am Soc Nephrol. 2010,
Warnock DG, Valbuena C, West M, Oliveira JP: Renal manifestations of Fabry disease. Fabry disease. Edited by: Elstein D, Altarescu G, Beck M. Dordrecht, Heidelberg, London, New-York: Springer, 2010, 211-244. full_text.
Linhart A, Palecek T, Bultas J, Ferguson JJ, Hrudova J, Karetova D, Zeman J, Ledvinova J, Poupetova H, Elleder M, Aschermann M: New insights in cardiac structural changes in patients with Fabry's disease. Am Heart J. 2000, 139: 1101-1108. 10.1067/mhj.2000.105105.
Kampmann C, Baehner F, Whybra C, Martin C, Wiethoff CM, Ries M, Gal A, Beck M: Cardiac manifestations of Anderson-Fabry disease in heterozygous females. J Am Coll Cardiol. 2002, 40: 1668-1674. 10.1016/S0735-1097(02)02380-X.
Senechal M, Germain DP: Fabry disease: a functional and anatomical study of cardiac manifestations in 20 hemizygous male patients. Clin Genet. 2003, 63: 46-52. 10.1034/j.1399-0004.2003.630107.x.
Shah JS, Hughes DA, Sachdev B, Tome M, Ward D, Lee P, Mehta AB, Elliott PM: Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry disease. Am J Cardiol. 2005, 96: 842-846. 10.1016/j.amjcard.2005.05.033.
Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, Tome MT, McKenna WJ, Lee P, Camici PG: Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart. 2006, 92: 357-360. 10.1136/hrt.2004.054015.
Hasegawa H, Takano H, Shindo S, Takeda S, Funabashi N, Nakagawa K, Toyozaki T, Kuwabara Y, Komuro I: Images in cardiovascular medicine. Transition from left ventricular hypertrophy to massive fibrosis in the cardiac variant of Fabry disease. Circulation. 2006, 113: e720-721. 10.1161/CIRCULATIONAHA.105.584292.
Linhart A, Kampmann C, Zamorano JL, Sunder-Plassmann G, Beck M, Mehta A, Elliott PM: Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J. 2007, 28: 1228-1235. 10.1093/eurheartj/ehm153.
Kampmann C, Linhart A, Baehner F, Palecek T, Wiethoff CM, Miebach E, Whybra C, Gal A, Bultas J, Beck M: Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int J Cardiol. 2008, 130: 367-373. 10.1016/j.ijcard.2008.03.007.
Takenaka T, Teraguchi H, Yoshida A, Taguchi S, Ninomiya K, Umekita Y, Yoshida H, Horinouchi M, Tabata K, Yonezawa S, Yoshimitsu M, Higuchi K, Nakao S, Anan R, Minagoe S, Tei C: Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol. 2008, 51: 50-59. 10.1016/j.jjcc.2007.12.001.
Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M, Sunder-Plassmann G: Natural course of Fabry disease: changing pattern of causes of death in FOS - Fabry Outcome Survey. J Med Genet. 2009, 46: 548-552. 10.1136/jmg.2008.065904.
Linhart A, Elliott PM: The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007, 93: 528-535. 10.1136/hrt.2005.063818.
Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A: Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation. 2003, 107: 1978-1984. 10.1161/01.CIR.0000061952.27445.A0.
Pieroni M, Chimenti C, Russo A, Russo MA, Maseri A, Frustaci A: Tissue Doppler imaging in Fabry disease. Curr Opin Cardiol. 2004, 19: 452-457. 10.1097/01.hco.0000131534.25034.43.
Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM: Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation. 2003, 108: 1299-1301. 10.1161/01.CIR.0000091253.71282.04.
Palecek T, Dostalova G, Kuchynka P, Karetova D, Bultas J, Elleder M, Linhart A: Right ventricular involvement in Fabry disease. J Am Soc Echocardiogr. 2008, 21: 1265-1268. 10.1016/j.echo.2008.09.002.
Niemann M, Breunig F, Beer M, Herrmann S, Strotmann J, Hu K, Emmert A, Voelker W, Ertl G, Wanner C, Weidemann F: The right ventricle in Fabry disease: natural history and impact of enzyme replacement therapy. Heart. 2010,
Kampmann C, Baehner FA, Whybra C, Bajbouj M, Baron K, Knuf M, Wiethoff CM, Trubel H, Beck M: The right ventricle in Fabry disease. Acta Paediatr Suppl. 2005, 94: 15-18. 10.1080/08035320510028049.
Sheth KJ, Thomas JP: Electrocardiograms in Fabry's disease. J Electrocardiol. 1982, 15: 153-156. 10.1016/S0022-0736(82)80010-1.
Yokoyama A, Yamazoe M, Shibata A: A case of heterozygous Fabry's disease with a short PR interval and giant negative T waves. Br Heart J. 1987, 57: 296-299. 10.1136/hrt.57.3.296.
Wise D: Short P-R intervals and tachyarrhythmias in Fabry's disease. Postgrad Med J. 1986, 62: 969-10.1136/pgmj.62.732.969.
Ikari Y, Kuwako K, Yamaguchi T: Fabry's disease with complete atrioventricular block: histological evidence of involvement of the conduction system. British Heart J. 1992, 68: 323-325. 10.1136/hrt.68.9.323.
Mehta J, Tuna N, Moller JH, Desnick RJ: Electrocardiographic and vectorcardiographic abnormalities in Fabry's disease. Am Heart J. 1977, 93: 699-705. 10.1016/S0002-8703(77)80064-1.
von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, Olsen EG, Christomanou H, Kandolf R, Bishop DF, Desnick RJ: An atypical variant of Fabry's disease with manifestations confined to the myocardium. N Engl J Med. 1991, 324: 395-399. 10.1056/NEJM199102073240607.
Goldman ME, Cantor R, Schwartz MF, Baker M, Desnick RJ: Echocardiographic abnormalities and disease severity in Fabry's disease. J Am Coll Cardiol. 1986, 7: 1157-1161. 10.1016/S0735-1097(86)80238-8.
Weidemann F, Strotmann JM, Niemann M, Herrmann S, Wilke M, Beer M, Voelker W, Ertl G, Emmert A, Wanner C, Breunig F: Heart valve involvement in Fabry cardiomyopathy. Ultrasound Med Biol. 2008, 35: 730-735. 10.1016/j.ultrasmedbio.2008.10.010.
Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, Kantola I, Raitakari OT, Knuuti J, Nuutila P: Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis. 2005, 28: 563-573. 10.1007/s10545-005-0563-2.
Bierer G, Kamangar N, Balfe D, Wilcox WR, Mosenifar Z: Cardiopulmonary exercise testing in Fabry disease. Respiration. 2005, 72: 504-511. 10.1159/000087675.
Lobo T, Morgan J, Bjorksten A, Nicholls K, Grigg L, Centra E, Becker G: Cardiovascular testing in Fabry disease: exercise capacity reduction, chronotropic incompetence and improved anaerobic threshold after enzyme replacement. Intern Med J. 2008, 38: 407-414. 10.1111/j.1445-5994.2008.01669.x.
Hilz MJ, Marthol H, Schwab S, Kolodny EH, Brys M, Stemper B: Enzyme replacement therapy improves cardiovascular responses to orthostatic challenge in Fabry patients. J Hypertens. 2010, 28: 1438-1448. 10.1097/HJH.0b013e328336a077.
Germain DP, Diebold B, Peyrard S, Martin-Mista AI, Benistan K: Aortic root dilatation is highly prevalent in male patients affected with Fabry disease and correlates with the presence of a megadolicho-ectatic basilar artery [abstract]. Am J Hum Genet. 2007, 81: 300-
Kahn P: Anderson-Fabry disease: a histopathological study of three cases with observations on the mechanism of production of pain. J Neurol Neurosurg Psychiatry. 1973, 36: 1053-1062. 10.1136/jnnp.36.6.1053.
Maag R, Binder A, Maier C, Scherens A, Toelle T, Treede RD, Baron R: Detection of a characteristic painful neuropathy in Fabry disease: A pilot study. Pain Med. 2008, 9: 1217-1223. 10.1111/j.1526-4637.2008.00470.x.
Fellgiebel A, Muller MJ, Ginsberg L: CNS manifestations of Fabry's disease. Lancet Neurol. 2006, 5: 791-795. 10.1016/S1474-4422(06)70548-8.
Sims K, Politei J, Banikazemi M, Lee P: Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: natural history data from the Fabry Registry. Stroke. 2009, 40: 788-794. 10.1161/STROKEAHA.108.526293.
Mitsias P, Levine SL: Cerebrovascular complications of Fabry's disease. Ann Neurol. 1996, 40: 8-17. 10.1002/ana.410400105.
Clavelou P, Besson G, Elziere C, Ferrier A, Pinard JM, Hermier M, Artigou JY, Germain DP: [Neurological aspects of Fabry's disease]. Rev Neurol (Paris). 2006, 162: 569-580.
Mendez MF, Stanley TM, Medel NM, Li Z, Tedesco DT: The vascular dementia of Fabry's disease. Dement Geriatr Cogn Disord. 1997, 8: 252-257. 10.1159/000106640.
Okeda R, Nisihara M: An autopsy case of Fabry disease with neuropathological investigation of the pathogenesis of associated dementia. Neuropathology. 2008, 28: 532-540. 10.1111/j.1440-1789.2008.00883.x.
Fellgiebel A, Keller I, Marin D, Muller MJ, Schermuly I, Yakushev I, Albrecht J, Bellhauser H, Kinateder M, Beck M, Stoeter P: Diagnostic utility of different MRI and MR angiography measures in Fabry disease. Neurology. 2009, 72: 63-68. 10.1212/01.wnl.0000338566.54190.8a.
DeGraba T, Azhar S, Dignat-George F, Brown E, Boutiere B, Altarescu G, McCarron R, Schiffmann R: Profile of endothelial and leukocyte activation in Fabry patients. Ann Neurol. 2000, 47: 229-233. 10.1002/1531-8249(200002)47:2<229::AID-ANA13>3.0.CO;2-T.
Moore DF, Scott LT, Gladwin MT, Altarescu G, Kaneski C, Suzuki K, Pease-Fye M, Ferri R, Brady RO, Herscovitch P, Schiffmann R: Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation. 2001, 104: 1506-1512. 10.1161/hc3801.096352.
Moore DF, Altarescu G, Ling GS, Jeffries N, Frei KP, Weibel T, Charria-Ortiz G, Ferri R, Arai AE, Brady RO, Schiffmann R: Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke. 2002, 33: 525-531. 10.1161/hs0202.102601.
Schiffmann R: Fabry disease. Pharmacol Ther. 2009, 122: 65-77. 10.1016/j.pharmthera.2009.01.003.
Kaneski CR, Moore DF, Ries M, Zirzow GC, Schiffmann R: Myeloperoxidase predicts risk of vasculopathic events in hemizgygous males with Fabry disease. Neurology. 2006, 67: 2045-2047. 10.1212/01.wnl.0000247278.88077.09.
Hilz MJ, Kolodny EH, Brys M, Stemper B, Haendl T, Marthol H: Reduced cerebral blood flow velocity and impaired cerebral autoregulation in patients with Fabry disease. J Neurol. 2004, 251: 564-570. 10.1007/s00415-004-0364-9.
Fellgiebel A, Albrecht J, Dellani PR, Schermuly I, Stoeter P, Muller MJ: Quantification of brain tissue alterations in Fabry disease using diffusion-tensor imaging. Acta Paediatr Suppl. 2007, 96: 33-36. 10.1111/j.1651-2227.2007.00203.x.
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H: Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993, 43: 1683-1689.
Ginsberg L, Manara R, Valentine AR, Kendall B, Burlina AP: Magnetic resonance imaging changes in Fabry disease. Acta Paediatr Suppl. 2006, 95: 57-62. 10.1080/08035320600618908.
Tedeschi G, Bonavita S, Banerjee TK, Virta A, Schiffmann R: Diffuse central neuronal involvement in Fabry disease: a proton MRS imaging study. Neurology. 1999, 52: 1663-1667.
Crutchfield KE, Patronas NJ, Dambrosia JM, Frei KP, Banerjee TK, Barton NW, Schiffmann R: Quantitative analysis of cerebral vasculopathy in patients with Fabry disease. Neurology. 1998, 50: 1746-1749.
Jardim L, Vedolin L, Schwartz IV, Burin MG, Cecchin C, Kalakun L, Matte U, Aesse F, Pitta-Pinheiro C, Marconato J, Giugliani R: CNS involvement in Fabry disease: clinical and imaging studies before and after 12 months of enzyme replacement therapy. J Inherit Metab Dis. 2004, 27: 229-240. 10.1023/B:BOLI.0000028794.04349.91.
Fellgiebel A, Muller MJ, Mazanek M, Baron K, Beck M, Stoeter P: White matter lesion severity in male and female patients with Fabry disease. Neurology. 2005, 65: 600-602. 10.1212/01.wnl.0000173030.70057.eb.
Buechner S, Moretti M, Burlina AP, Cei G, Manara R, Ricci R, Mignani R, Parini R, Di Vito R, Giordano GP, Simonelli P, Siciliano G, Borsini W: Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry. 2008, 79: 1249-1254. 10.1136/jnnp.2008.143693.
Schreiber W, Udvardi A, Kristoferitsch W: Chronic meningitis and lacunar stroke in Fabry disease. J Neurol. 2007, 254: 1447-1449. 10.1007/s00415-007-0533-8.
Lidove O, Chauveheid MP, Benoist L, Alexandra JF, Klein I, Papo T: Chronic meningitis and thalamic involvement in a woman: Fabry disease expanding phenotype. J Neurol Neurosurg Psychiatry. 2007, 78: 1007-10.1136/jnnp.2006.108464.
Moore DF, Ye F, Schiffmann R, Butman JA: Increased signal intensity in the pulvinar on T1-weighted images: a pathognomonic MR imaging sign of Fabry disease. AJNR Am J Neuroradiol. 2003, 24: 1096-1101.
Takanashi J, Barkovich AJ, Dillon WP, Sherr EH, Hart KA, Packman S: T1 hyperintensity in the pulvinar: key imaging feature for diagnosis of Fabry disease. AJNR Am J Neuroradiol. 2003, 24: 916-921.
Burlina AP, Manara R, Caillaud C, Laissy JP, Severino M, Klein I, Burlina A, Lidove O: The pulvinar sign: frequency and clinical correlations in Fabry disease. J Neurol. 2008, 255: 738-744. 10.1007/s00415-008-0786-x.
Germain DP, Benistan K, Halimi P: Chiari type I malformation in four unrelated patients affected with Fabry disease. Eur J Med Genet. 2006, 49: 419-425. 10.1016/j.ejmg.2006.01.007.
Germain DP, Avan P, Chassaing A, Bonfils P: Patients affected with Fabry disease have an increased incidence of progressive hearing loss and sudden deafness: an investigation of twenty-two hemizygous male patients. BMC Med Genet. 2002, 3: 10-10.1186/1471-2350-3-10.
Sakurai Y, Kojima H, Shiwa M, Ohashi T, Eto Y, Moriyama H: The hearing status in 12 female and 15 male Japanese Fabry patients. Auris Nasus Larynx. 2009, 36: 627-632. 10.1016/j.anl.2009.01.001.
Conti G, Sergi B: Auditory and vestibular findings in Fabry disease: a study of hemizygous males and heterozygous females. Acta Paediatr Suppl. 2003, 92: 33-37. 10.1111/j.1651-2227.2003.tb00219.x.
Ries M, Kim HJ, Zalewski CK, Mastroianni MA, Moore DF, Brady RO, Dambrosia JM, Schiffmann R, Brewer CC: Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain. 2007, 130: 143-150. 10.1093/brain/awl310.
Palla A, Hegemann S, Widmer U, Straumann D: Vestibular and auditory deficits in Fabry disease and their response to enzyme replacement therapy. J Neurol. 2007, 254: 1433-1442. 10.1007/s00415-007-0575-y.
Orssaud C, Dufier J, Germain DP: Ocular manifestations in Fabry disease: a survey of 32 hemizygous male patients. Ophthalmic Genet. 2003, 24: 129-139. 10.1076/opge.24.3.129.15609.
Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A: Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Experiment Ophthalmol. 2005, 33: 164-168. 10.1111/j.1442-9071.2005.00990.x.
Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S: Ocular manifestations of Fabry's disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 2007, 91: 210-214. 10.1136/bjo.2006.100602.
Falke K, Buttner A, Schittkowski M, Stachs O, Kraak R, Zhivov A, Rolfs A, Guthoff R: The microstructure of cornea verticillata in Fabry disease and amiodarone-induced keratopathy: a confocal laser-scanning microscopy study. Graefes Arch Clin Exp Ophthalmol. 2009, 247: 523-534. 10.1007/s00417-008-0962-9.
Sher NA, Letson RD, Desnick RJ: The ocular manifestations in Fabry's disease. Arch Ophthalmol. 1979, 97: 671-676.
Rosenberg DM, Ferrans VJ, Fulmer JD, Line BR, Barranger JA, Brady RO, Crystal RG: Chronic airflow obstruction in Fabry's disease. Am J Med. 1980, 68: 898-905. 10.1016/0002-9343(80)90224-7.
Brown LK, Miller A, Bhuptani A, Sloane MF, Zimmerman MI, Schilero G, Eng CM, Desnick RJ: Pulmonary involvement in Fabry disease. Am J Respir Crit Care Med. 1997, 155: 1004-1010.
Magage S, Lubanda JC, Germain DP, Bultas J, Karetova D, Linhart A: [Respiratory involvement in patients with Fabry disease]. Med Sci (Paris). 2005, 21: 37-39.
Magage S, Lubanda JC, Susa Z, Bultas J, Karetova D, Dobrovolny R, Hrebicek M, Germain DP, Linhart A: Natural history of the respiratory involvement in Anderson-Fabry disease. J Inherit Metab Dis. 2007, 30: 790-799. 10.1007/s10545-007-0616-9.
Wang RY, Abe JT, Cohen AH, Wilcox WR: Enzyme replacement therapy stabilizes obstructive pulmonary Fabry disease associated with respiratory globotriaosylceramide storage. J Inherit Metab Dis. 2008, Short Report #126
Germain DP, Benistan K, Khatchikian L, Mutschler C: [Bone involvement in Fabry disease.]. Med Sci (Paris). 2005, 21: 43-44.
Germain DP, Benistan K, Boutouyrie P, Mutschler C: Osteopenia and osteoporosis: previously unrecognized symptoms of Fabry disease. Clin Genet. 2005, 68: 93-95. 10.1111/j.1399-0004.2005.00457.x.
Mersebach H, Johansson JO, Rasmussen AK, Bengtsson BA, Rosenberg K, Hasholt L, Sorensen SA, Sorensen SS, Feldt-Rasmussen U: Osteopenia: a common aspect of Fabry disease. Predictors of bone mineral density. Genet Med. 2007, 9: 812-818. 10.1097/GIM.0b013e31815cb197.
Germain DP: Bone and muscle involvement in Fabry disease. Fabry disease. Edited by: Elstein D, Altarescu G, Beck M. Dordrecht, Heidelberg, London, New-York: Springer, 2010, 293-298. full_text.
Sadek J, Shellhaas R, Camfield CS, Camfield PR, Burley J: Psychiatric findings in four female carriers of Fabry disease. Psychiatr Genet. 2004, 14: 199-201. 10.1097/00041444-200412000-00006.
Gold KF, Pastores GM, Botteman MF, Yeh JM, Sweeney S, Aliski W, Pashos CL: Quality of life of patients with Fabry disease. Qual Life Res. 2002, 11: 317-327. 10.1023/A:1015511908710.
Street NJ, Yi MS, Bailey LA, Hopkin RJ: Comparison of health-related quality of life between heterozygous women with Fabry disease, a healthy control population, and patients with other chronic disease. Genet Med. 2006, 8: 346-353. 10.1097/01.gim.0000223545.63012.5a.
Crosbie TW, Packman W, Packman S: Psychological aspects of patients with Fabry disease. J Inherit Metab Dis. 2009, 32: 745-753. 10.1007/s10545-009-1254-1.
Segal P, Kohn Y, Pollak Y, Altarescu G, Galili-Weisstub E, Raas-Rothschild A: Psychiatric and cognitive profile in Anderson-Fabry patients: a preliminary study. J Inherit Metab Dis. 2010, 33: 429-436. 10.1007/s10545-010-9133-3.
Laney DA, Gruskin DJ, Fernhoff PM, Cubells JF, Ousley OY, Hipp H, Mehta AJ: Social-adaptive and psychological functioning of patients affected by Fabry disease. J Inherit Metab Dis. 2010
Kleinert J, Dehout F, Schwarting A, de Lorenzo AG, Ricci R, Kampmann C, Beck M, Ramaswami U, Linhart A, Gal A, Houge G, Widmer U, Mehta A, Sunder-Plassmann G: Anemia is a new complication in Fabry disease: data from the Fabry Outcome Survey. Kidney Int. 2005, 67: 1955-1960. 10.1111/j.1523-1755.2005.00294.x.
Oliveira JP, Valbuena C, Baldaia Moreira A, Fonseca E, Soares C, Leao Teles E, Waldek S: Splenomegaly, hypersplenism and peripheral blood cytopaenias in patients with classical Anderson-Fabry disease. Virchows Arch. 2008, 453: 291-300. 10.1007/s00428-008-0651-4.
Boutouyrie P, Laurent S, Laloux B, Lidove O, Grunfeld JP, Germain DP: Non-invasive evaluation of arterial involvement in patients affected with Fabry disease. J Med Genet. 2001, 38: 629-631. 10.1136/jmg.38.9.629.
Boutouyrie P, Laurent S, Laloux B, Lidove O, Grunfeld JP, Germain DP: Arterial remodelling in Fabry disease. Acta Paediatr Suppl. 2002, 91: 62-66. 10.1080/080352502762457950.
Barbey F, Brakch N, Linhart A, Jeanrenaud X, Palecek T, Bultas J, Burnier M, Hayoz D: Increased carotid intima-media thickness in the absence of atherosclerotic plaques in an adult population with Fabry disease. Acta Paediatr Suppl. 2006, 95: 63-68. 10.1080/08035320600618924.
Barbey F, Brakch N, Linhart A, Rosenblatt-Velin N, Jeanrenaud X, Qanadli S, Steinmann B, Burnier M, Palecek T, Bultas J, Hayoz D: Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol. 2006, 26: 839-844. 10.1161/01.ATV.0000209649.60409.38.
Papaxanthos-Roche A, Deminiere C, Bauduer F, Hocke C, Mayer G, Lacombe D: Azoospermia as a new feature of Fabry disease. Fertil Steril. 2007, 88: 212-e215-218
Cox-Brinkman J, Vedder A, Hollak C, Richfield L, Mehta A, Orteu K, Wijburg F, Hammond P: Three-dimensional face shape in Fabry disease. Eur J Hum Genet. 2007, 15: 535-542. 10.1038/sj.ejhg.5201798.
Hauser AC, Gessl A, Lorenz M, Voigtlander T, Fodinger M, Sunder-Plassmann G: High prevalence of subclinical hypothyroidism in patients with Anderson-Fabry disease. J Inherit Metab Dis. 2005, 28: 715-722. 10.1007/s10545-005-0003-3.
Faggiano A, Pisani A, Milone F, Gaccione M, Filippella M, Santoro A, Vallone G, Tortora F, Sabbatini M, Spinelli L, Lombardi G, Cianciaruso B, Colao A: Endocrine dysfunction in patients with Fabry disease. J Clin Endocrinol Metab. 2006, 91: 4319-4325. 10.1210/jc.2006-0858.
Amann-Vesti BR, Gitzelmann G, Widmer U, Bosshard NU, Steinmann B, Koppensteiner R: Severe lymphatic microangiopathy in Fabry disease. Lymphat Res Biol. 2003, 1: 185-189. 10.1089/153968503768330229.
Ries M, Bettis KE, Choyke P, Kopp JB, Austin HA, Brady RO, Schiffmann R: Parapelvic kidney cysts: a distinguishing feature with high prevalence in Fabry disease. Kidney Int. 2004, 66: 978-982. 10.1111/j.1523-1755.2004.00846.x.
Sayer JA, Haslam P, Brennan P: Parapelvic cysts leading to a diagnosis of Fabry disease. Kidney Int. 2008, 74: 1366-10.1038/ki.2008.141.
Foda MM, Mahmood K, Rasuli P, Dunlap H, Kiruluta G, Schillinger JF: High-flow priapism associated with Fabry's disease in a child: a case report and review of the literature. Urology. 1996, 48: 949-952. 10.1016/S0090-4295(96)00320-2.
Backenroth R, Landau EH, Goren M, Raas-Rothschild A: Fabry disease and G6PD in three family members with priapism: is the nitric oxide pathway to blame?. J Sex Med. 2010, 7: 1588-1591. 10.1111/j.1743-6109.2009.01665.x.
Germain DP: General aspects of X-linked diseases. Fabry disease. Perspectives from 5 years of FOS. Edited by: Mehta AB, Beck M, Sunder-Plassman G. 2006, Oxford: Oxford Pharmagenesis, 63-68.
Germain DP: [Genetics of Fabry disease: diagnostic and therapeutic implications]. Presse Med. 2007, 36: S14-19.
Lyon MF: Gene action in the X-chromosome of the mouse (mus musculus L.). Nature. 1961, 190: 372-373. 10.1038/190372a0.
Dobyns WB, Filauro A, Tomson BN, Chan AS, Ho AW, Ting NT, Oosterwijk JC, Ober C: Inheritance of most X-linked traits is not dominant or recessive, just X-linked. Am J Med Genet A. 2004, 129: 136-143. 10.1002/ajmg.a.30123.
Germain DP: [Fabry disease. Clinical and genetic aspects. Therapeutic perspectives]. Rev Med Interne. 2000, 21: 1086-1103. 10.1016/S0248-8663(00)00269-1.
Whybra C, Kampmann C, Willers I, Davies J, Winchester B, Kriegsmann J, Bruhl K, Gal A, Bunge S, Beck M: Anderson-Fabry disease: clinical manifestations of disease in female heterozygotes. J Inherit Metab Dis. 2001, 24: 715-724. 10.1023/A:1012993305223.
Whybra C, Wendrich K, Ries M, Gal A, Beck M: Clinical manifestations in female Fabry disease patients. Contrib Nephrol. 2001, 136: 245-250. full_text.
Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, Breunig F, Charrow J, Germain DP, Nicholls K, Banikazemi M: Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007, 30: 184-192. 10.1007/s10545-007-0521-2.
Mutoh T, Senda Y, Sugimura K, Koike Y, Matsuoka Y, Sobue I, Takahashi A, Naoi M: Severe orthostatic hypotension in a female carrier of Fabry's disease. Arch Neurol. 1988, 45: 468-472.
Galanos J, Nicholls K, Grigg L, Kiers L, Crawford A, Becker G: Clinical features of Fabry's disease in Australian patients. Intern Med J. 2002, 32: 575-584. 10.1046/j.1445-5994.2002.00291.x.
Germain DP: [Fabry disease in 2004]. Rev Prat. 2003, 53: 2215-2220.
Igawa O, Miake J, Hisatome I: Ventricular tachycardias and dilated cardiomyopathy caused by Fabry disease. Pacing Clin Electrophysiol. 2005, 28: 1142-1143. 10.1111/j.1540-8159.2005.00241.x.
Grewal RP, McLatchey SK: Cerebrovascular manifestations in a female carrier of Fabry's disease. Acta Neurol Belg. 1992, 92: 36-40.
Wendrich K, Whybra C, Ries M, Gal A, Beck M: Neurological manifestations of Fabry disease in females. Contrib Nephrol. 2001, 136: 241-244. full_text.
Giacomini PS, Shannon PT, Clarke JT, Jaigobin C: Fabry's disease presenting as stroke in a young female. Can J Neurol Sci. 2004, 31: 112-114.
Kotanko P, Kramar R, Devrnja D, Paschke E, Voigtlander T, Auinger M, Pagliardini S, Spada M, Demmelbauer K, Lorenz M, Hauser AC, Kofler HJ, Lhotta K, Neyer U, Pronai W, Wallner M, Wieser C, Wiesholzer M, Zodl H, Fodinger M, Sunder-Plassmann G: Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol. 2004, 15: 1323-1329. 10.1097/01.ASN.0000124671.61963.1E.
Ichinose M, Nakayama M, Ohashi T, Utsunomiya Y, Kobayashi M, Eto Y: Significance of screening for Fabry disease among male dialysis patients. Clin Exp Nephrol. 2005, 9: 228-232. 10.1007/s10157-005-0369-4.
Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM: Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002, 105: 1407-1411. 10.1161/01.CIR.0000012626.81324.38.
Monserrat L, Gimeno-Blanes JR, Marin F, Hermida-Prieto M, Garcia-Honrubia A, Perez I, Fernandez X, de Nicolas R, de la Morena G, Paya E, Yague J, Egido J: Prevalence of Fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2007, 50: 2399-2403. 10.1016/j.jacc.2007.06.062.
Hagege AA, Caudron E, Damy T, Roudaut R, Millaire A, Etchecopar-Chevreuil C, Tran TC, Jabbour F, Boucly C, Prognon P, Charron P, Germain DP: Screening patients with hypertrophic cardiomyopathy for Fabry disease using a filter-paper test: the FOCUS study. Heart. 2010,
Rolfs A, Bottcher T, Zschiesche M, Morris P, Winchester B, Bauer P, Walter U, Mix E, Lohr M, Harzer K, Strauss U, Pahnke J, Grossmann A, Benecke R: Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005, 366: 1794-1796. 10.1016/S0140-6736(05)67635-0.
Brouns R, Sheorajpanday R, Braxel E, Eyskens F, Baker R, Hughes D, Mehta A, Timmerman T, Vincent MF, De Deyn PP: Middelheim Fabry Study (MiFaS): a retrospective Belgian study on the prevalence of Fabry disease in young patients with cryptogenic stroke. Clin Neurol Neurosurg. 2007, 109: 479-484. 10.1016/j.clineuro.2007.03.008.
Brouns R, Thijs V, Eyskens F, Van den Broeck M, Belachew S, Van Broeckhoven C, Redondo P, Hemelsoet D, Fumal A, Jeangette S, Verslegers W, Baker R, Hughes D, De Deyn PP: Belgian Fabry study: prevalence of Fabry disease in a cohort of 1000 young patients with cerebrovascular disease. Stroke. 2010, 41: 863-868. 10.1161/STROKEAHA.110.579409.
Wozniak MA, Kittner SJ, Tuhrim S, Cole JW, Stern B, Dobbins M, Grace ME, Nazarenko I, Dobrovolny R, McDade E, Desnick RJ: Frequency of unrecognized Fabry disease among young European-American and African-American men with first ischemic stroke. Stroke. 2010, 41: 78-81. 10.1161/STROKEAHA.109.558320.
Vedder AC, Gerdes VE, Poorthuis BJ, Helmond M, Trip MD, Aerts JM, Hollak CE: Failure to detect Fabry patients in a cohort of prematurely atherosclerotic males. J Inherit Metab Dis. 2007, 30: 988-10.1007/s10545-007-0721-9.
Hauser AC, Lorenz M, Voigtlander T, Fodinger M, Sunder-Plassmann G: Results of an ophthalmologic screening programme for identification of cases with Anderson-Fabry disease. Ophthalmologica. 2004, 218: 207-209. 10.1159/000076846.
Ishii S, Kase R, Sakuraba H, Suzuki Y: Characterization of a mutant alpha-galactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem Biophys Res Commun. 1993, 197: 1585-1589. 10.1006/bbrc.1993.2659.
Ishii S, Chang HH, Kawasaki K, Yasuda K, Wu HL, Garman SC, Fan JQ: Mutant alpha-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J. 2007, 406: 285-295. 10.1042/BJ20070479.
Kleinert J, Kotanko P, Spada M, Pagliardini S, Paschke E, Paul K, Voigtlander T, Wallner M, Kramar R, Stummvoll HK, Schwarz C, Horn S, Holzer H, Fodinger M, Sunder-Plassmann G: Anderson-Fabry disease: a case-finding study among male kidney transplant recipients in Austria. Transpl Int. 2009, 22: 287-292. 10.1111/j.1432-2277.2008.00791.x.
Linthorst GE, Hollak CE, Korevaar JC, Van Manen JG, Aerts JM, Boeschoten EW: alpha-Galactosidase A deficiency in Dutch patients on dialysis: a critical appraisal of screening for Fabry disease. Nephrol Dial Transplant. 2003, 18: 1581-1584. 10.1093/ndt/gfg194.
Germain DP: A new phenotype of Fabry disease with intermediate severity between the classical form and the cardiac variant. Contrib Nephrol. 2001, 136: 234-240. full_text.
Germain DP, Benistan K, Angelova L: [X-linked inheritance and its implication in the diagnosis and management of female patients in Fabry disease]. Rev Med Int. 2010, 31: S209-S214.
Sakuraba H, Oshima A, Fukuhara Y, Shimmoto M, Nagao Y, Bishop DF, Desnick RJ, Suzuki Y: Identification of point mutations in the alpha-galactosidase A gene in classical and atypical hemizygotes with Fabry disease. Am J Hum Genet. 1990, 47: 784-789.
Eng CM, Resnick-Silverman LA, Niehaus DJ, Astrin KH, Desnick RJ: Nature and frequency of mutations in the alpha-galactosidase A gene that cause Fabry disease. Am J Hum Genet. 1993, 53: 1186-1197.
Eng CM, Desnick RJ: Molecular basis of Fabry disease: mutations and polymorphisms in the human alpha-galactosidase A gene. Hum Mutat. 1994, 3: 103-111. 10.1002/humu.1380030204.
Ploos van Amstel JK, Jansen RP, de Jong JG, Hamel BC, Wevers RA: Six novel mutations in the alpha-galactosidase A gene in families with Fabry disease. Hum Mol Genet. 1994, 3: 503-505. 10.1093/hmg/3.3.503.
Blanch LC, Meaney C, Morris CP: A sensitive mutation screening strategy for Fabry disease: detection of nine mutations in the alpha-galactosidase A gene. Hum Mutat. 1996, 8: 38-43. 10.1002/(SICI)1098-1004(1996)8:1<38::AID-HUMU5>3.0.CO;2-L.
Davies JP, Eng CM, Hill JA, Malcolm S, MacDermot K, Winchester B, Desnick RJ: Fabry disease: fourteen alpha-galactosidase A mutations in unrelated families from the United Kingdom and other European countries. Eur J Hum Genet. 1996, 4: 219-224.
Germain DP, Biasotto M, Tosi M, Meo T, Kahn A, Poenaru L: Fluorescence-assisted mismatch analysis (FAMA) for exhaustive screening of the alpha-galactosidase A gene and detection of carriers in Fabry disease. Hum Genet. 1996, 98: 719-726. 10.1007/s004390050292.
Redonnet-Vernhet I, Ploos van Amstel JK, Jansen RP, Wevers RA, Salvayre R, Levade T: Uneven × inactivation in a female monozygotic twin pair with Fabry disease and discordant expression of a novel mutation in the alpha-galactosidase A gene. J Med Genet. 1996, 33: 682-688. 10.1136/jmg.33.8.682.
Eng CM, Ashley GA, Burgert TS, Enriquez AL, D'Souza M, Desnick RJ: Fabry disease: thirty-five mutations in the alpha-galactosidase A gene in patients with classic and variant phenotypes. Mol Med. 1997, 3: 174-182.
Guffon N, Froissart R, Chevalier-Porst F, Maire I: Mutation analysis in 11 French patients with Fabry disease. Hum Mutat. 1998, S288-290. Suppl 1
Germain DP, Poenaru L: Fabry disease: identification of novel alpha-galactosidase A mutations and molecular carrier detection by use of fluorescent chemical cleavage of mismatches. Biochem Biophys Res Commun. 1999, 257: 708-713. 10.1006/bbrc.1999.0310.
Topaloglu AK, Ashley GA, Tong B, Shabbeer J, Astrin KH, Eng CM, Desnick RJ: Twenty novel mutations in the alpha-galactosidase A gene causing Fabry disease. Mol Med. 1999, 5: 806-811.
Ashton-Prolla P, Tong B, Shabbeer J, Astrin KH, Eng CM, Desnick RJ: Fabry disease: twenty-two novel mutations in the alpha-galactosidase A gene and genotype/phenotype correlations in severely and mildly affected hemizygotes and heterozygotes. J Investig Med. 2000, 48: 227-235.
Kase R, Bierfreund U, Klein A, Kolter T, Utsumi K, Itoha K, Sandhoff K, Sakuraba H: Characterization of two alpha-galactosidase mutants (Q279E and R301Q) found in an atypical variant of Fabry disease. Biochim Biophys Acta. 2000, 1501: 227-235.
Lee JK, Kim GH, Kim JS, KK K, Lee MC, Yoo HW: Identification of four novel mutations in five unrelated Korean families with Fabry disease. Clin Genet. 2000, 58: 228-233. 10.1034/j.1399-0004.2000.580311.x.
Altarescu GM, Goldfarb LG, Park KY, Kaneski C, Jeffries N, Litvak S, Nagle JW, Schiffmann R: Identification of fifteen novel mutations and genotype-phenotype relationship in Fabry disease. Clin Genet. 2001, 60: 46-51. 10.1034/j.1399-0004.2001.600107.x.
Ashley GA, Shabbeer J, Yasuda M, Eng CM, Desnick RJ: Fabry disease: twenty novel alpha-galactosidase A mutations causing the classical phenotype. J Hum Genet. 2001, 46: 192-196. 10.1007/s100380170088.
Blaydon D, Hill J, Winchester B: Fabry disease: 20 novel GLA mutations in 35 families. Hum Mutat. 2001, 18: 459-10.1002/humu.1219.
Germain DP, Salard D, Fellmann F, Azibi K, Caillaud C, Bernard MC, Poenaru L: Identification of a novel de novo mutation (G373D) in the alpha-galactosidase A gene (GLA) in a patient affected with Fabry disease. Hum Mutat. 2001, 17: 353-10.1002/humu.41.
Germain DP: Co-occurrence and contribution of Fabry disease and Klippel-Trenaunay-Weber syndrome to a patient with atypical skin lesions. Clin Genet. 2001, 60: 63-67. 10.1034/j.1399-0004.2001.600110.x.
Germain DP, Shabbeer J, Cotigny S, Desnick RJ: Fabry disease: twenty novel alpha-galactosidase A mutations and genotype-phenotype correlations in classical and variant phenotypes. Mol Med. 2002, 8: 306-312.
Yasuda M, Shabbeer J, Osawa M, Desnick RJ: Fabry disease: novel alpha-galactosidase A 3'-terminal mutations result in multiple transcripts due to aberrant 3'-end formation. Am J Hum Genet. 2003, 73: 162-173. 10.1086/376608.
Garman SC, Garboczi DN: The molecular defect leading to Fabry disease: structure of human alpha-galactosidase. J Mol Biol. 2004, 337: 319-335. 10.1016/j.jmb.2004.01.035.
Dobrovolny R, Dvorakova L, Ledvinova J, Magage S, Bultas J, Lubanda JC, Poupetova H, Elleder M, Karetova D, Hrebicek M: Recurrence of Fabry disease as a result of paternal germline mosaicism for alpha-galactosidase A gene mutation. Am J Med Genet A. 2005, 134: 84-87.
Shabbeer J, Robinson M, Desnick RJ: Detection of alpha-galactosidase A mutations causing Fabry disease by denaturing high performance liquid chromatography. Hum Mutat. 2005, 25: 299-305. 10.1002/humu.20144.
Schaefer E, Mehta A, Gal A: Genotype and phenotype in Fabry disease: analysis of the Fabry Outcome Survey. Acta Paediatr Suppl. 2005, 94: 87-92. 10.1080/08035320510031045.
Shabbeer J, Yasuda M, Benson SD, Desnick RJ: Fabry disease: identification of 50 novel alpha-galactosidase A mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum Genomics. 2006, 2: 297-309.
Shimotori M, Maruyama H, Nakamura G, Suyama T, Sakamoto F, Itoh M, Miyabayashi S, Ohnishi T, Sakai N, Wataya-Kaneda M, Kubota M, Takahashi T, Mori T, Tamura K, Kageyama S, Shio N, Maeba T, Yahagi H, Tanaka M, Oka M, Sugiyama H, Sugawara T, Mori N, Tsukamoto H, Tamagaki K, Tanda S, Suzuki Y, Shinonaga C, Miyazaki J, Ishii S, Gejyo F: Novel mutations of the GLA gene in Japanese patients with Fabry disease and their functional characterization by active site specific chaperone. Hum Mutat. 2008, 29: 331-10.1002/humu.9520.
Bernstein HS, Bishop DF, Astrin KH, Kornreich R, Eng CM, Sakuraba H, Desnick RJ: Fabry disease: six gene rearrangements and an exonic point mutation in the alpha-galactosidase gene. J Clin Invest. 1989, 83: 1390-1399. 10.1172/JCI114027.
Kornreich R, Bishop DF, Desnick RJ: α-Galactosidase A gene rearrangement causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990, 265: 9319-9326.
Gal A: Molecular genetics of Fabry disease and Genotype-phenotype correlation. Fabry disease. Edited by: Elstein D, Altarescu G, Beck M. Dordrecht, Heidelberg, London, New-York: Springer, 2010, 3-19. full_text.
The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff. [http://www.hgmd.cf.ac.uk]
Froissart R, Guffon N, Vanier MT, Desnick RJ, Maire I: Fabry disease: D313Y is an alpha-galactosidase A sequence variant that causes pseudodeficient activity in plasma. Mol Genet Metab. 2003, 80: 307-314. 10.1016/S1096-7192(03)00136-7.
Davies JP, Winchester BG, Malcolm S: Sequence variations in the first exon of alpha-galactosidase A. J Med Genet. 1993, 30: 658-663. 10.1136/jmg.30.8.658.
Fitzmaurice TF, Desnick RJ, Bishop DF: Human alpha-galactosidase A: high plasma activity expressed by the -30G > A allele. J Inherit Metab Dis. 1997, 20: 643-657. 10.1023/A:1005366224351.
Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, van Roomen C, Mirzaian M, Wijburg FA, Linthorst GE, Vedder AC, Rombach SM, Cox-Brinkman J, Somerharju P, Boot RG, Hollak CE, Brady RO, Poorthuis BJ: Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci USA. 2008, 105: 2812-2817. 10.1073/pnas.0712309105.
Guce AI, Garman SC: The structure of human α-galactosidase A and implications for Fabry disease. Fabry disease. Edited by: Elstein D, Altarescu G, Beck M. Dordrecht, Heidelberg, London, New-York: Springer, 2010, 21-38. full_text.
Guce AI, Clark NE, Salgado EN, Ivanen DR, Kulminskaya AA, Brumer H, Garman SC: Catalytic mechanism of human alpha-galactosidase. J Biol Chem. 2010, 285: 3625-3632. 10.1074/jbc.M109.060145.
Linthorst GE, De Rie MA, Tjiam KH, Aerts JM, Dingemans KP, Hollak CE: Misdiagnosis of Fabry disease: importance of biochemical confirmation of clinical or pathological suspicion. Br J Dermatol. 2004, 150: 575-577. 10.1046/j.1365-2133.2004.05813.x.
Mayes JS, Scheerer JB, Sifers RN, Donaldson ML: Differential assay for lysosomal alpha-galactosidases in human tissues and its application to Fabry's disease. Clin Chim Acta. 1981, 112: 247-251. 10.1016/0009-8981(81)90384-3.
Hoffmann B, Georg Koch H, Schweitzer-Krantz S, Wendel U, Mayatepek E: Deficient alpha-galactosidase A activity in plasma but no Fabry disease--a pitfall in diagnosis. Clin Chem Lab Med. 2005, 43: 1276-1277. 10.1515/CCLM.2005.219.
Linthorst GE, Vedder AC, Aerts JM, Hollak CE: Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin Chim Acta. 2005, 353: 201-203. 10.1016/j.cccn.2004.10.019.
Chamoles NA, Blanco M, Gaggioli D: Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001, 308: 195-196. 10.1016/S0009-8981(01)00478-8.
Caudron E, Moliere D, Zhou JY, Prognon P, Germain DP: [Recent advances of Fabry disease screening for at risk population]. Med Sci (Paris). 2005, 21: 48-50.
Lukacs Z, Keil A, Kohlschutter A, Beck M, Mengel E: The ratio of alpha-galactosidase to beta-glucuronidase activities in dried blood for the identification of female Fabry disease patients. J Inherit Metab Dis. 2005, 28: 803-805. 10.1007/s10545-005-0039-4.
Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, Keutzer JM: Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008, 54: 1725-1728. 10.1373/clinchem.2008.104711.
Olivova P, der Veen KV, Cullen E, Rose M, Zhang XK, Sims KB, Keutzer J, Browning MF: Effect of sample collection on alpha-galactosidase A enzyme activity measurements in dried blood spots on filter paper. Clin Chim Acta. 2009, 403: 159-162. 10.1016/j.cca.2009.02.008.
Vedder AC, Linthorst GE, van Breemen MJ, Groener JE, Bemelman FJ, Strijland A, Mannens MM, Aerts JM, Hollak CE: The Dutch Fabry cohort: diversity of clinical manifestations and Gb3 levels. J Inherit Metab Dis. 2007, 30: 68-78. 10.1007/s10545-006-0484-8.
Roy S, Gaudin K, Germain DP, Baillet A, Prognon P, Chaminade P: Optimisation of the separation of four major neutral glycosphingolipids: application to a rapid and simple detection of urinary globotriaosylceramide in Fabry disease. J Chromatogr B Analyt Technol Biomed Life Sci. 2004, 805: 331-337. 10.1016/j.jchromb.2004.03.037.
Auray-Blais C, Cyr D, Mills K, Giguere R, Drouin R: Development of a filter paper method potentially applicable to mass and high-risk urinary screenings for Fabry disease. J Inherit Metab Dis. 2007, 30: 106-10.1007/s10545-006-0444-3.
Auray-Blais C, Cyr D, Ntwari A, West ML, Cox-Brinkman J, Bichet DG, Germain DP, Laframboise R, Melancon SB, Stockley T, Clarke JT, Drouin R: Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol Genet Metab. 2008, 93: 331-340. 10.1016/j.ymgme.2007.10.001.
Touboul D, Roy S, Germain DP, Baillet A, Brion F, Prognon P, Chaminade P, Laprevote O: Fast fingerprinting by MALDI-TOF mass spectrometry of urinary sediment glycosphingolipids in Fabry disease. Anal Bioanal Chem. 2005, 382: 1209-1216. 10.1007/s00216-005-3239-8.
Mills K, Morris P, Lee P, Vellodi A, Waldek S, Young E, Winchester B: Measurement of urinary CDH and CTH by tandem mass spectrometry in patients hemizygous and heterozygous for Fabry disease. J Inherit Metab Dis. 2005, 28: 35-48. 10.1007/s10545-005-5263-4.
Young E, Mills K, Morris P, Vellodi A, Lee P, Waldek S, Winchester B: Is globotriaosylceramide a useful biomarker in Fabry disease?. Acta Paediatr Suppl. 2005, 94: 51-54. 10.1080/08035320510028111.
Piraud M, de Goiffon F, Froissart R, Maire I, Vanier MT: [Globotriaosylceramide measurement in urine]. Med Sci (Paris). 2005, 21: 45-47.
Hozumi I, Nishizawa M, Ariga T, Miyatake T: Biochemical and clinical analysis of accumulated glycolipids in symptomatic heterozygotes of angiokeratoma corporis diffusum (Fabry's disease) in comparison with hemizygotes. J Lipid Res. 1990, 31: 335-340.
Delobel A, Roy S, Touboul D, Gaudin K, Germain DP, Baillet A, Brion F, Prognon P, Chaminade P, Laprevote O: Atmospheric pressure photoionization coupled to porous graphitic carbon liquid chromatography for the analysis of globotriaosylceramides. Application to Fabry disease. J Mass Spectrom. 2006, 41: 50-58. 10.1002/jms.945.
Touboul D, Roy S, Germain DP, Chaminade P, Brunelle A, Laprevote O: MALDI-TOF and cluster-TOF-SIMS imaging of Fabry disease biomarkers. Int J Mass Spectrometry. 2007, 260: 158-165. 10.1016/j.ijms.2006.09.027.
Bishop DF, Calhoun DH, Bernstein HS, Hantzopoulos P, Quinn M, Desnick RJ: Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci USA. 1986, 83: 4859-4863. 10.1073/pnas.83.13.4859.
Kornreich R, Desnick RJ, Bishop DF: Nucleotide sequence of the human alpha-galactosidase A gene. Nucl Acids Res. 1989, 17: 3301-3302. 10.1093/nar/17.8.3301.
Rodriguez-Mari A, Coll MJ, Chabas A: Molecular analysis in Fabry disease in Spain: fifteen novel GLA mutations and identification of a homozygous female. Hum Mutat. 2003, 22: 258-10.1002/humu.9172.
Schirinzi A, Centra M, Prattichizzo C, Gigante M, De Fabritiis M, Giancaspro V, Petrarulo F, Santacroce R, Margaglione M, Gesualdo L, Ranieri E: Identification of GLA gene deletions in Fabry patients by Multiplex Ligation-dependent Probe Amplification (MLPA). Mol Genet Metab. 2008, 94: 382-385. 10.1016/j.ymgme.2008.03.017.
Bekri S, Lidove O, Jaussaud R, Knebelmann B, Barbey F: The role of ceramide trihexoside (globotriaosylceramide) in the diagnosis and follow-up of the efficacy of treatment of Fabry disease: a review of the literature. Cardiovasc Hematol Agents Med Chem. 2006, 4: 289-297. 10.2174/187152506778520718.
Andrade J, Waters PJ, Singh RS, Levin A, Toh BC, Vallance HD, Sirrs S: Screening for Fabry disease in patients with chronic kidney disease: limitations of plasma alpha-galactosidase assay as a screening test. Clin J Am Soc Nephrol. 2008, 3: 139-145. 10.2215/CJN.02490607.
Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH: Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004, 50: 1785-1796. 10.1373/clinchem.2004.035907.
Gelb MH, Turecek F, Scott CR, Chamoles NA: Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis. 2006, 29: 397-404. 10.1007/s10545-006-0265-4.
Navarro C, Teijeira S, Dominguez C, Fernandez JM, Rivas E, Fachal C, Barrera S, Rodriguez C, Iranzo P: Fabry disease: an ultrastructural comparative study of skin in hemizygous and heterozygous patients. Acta Neuropathol. 2006, 111: 178-185. 10.1007/s00401-005-0026-8.
Albay D, Adler SG, Philipose J, Calescibetta CC, Romansky SG, Cohen AH: Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol. 2005, 733-738. 10.1038/modpathol.3800344.
Demuth K, Germain DP: Endothelial markers and homocysteine in patients with classic Fabry disease. Acta Paediatr Suppl. 2002, 91: 57-61. 10.1080/080352502762457941.
Cartwright DJ, Cole AL, Cousins AJ, Lee PJ: Raised HDL cholesterol in Fabry disease: response to enzyme replacement therapy. J Inherit Metab Dis. 2004, 27: 791-793. 10.1023/B:BOLI.0000045841.27968.06.
Vedder AC, Cox-Brinkman J, Hollak CE, Linthorst GE, Groener JE, Helmond MT, Scheij S, Aerts JM: Plasma chitotriosidase in male Fabry patients: a marker for monitoring lipid-laden macrophages and their correction by enzyme replacement therapy. Mol Genet Metab. 2006, 89: 239-244. 10.1016/j.ymgme.2006.04.013.
van Breemen MJ, Rombach SM, Dekker N, Poorthuis BJ, Linthorst GE, Zwinderman AH, Breunig F, Wanner C, Aerts JM, Hollak CE: Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta. 2010,
Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, Kuiper S, Vd Bergh Weerman MA, Groener JE, Poorthuis BJ, Hollak CE, Aerts JM: Plasma globotriaosylsphingosine: Diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta. 2010, 180: 741-748.
Togawa T, Kodama T, Suzuki T, Sugawara K, Tsukimura T, Ohashi T, Ishige N, Suzuki K, Kitagawa T, Sakuraba H: Plasma globotriaosylsphingosine as a biomarker of Fabry disease. Mol Genet Metab. 2010, 100: 257-261. 10.1016/j.ymgme.2010.03.020.
Auray-Blais C, Ntwari A, Clarke JT, Warnock DG, Oliveira JP, Young SP, Millington DS, Bichet DG, Sirrs S, West ML, Casey R, Hwu WL, Keutzer JM, Zhang XK, Gagnon R: How well does urinary lyso-Gb3 function as a biomarker in Fabry disease?. Clin Chim Acta. 2010, 411: 1906-1914. 10.1016/j.cca.2010.07.038.
Sanchez-Nino MD, Sanz AB, Carrasco S, Saleem MA, Mathieson PW, Valdivielso JM, Ruiz-Ortega M, Egido J, Ortiz A: Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 2010,
Brakch N, Dormond O, Bekri S, Golshayan D, Correvon M, Mazzolai L, Steinmann B, Barbey F: Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J. 2010, 31: 67-76. 10.1093/eurheartj/ehp387.
Lacomis D, Roeske-Anderson L, Mathie L: Neuropathy and Fabry's disease. Muscle Nerve. 2005, 31: 102-107. 10.1002/mus.20130.
Saip S, Uluduz D, Erkol G: Fabry disease mimicking multiple sclerosis. Clin Neurol Neurosurg. 2007, 109: 361-363. 10.1016/j.clineuro.2006.12.006.
Linthorst GE, Hollak CE: Chloroquine-induced phospholipidosis of the kidney mimicking Fabry's disease. Hum Pathol. 2003, 34: 1358-10.1016/S0046-8177(03)00396-4.
Bennett RL, Hart KA, O'Rourke E, Barranger JA, Johnson J, MacDermot KD, Pastores GM, Steiner RD, Thadhani R: Fabry disease in genetic counseling practice: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2002, 11: 121-146. 10.1023/A:1014545521753.
Laney DA, Fernhoff PM: Diagnosis of Fabry disease via analysis of family history. J Genet Couns. 2008, 17: 79-83. 10.1007/s10897-007-9128-x.
Desnick RJ: Prenatal diagnosis of Fabry disease. Prenat Diagn. 2007, 27: 693-694. 10.1002/pd.1767.
Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR: Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003, 138: 338-346.
Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M: Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004, 34: 236-242. 10.1111/j.1365-2362.2004.01309.x.
Beck M: Agalsidase alfa - a preparation for enzyme replacement therapy in Anderson-Fabry disease. Expert Opin Investig Drugs. 2002, 11: 851-858. 10.1517/13543784.11.6.851.
Germain DP: Fabry disease: recent advances in enzyme replacement therapy. Expert Opin Investig Drugs. 2002, 11: 1467-1476. 10.1517/13543784.11.10.1467.
Desnick RJ: Enzyme replacement therapy for Fabry disease: lessons from two alpha-galactosidase A orphan products and one FDA approval. Expert Opin Biol Ther. 2004, 4: 1167-1176. 10.1517/14712598.4.7.1167.
Beck M: Agalsidase alfa for the treatment of Fabry disease: new data on clinical efficacy and safety. Expert Opin Biol Ther. 2009, 9: 255-261. 10.1517/14712590802658428.
Morel CF, Clarke JT: The use of agalsidase alfa enzyme replacement therapy in the treatment of Fabry disease. Expert Opin Biol Ther. 2009, 9: 631-639. 10.1517/14712590902902296.
Germain DP: [Fabry's disease (alpha-galactosidase-A deficiency): recent therapeutic innovations]. J Soc Biol. 2002, 196: 183-190.
Germain DP: [Current practice in Fabry disease: a comprehensive multidisciplinary approach]. Presse Med. 2007, 36: S3-6. 10.1016/j.lpm.2006.10.024.
Weidemann F, Sommer C, Duning T, Lanzl I, Mohrenschlager M, Naleschinski D, Arning K, Baron R, Niemann M, Breunig F, Schaefer R, Strotmann J, Wanner C: Department-related tasks and organ-targeted therapy in Fabry disease: an interdisciplinary challenge. Am J Med. 2010, 123: 658e1-658e10. 10.1016/j.amjmed.2009.12.022.
Gordon KE, Ludman MD, Finley GA: Successful treatment of painful crises of Fabry disease with low dose morphine. Pediatr Neurol. 1995, 12: 250-251. 10.1016/0887-8994(95)00007-3.
Lenoir G, Rivron M, Gubler MC, Dufier JL, Tome FS, Guivarch M: [Fabry's disease. Carbamazepine therapy in acrodyniform syndrome]. Arch Fr Pediatr. 1977, 34: 704-716.
Filling-Katz MR, Merrick HF, Fink JK, Miles RB, Sokol J, Barton NW: Carbamazepine in Fabry's disease: effective analgesia with dose- dependent exacerbation of autonomic dysfunction. Neurology. 1989, 39: 598-600.
Ries M, Mengel E, Kutschke G, Kim KS, Birklein F, Krummenauer F, Beck M: Use of gabapentin to reduce chronic neuropathic pain in Fabry disease. J Inherit Metab Dis. 2003, 26: 413-414. 10.1023/A:1025127723729.
Lockman LA, Hunninghake DB, Krivit W, Desnick RJ: Relief of pain of Fabry's disease by diphenylhydantoin. Neurology. 1973, 23: 871-875.
Argoff CE, Barton NW, Brady RO, Ziessman HA: Gastrointestinal symptoms and delayed gastric emptying in Fabry's disease: response to metoclopramide. Nucl Med Commun. 1998, 19: 887-891. 10.1097/00006231-199809000-00009.
Warnock DG: Fabry disease: diagnosis and management, with emphasis on the renal manifestations. Curr Opin Nephrol Hypertens. 2005, 14: 87-95. 10.1097/00041552-200503000-00002.
Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N: Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007, 18: 1547-1557. 10.1681/ASN.2006080816.
Wanner C, Breunig F: Fabry nephropathy and the case for adjunctive renal therapy. J Am Soc Nephrol. 2007, 18: 2426-2428. 10.1681/ASN.2007070783.
Warnock DG, Remuzzi G, Brenner BM, Levin A, Wanner C: Introduction to Focus on Fabry nephropathy: biomarkers, progression, and disease severity. Clin J Am Soc Nephrol. 2010, 5: 359-10.2215/CJN.08191109.
Tahir H, Jackson LL, Warnock DG: Antiproteinuric therapy and Fabry nephropathy: sustained reduction of proteinuria in patients receiving enzyme replacement therapy with agalsidase-beta. J Am Soc Nephrol. 2007, 18: 2609-2617. 10.1681/ASN.2006121400.
Kleinert J, Dehout F, Schwarting A, de Lorenzo AG, Ricci R, Kampmann C, Beck M, Ramaswami U, Linhart A, Gal A, Houge G, Widmer U, Mehta A, Sunder-Plassmann G: Prevalence of uncontrolled hypertension in patients with Fabry disease. Am J Hypertens. 2006, 19: 782-787. 10.1016/j.amjhyper.2006.01.011.
Thadhani R, Wolf M, West ML, Tonelli M, Ruthazer R, Pastores GM, Obrador GT: Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002, 61: 249-255. 10.1046/j.1523-1755.2002.00097.x.
Mignani R, Feriozzi S, Pisani A, Cioni A, Comotti C, Cossu M, Foschi A, Giudicissi A, Gotti E, Lozupone VA, Marchini F, Martinelli F, Bianco F, Panichi V, Procaccini DA, Ragazzoni E, Serra A, Soliani F, Spinelli L, Torti G, Veroux M, Cianciaruso B, Cagnoli L: Agalsidase therapy in patients with Fabry disease on renal replacement therapy: a nationwide study in Italy. Nephrol Dial Transplant. 2008, 23: 1628-1635. 10.1093/ndt/gfm813.
Cybulla M, Walter KN, Schwarting A, Divito R, Feriozzi S, Sunder-Plassmann G: Kidney transplantation in patients with Fabry disease. Transpl Int. 2009, 22: 475-481. 10.1111/j.1432-2277.2008.00824.x.
Ojo A, Meier-Kriesche HU, Friedman G, Hanson J, Cibrik D, Leichtman A, Kaplan B: Excellent outcome of renal transplantation in patients with Fabry's disease. Transplantation. 2000, 69: 2337-2339. 10.1097/00007890-200006150-00020.
Shah T, Gill J, Malhotra N, Takemoto SK, Bunnapradist S: Kidney transplant outcomes in patients with Fabry disease. Transplantation. 2009, 87: 280-285. 10.1097/TP.0b013e318191a842.
Politei JM: Can we use statins to prevent stroke in Fabry disease?. J Inherit Metab Dis. 2009, 32: 481-487. 10.1007/s10545-009-1156-2.
Cantor WJ, Daly P, Iwanochko M, Clarke JT, Cusimano RJ, Butany J: Cardiac transplantation for Fabry's disease. Can J Cardiol. 1998, 14: 81-84.
Lee K, Jin X, Zhang K, Copertino L, Andrews L, Baker-Malcolm J, Geagan L, Qiu H, Seiger K, Barngrover D, McPherson JM, Edmunds T: A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology. 2003, 13: 305-313. 10.1093/glycob/cwg034.
Sakuraba H, Murata-Ohsawa M, Kawashima I, Tajima Y, Kotani M, Ohshima T, Chiba Y, Takashiba M, Jigami Y, Fukushige T, Kanzaki T, Itoh K: Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J Hum Genet. 2006, 51: 180-188. 10.1007/s10038-005-0342-9.
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldeck S, Caplan L, Linthorst GE, Desnick RJ: Safety and efficacy of recombinant human α-galactosidase A - replacement therapy in Fabry's disease. N Engl J Med. 2001, 345: 9-16. 10.1056/NEJM200107053450102.
Schiffmann R, Kopp JB, Austin HA, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001, 285: 2743-2749. 10.1001/jama.285.21.2743.
Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ: Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007, 146: 77-86.
Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, Mehta AB: Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: a randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart. 2008, 94: 153-158. 10.1136/hrt.2006.104026.
Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO: Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol Dial Transplant. 2006, 21: 345-354. 10.1093/ndt/gfi152.
Schiffmann R, Askari H, Timmons M, Robinson C, Benko W, Brady RO, Ries M: Weekly enzyme replacement therapy may slow decline of renal function in patients with Fabry disease who are on long-term biweekly dosing. J Am Soc Nephrol. 2007, 18: 1576-1583. 10.1681/ASN.2006111263.
Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP: Long-term safety and efficacy of enzyme replacement therapy for Fabry's Disease. Am J Hum Genet. 2004, 75: 65-74. 10.1086/422366.
Beck M, Ricci R, Widmer U, Dehout F, de Lorenzo AG, Kampmann C, Linhart A, Sunder-Plassmann G, Houge G, Ramaswami U, Gal A, Mehta A: Fabry disease: overall effects of agalsidase alfa treatment. Eur J Clin Invest. 2004, 34: 838-844. 10.1111/j.1365-2362.2004.01424.x.
Hoffmann B, Garcia de Lorenzo A, Mehta A, Beck M, Widmer U, Ricci R: Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: data from FOS (Fabry Outcome Survey). J Med Genet. 2005, 42: 247-252. 10.1136/jmg.2004.025791.
Schwarting A, Dehout F, Feriozzi S, Beck M, Mehta A, Sunder-Plassmann G: Enzyme replacement therapy and renal function in 201 patients with Fabry disease. Clin Nephrol. 2006, 66: 77-84.
Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassman G, Schiffmann R, Barbey F, Ries M, Clarke JT: Enzyme replacement therapy with agalsidase alfa in patients with Fabry's disease: an analysis of registry data. Lancet. 2009, 374: 1886-1896. 10.1016/S0140-6736(09)61493-8.
Breunig F, Weidemann F, Strotmann J, Knoll A, Wanner C: Clinical benefit of enzyme replacement therapy in Fabry disease. Kidney Int. 2006, 69: 1216-1221. 10.1038/sj.ki.5000208.
Kalliokoski RJ, Kantola I, Kalliokoski KK, Engblom E, Sundell J, Hannukainen JC, Janatuinen T, Raitakari OT, Knuuti J, Penttinen M, Viikari J, Nuutila P: The effect of 12-month enzyme replacement therapy on myocardial perfusion in patients with Fabry disease. J Inherit Metab Dis. 2006, 29: 112-118. 10.1007/s10545-006-0221-3.
Vedder AC, Linthorst GE, Houge G, Groener JE, Ormel EE, Bouma BJ, Aerts JM, Hirth A, Hollak CE: Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2007, 2: e598-10.1371/journal.pone.0000598.
Vedder AC, Breunig F, Donker-Koopman WE, Mills K, Young E, Winchester B, Ten Berge IJ, Groener JE, Aerts JM, Wanner C, Hollak CE: Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol Genet Metab. 2008, 94: 319-325. 10.1016/j.ymgme.2008.03.003.
Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S, Voelker W, Ertl G, Wanner C, Strotmann J: Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009, 119: 524-529. 10.1161/CIRCULATIONAHA.108.794529.
Fabrazyme (agalsidase beta) European Public Assessment Report (EPAR). [http://www.emea.europa.eu/humandocs/Humans/EPAR/fabrazyme/fabrazyme.htm]
Replagal (agalsidase alfa) European Public Assessment Report (EPAR). [http://www.emea.europa.eu/humandocs/Humans/EPAR/replagal/replagal.htm]
Pastores GM, Thadani R: Advances in the management of Anderson-Fabry disease: enzyme replacement therapy. Expert Opin Biol Ther. 2002, 2: 1-9. 10.1517/14712598.2.1.1.
Ries M, Clarke JT, Whybra C, Timmons M, Robinson C, Schlaggar BL, Pastores G, Lien YH, Kampmann C, Brady RO, Beck M, Schiffmann R: Enzyme-replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics. 2006, 118: 924-932. 10.1542/peds.2005-2895.
Ramaswami U, Wendt S, Pintos-Morell G, Parini R, Whybra C, Leon Leal JA, Santus F, Beck M: Enzyme replacement therapy with agalsidase alfa in children with Fabry disease. Acta Paediatr. 2007, 96: 122-127. 10.1111/j.1651-2227.2007.00029.x.
Pintos-Morell G, Beck M: Fabry disease in children and the effects of enzyme replacement treatment. Eur J Pediatr. 2009, 168: 1355-1363. 10.1007/s00431-009-0937-9.
Ries M, Clarke JT, Whybra C, Mehta A, Loveday KS, Brady RO, Beck M, Schiffmann R: Enzyme replacement in Fabry disease: pharmacokinetics and pharmacodynamics of agalsidase alpha in children and adolescents. J Clin Pharmacol. 2007, 47: 1222-1230. 10.1177/0091270007305299.
Schiffmann R, Martin RA, Reimschisel T, Johnson K, Castaneda V, Lien YH, Pastores GM, Kampmann C, Ries M, Clarke JT: Four-year prospective clinical trial of agalsidase alfa in children with Fabry disease. J Pediatr. 2010, 156: 832-837. 10.1016/j.jpeds.2009.11.007.
Jardim LB, Gomes I, Netto CB, Nora DB, Matte US, Pereira F, Burin MG, Kalakun L, Giugliani R, Becker J: Improvement of sympathetic skin responses under enzyme replacement therapy in Fabry disease. J Inherit Metab Dis. 2006, 29: 653-659. 10.1007/s10545-006-0339-3.
Dehout F, Schwarting A, Beck M, Mehta A, Ricci R, Widmer U: Effects of enzyme replacement therapy with agalsidase alfa on glomerular filtration rate in patients with Fabry disease: preliminary data. Acta Paediatr Suppl. 2003, 92: 14-15. 10.1111/j.1651-2227.2003.tb00214.x.
Feriozzi S, Schwarting A, Sunder-Plassmann G, West M, Cybulla M: Agalsidase alfa slows the decline in renal function in patients with Fabry disease. Am J Nephrol. 2008, 29: 353-361. 10.1159/000168482.
West M, Nicholls K, Mehta A, Clarke JT, Steiner R, Beck M, Barshop BA, Rhead W, Mensah R, Ries M, Schiffmann R: Agalsidase alfa and kidney dysfunction in Fabry disease. J Am Soc Nephrol. 2009, 20: 1132-1139. 10.1681/ASN.2008080870.
Baehner F, Kampmann C, Whybra C, Miebach E, Wiethoff CM, Beck M: Enzyme replacement therapy in heterozygous females with Fabry disease: results of a phase IIIB study. J Inherit Metab Dis. 2003, 26: 617-627. 10.1023/B:BOLI.0000005658.14563.77.
Germain DP: Fabry disease: the need to stratify patient populations to better understand the outcome of enzyme replacement therapy. Clin Ther. 2007, 29: S17-S18. 10.1016/S0149-2918(07)80122-6.
Brady RO, Schiffmann R: Enzyme-replacement therapy for metabolic storage disorders. Lancet Neurol. 2004, 3: 752-756. 10.1016/S1474-4422(04)00938-X.
Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M: The Mainz Severity Score Index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet. 2004, 65: 299-307. 10.1111/j.1399-0004.2004.00219.x.
Parini R, Rigoldi M, Santus F, Furlan F, De Lorenzo P, Valsecchi G, Concolino D, Strisciuglio P, Feriozzi S, Di Vito R, Ravaglia R, Ricci R, Morrone A: Enzyme replacement therapy with agalsidase alfa in a cohort of Italian patients with Anderson-Fabry disease: testing the effects with the Mainz Severity Score Index. Clin Genet. 2008, 74: 260-266. 10.1111/j.1399-0004.2008.01012.x.
Whybra C, Miebach E, Mengel E, Gal A, Baron K, Beck M, Kampmann C: A 4-year study of the efficacy and tolerability of enzyme replacement therapy with agalsidase alfa in 36 women with Fabry disease. Genet Med. 2009, 11: 441-449. 10.1097/GIM.0b013e3181a23bec.
Thurberg BL, Rennke H, Colvin RB, Dikman S, Gordon RE, Collins AB, Desnick RJ, O'Callaghan M: Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002, 62: 1933-1946. 10.1046/j.1523-1755.2002.00675.x.
Eto Y, Ohashi T, Utsunomiya Y, Fujiwara M, Mizuno A, Inui K, Sakai N, Kitagawa T, Suzuki Y, Mochizuki S, Kawakami M, Hosoya T, Owada M, Sakuraba H, Saito H: Enzyme replacement therapy in Japanese Fabry disease patients: the results of a phase 2 bridging study. J Inherit Metab Dis. 2005, 28: 575-583. 10.1007/s10545-005-0575-y.
Thurberg BL, Fallon JT, Mitchell R, Aretz T, Gordon RE, O'Callaghan MW: Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation. 2009, 119: 2561-2567. 10.1161/CIRCULATIONAHA.108.841494.
Schiffmann R, Rapkiewicz A, Abu-Asab M, Ries M, Askari H, Tsokos M, Quezado M: Pathological findings in a patient with Fabry disease who died after 2.5 years of enzyme replacement. Virchows Arch. 2006, 448: 337-343. 10.1007/s00428-005-0089-x.
Keslova-Veselikova J, Hulkova H, Dobrovolny R, Asfaw B, Poupetova H, Berna L, Sikora J, Golan L, Ledvinova J, Elleder M: Replacement of alpha-galactosidase A in Fabry disease: effect on fibroblast cultures compared with biopsied tissues of treated patients. Virchows Arch. 2008, 452: 651-665. 10.1007/s00428-008-0586-9.
Wraith JE, Tylki-Szymanska A, Guffon N, Lien YH, Tsimaratos M, Vellodi A, Germain DP: Safety and efficacy of enzyme replacement therapy with agalsidase beta: an international, open-label study in pediatric patients with Fabry disease. J Pediatr. 2008, 152: 563-570. 10.1016/j.jpeds.2007.09.007.
Hilz MJ, Brys M, Marthol H, Stemper B, Dutsch M: Enzyme replacement therapy improves function of C-, Adelta-, and Abeta-nerve fibers in Fabry neuropathy. Neurology. 2004, 62: 1066-1072.
Watt T, Burlina AP, Cazzorla C, Schonfeld D, Banikazemi M, Hopkin RJ, Martins AM, Sims K, Beitner-Johnson D, O'Brien F, Feldt-Rasmussen U: Agalsidase beta treatment is associated with improved quality of life in patients with Fabry disease: Findings from the Fabry Registry. Genet Med. 2010,
Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985, 33: 278-285.
Koskenvuo JW, Hartiala JJ, Nuutila P, Kalliokoski R, Viikari JS, Engblom E, Penttinen M, Knuuti J, Mononen I, Kantola IM: Twenty-four-month alpha-galactosidase A replacement therapy in Fabry disease has only minimal effects on symptoms and cardiovascular parameters. J Inherit Metab Dis. 2008, 31: 432-441. 10.1007/s10545-008-0848-3.
Imbriaco M, Pisani A, Spinelli L, Cuocolo A, Messalli G, Capuano E, Marmo M, Liuzzi R, Visciano B, Cianciaruso B, Salvatore M: Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: a prospective long-term cardiac magnetic resonance imaging study. Heart. 2009, 95: 1103-1107. 10.1136/hrt.2008.162800.
Lubanda JC, Anijalg E, Bzduch V, Thurberg BL, Benichou B, Tylki-Szymanska A: Evaluation of a low dose, after a standard therapeutic dose, of agalsidase beta during enzyme replacement therapy in patients with Fabry disease. Genet Med. 2009, 11: 256-264. 10.1097/GIM.0b013e3181981d82.
Mehta A, Beck M, Kampmann C, Frustaci A, Germain DP, Pastores GM, Sunder-Plassmann G: Enzyme replacement therapy in Fabry disease: comparison of agalsidase alfa and agalsidase beta. Mol Genet Metab. 2008, 95: 114-115. 10.1016/j.ymgme.2008.07.002.
Sirrs S, Clarke JT, Bichet DG, Casey R, Lemoine K, Flowerdew G, Sinasac DS, West ML: Baseline characteristics of patients enrolled in the Canadian Fabry Disease Initiative. Mol Genet Metab. 2010, 99: 367-373. 10.1016/j.ymgme.2009.11.001.
Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM: Enzyme therapy for Fabry disease: neutralizing antibodies toward agalsidase alpha and beta. Kidney Int. 2004, 66: 1589-1595. 10.1111/j.1523-1755.2004.00924.x.
Benichou B, Goyal S, Sung C, Norfleet AM, O'Brien F: A retrospective analysis of the potential impact of IgG antibodies to agalsidase beta on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab. 2009, 96: 4-12. 10.1016/j.ymgme.2008.10.004.
Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function measured and estimated glomerular filtration rate. N Engl J Med. 2006, 354: 2473-2483. 10.1056/NEJMra054415.
Waldek S, Germain DP, Wanner C, Warnock DG: Enzyme replacement therapy for Fabry's disease. Lancet. 2010, 375: 1523-10.1016/S0140-6736(10)60653-8.
Centre de référence pour la maladie de Fabry et les maladies héréditaires du tissu conjonctif. [http://www.centre-geneo.com]
Bodensteiner D, Scott CR, Sims KB, Shepherd GM, Cintron RD, Germain DP: Successful reinstitution of agalsidase beta therapy in Fabry disease patients with previous IgE-antibody or skin-test reactivity to the recombinant enzyme. Genet Med. 2008, 10: 353-358. 10.1097/GIM.0b013e318170f868.
Ohashi T, Iizuka S, Ida H, Eto Y: Reduced alpha-Gal A enzyme activity in Fabry fibroblast cells and Fabry mice tissues induced by serum from antibody positive patients with Fabry disease. Mol Genet Metab. 2008, 94: 313-318. 10.1016/j.ymgme.2008.03.008.
Germain DP: Gaucher's disease: a paradigm for interventional genetics. Clin Genet. 2004, 65: 77-86. 10.1111/j.0009-9163.2004.00217.x.
Germain DP, Puech JP, Caillaud C, Kahn A, Poenaru L: Exhaustive screening of the acid beta-glucosidase gene, by fluorescence-assisted mismatch analysis using universal primers: mutation profile and genotype/phenotype correlations in Gaucher disease. Am J Hum Genet. 1998, 63: 415-427. 10.1086/301969.
Germain DP, Kaneski CR, Brady RO: Mutation analysis of the acid beta-glucosidase gene in a patient with type 3 Gaucher disease and neutralizing antibody to alglucerase. Mutat Res. 2001, 483: 89-94.
Garman RD, Munroe K, Richards SM: Methotrexate reduces antibody responses to recombinant human alpha-galactosidase A therapy in a mouse model of Fabry disease. Clin Exp Immunol. 2004, 137: 496-502. 10.1111/j.1365-2249.2004.02567.x.
Kosch M, Koch HG, Oliveira JP, Soares C, Bianco F, Breuning F, Rasmussen AK, Schaefer RM: Enzyme replacement therapy administered during hemodialysis in patients with Fabry disease. Kidney Int. 2004, 66: 1279-1282. 10.1111/j.1523-1755.2004.00883.x.
Wendt S, Whybra C, Kampmann C, Teichmann E, Beck M: Successful pregnancy outcome in a patient with Fabry disease receiving enzyme replacement therapy with agalsidase alfa. J Inherit Metab Dis. 2005, 28: 787-788. 10.1007/s10545-005-0018-9.
Kalkum G, Macchiella D, Reinke J, Kolbl H, Beck M: Enzyme replacement therapy with agalsidase alfa in pregnant women with Fabry disease. Eur J Obstet Gynecol Reprod Biol. 2009, 144: 92-93. 10.1016/j.ejogrb.2009.01.007.
Germain DP, Bruneval P, Tran TC, Balouet P, Richalet B, Benistan K: Uneventful pregnancy outcome after enzyme replacement therapy with agalsidase beta in a heterozygous female with Fabry disease: A case report. Eur J Med Genet. 2010, 53: 111-112. 10.1016/j.ejmg.2009.12.004.
Politei JM: Treatment with agalsidase beta during pregnancy in Fabry disease. J Obstet Gynaecol Res. 2010, 36: 428-429. 10.1111/j.1447-0756.2009.01164.x.
Cousins A, Lee P, Rorman D, Raas-Rothschild A, Banikazemi M, Waldek S, Thompson L: Home-based infusion therapy for patients with Fabry disease. Br J Nurs. 2008, 17: 653-657.
Linthorst GE, Vedder AC, Ormel EE, Aerts JM, Hollak CE: Home treatment for Fabry disease: practice guidelines based on 3 years experience in The Netherlands. Nephrol Dial Transplant. 2006, 21: 355-360. 10.1093/ndt/gfi221.
Thomaidis T, Relle M, Golbas M, Brochhausen C, Galle PR, Beck M, Schwarting A: Downregulation of alpha-galactosidase A upregulates CD77: functional impact for Fabry nephropathy. Kidney Int. 2009, 75: 399-407. 10.1038/ki.2008.576.
Pastores GM, Hughes DA: To see a world in a grain of sand: elucidating the pathophysiology of Anderson-Fabry disease through investigations of a cellular model. Kidney Int. 2009, 75: 351-353. 10.1038/ki.2008.606.
Suzuki K, Proia RL: Mouse models of human lysosomal diseases. Brain Pathol. 1998, 8: 195-215. 10.1111/j.1750-3639.1998.tb00145.x.
Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB: Alpha-galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA. 1997, 94: 2540-2544. 10.1073/pnas.94.6.2540.
Gotlib RW, Bishop DF, Wang AM, Zeidner KM, Ioannou YA, Adler DA, Disteche CM, Desnick RJ: The entire genomic sequence and cDNA expression of mouse alpha-galactosidase A. Biochem Mol Med. 1996, 57: 139-48. 10.1006/bmme.1996.0020.
Ishii S, Yoshioka H, Mannen K, Kulkarni AB, Fan JQ: Transgenic mouse expressing human mutant alpha-galactosidase A in an endogenous enzyme deficient background: a biochemical animal model for studying active-site specific chaperone therapy for Fabry disease. Biochim Biophys Acta. 2004, 1690: 250-257.
Shimmoto M, Kase R, Itoh K, Utsumi K, Ishii S, Taya C, Yonekawa H, Sakuraba H: Generation and characterization of transgenic mice expressing a human mutant alpha-galactosidase with an R301Q substitution causing a variant form of Fabry disease. FEBS Lett. 1997, 417: 89-91. 10.1016/S0014-5793(97)01263-5.
Eitzman DT, Bodary PF, Shen Y, Khairallah CG, Wild SR, Abe A, Shaffer-Hartman J, Shayman JA: Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol. 2003, 14: 298-302. 10.1097/01.ASN.0000043901.45141.D4.
Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA: Decreased nitric oxide bioavailability in a mouse model of Fabry disease. J Am Soc Nephrol. 2009, 20: 1975-1985. 10.1681/ASN.2008111190.
Ohshima T, Schiffmann R, Murray GJ, Kopp J, Quirk JM, Stahl S, Chan CC, Zerfas P, Tao-Cheng JH, Ward JM, Brady RO, Kulkarni AB: Aging accentuates and bone marrow transplantation ameliorates metabolic defects in Fabry disease mice. Proc Natl Acad Sci USA. 1999, 96: 6423-6427. 10.1073/pnas.96.11.6423.
Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, Shayman JA: Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000, 105: 1563-1571. 10.1172/JCI9711.
Ioannou YA, Zeidner KM, Gordon RE, Desnick RJ: Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am J Hum Genet. 2001, 68: 14-25. 10.1086/316953.
Ishii S, Chang HH, Yoshioka H, Shimada T, Mannen K, Higuchi Y, Taguchi A, Fan JQ: Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J Pharmacol Exp Ther. 2009, 328: 723-731. 10.1124/jpet.108.149054.
Takenaka T, Murray GJ, Qin G, Quirk JM, Ohshima T, Qasba P, Clark K, Kulkarni AB, Brady RO, Medin JA: Long-term enzyme correction and lipid reduction in multiple organs of primary and secondary transplanted Fabry mice receiving transduced bone marrow cells. Proc Natl Acad Sci USA. 2000, 97: 7515-7520. 10.1073/pnas.120177997.
Jung SC, Han IP, Limaye A, Xu R, Gelderman MP, Zerfas P, Tirumalai K, Murray GJ, During MJ, Brady RO, Qasba P: Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc Natl Acad Sci USA. 2001, 98: 2676-2681. 10.1073/pnas.051634498.
Takahashi H, Hirai Y, Migita M, Seino Y, Fukuda Y, Sakuraba H, Kase R, Kobayashi T, Hashimoto Y, Shimada T: Long-term systemic therapy of Fabry disease in a knockout mouse by adeno-associated virus-mediated muscle-directed gene transfer. Proc Natl Acad Sci USA. 2002, 99: 13777-13782. 10.1073/pnas.222221899.
Park J, Murray GJ, Limaye A, Quirk JM, Gelderman MP, Brady RO, Qasba P: Long-term correction of globotriaosylceramide storage in Fabry mice by recombinant adeno-associated virus-mediated gene transfer. Proc Natl Acad Sci USA. 2003, 100: 3450-3454. 10.1073/pnas.0537900100.
Nakamura G, Maruyama H, Ishii S, Shimotori M, Kameda S, Kono T, Miyazaki J, Kulkarni AB, Gejyo F: Naked plasmid DNA-based alpha-galactosidase A gene transfer partially reduces systemic accumulation of globotriaosylceramide in Fabry mice. Mol Biotechnol. 2008, 38: 109-119. 10.1007/s12033-007-9008-5.
Koeberl DD: Age-related efficacy with an AAV vector in Fabry disease mice. Mol Genet Metab. 2009, 96: 83-84. 10.1016/j.ymgme.2008.10.014.
Fabry Registry. [http://www.lsdregistry.net/fabryregistry/]
Fabry Outcome Survey. [http://www.globaloutcomesurveys.com]
Deegan PB, Baehner AF, Barba Romero MA, Hughes DA, Kampmann C, Beck M: Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006, 43: 347-352. 10.1136/jmg.2005.036327.
Giannini EH, Mehta AB, Hilz MJ, Beck M, Bichet DG, Brady RO, West M, Germain DP, Wanner C, Waldek S, Clarke JT, Mengel E, Strotmann JM, Warnock DG, Linhart A: A validated disease severity scoring system for Fabry disease. Mol Genet Metab. 2010, 99: 283-290. 10.1016/j.ymgme.2009.10.178.
Ortiz A, Cianciaruso B, Cizmarik M, Germain DP, Mignani R, Oliveira JP, Villalobos J, Vujkovac B, Waldek S, Wanner C, Warnock DG: End-stage renal disease in patients with Fabry disease: natural history data from the Fabry Registry. Nephrol Dial Transplant. 2010, 25: 769-775. 10.1093/ndt/gfp554.
Tomasic IB, Metcalf MC, Guce AI, Clark NE, Garman SC: Interconversion of the specificities of human lysosomal enzymes associated with Fabry and Schindler diseases. J Biol Chem. 2010, 285: 21560-21566. 10.1074/jbc.M110.118588.
Tajima Y, Kawashima I, Tsukimura T, Sugawara K, Kuroda M, Suzuki T, Togawa T, Chiba Y, Jigami Y, Ohno K, Fukushige T, Kanekura T, Itoh K, Ohashi T, Sakuraba H: Use of a modified alpha-N-acetylgalactosaminidase in the development of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2009, 85: 569-580. 10.1016/j.ajhg.2009.09.016.
Garman SC: Structure-function relationships in alpha-galactosidase A. Acta Paediatr Suppl. 2007, 96: 6-16. 10.1111/j.1651-2227.2007.00198.x.
Yam GH, Zuber C, Roth J: A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J. 2005, 19: 12-18. 10.1096/fj.04-2375com.
Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J: Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006, 290: C1076-1082. 10.1152/ajpcell.00426.2005.
Fan JQ, Ishii S: Active-site-specific chaperone therapy for Fabry disease. Yin and Yang of enzyme inhibitors. FEBS J. 2007, 274: 4962-4971. 10.1111/j.1742-4658.2007.06041.x.
Germain DP, Fan JQ: Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: in vitro and preclinical studies. Int J Clin Pharmacol Ther. 2009, 47: S111-117.
Okumiya T, Ishii S, Takenaka T, Kase R, Kamei S, Sakuraba H, Suzuki Y: Galactose stabilizes various missense mutants of alpha-galactosidase in Fabry disease. Biochem Biophys Res Commun. 1995, 214: 1219-1224. 10.1006/bbrc.1995.2416.
Fan JQ, Ishii S, Asano N, Suzuki Y: Accelerated transport and maturation of lysosomal a-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nature Med. 1999, 5: 112-115. 10.1038/4801.
Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, Eng CM, Desnick RJ: Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy. N Engl J Med. 2001, 345: 25-32. 10.1056/NEJM200107053450104.
Sugawara K, Tajima Y, Kawashima I, Tsukimura T, Saito S, Ohno K, Iwamoto K, Kobayashi T, Itoh K, Sakuraba H: Molecular interaction of imino sugars with human alpha-galactosidase: Insight into the mechanism of complex formation and pharmacological chaperone action in Fabry disease. Mol Genet Metab. 2009, 96: 233-238. 10.1016/j.ymgme.2008.12.017.
Benjamin ER, Flanagan JJ, Schilling A, Chang HH, Agarwal L, Katz E, Wu X, Pine C, Wustman B, Desnick RJ, Lockhart DJ, Valenzano KJ: The pharmacological chaperone 1-deoxygalactonojirimycin increases alpha-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis. 2009, 32: 424-440. 10.1007/s10545-009-1077-0.
MacDermot KD, Holmes A, Miners AH: Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001, 38: 750-760. 10.1136/jmg.38.11.750.
Ozkara HA, Topcu M: Sphingolipidoses in Turkey. Brain Dev. 2004, 26: 363-366. 10.1016/j.braindev.2004.01.005.
Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcao A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sa Miranda MC: Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004, 12: 87-92. 10.1038/sj.ejhg.5201044.
Acknowledgements
I am particularly grateful to my patients and their families. I thank my collaborator Dr. Karelle BENISTAN, MD. I am grateful to Genzyme Corporation and Shire HGT for their continuous scientific support. This work was supported by the French Ministry of Health within the "Plan National Maladies Rares" program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dominique P. GERMAIN is a consultant for Genzyme Corporation and Shire HGT. He has received speaker's fees, research support and honoraria from Genzyme Corporation and Shire HGT.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Germain, D.P. Fabry disease. Orphanet J Rare Dis 5, 30 (2010). https://doi.org/10.1186/1750-1172-5-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1172-5-30