Abstract
Purpose of Review
Bempedoic acid is a novel therapeutic agent that is designed to reduce levels of low-density lipoprotein cholesterol (LDL-C). The purpose of this review is to provide the background for development of bempedoic acid, findings from clinical trials and to discuss clinical implications.
Recent Findings
Bempedoic acid inhibits ATP citrate lyase within the liver and reduces cholesterol synthesis, with the potential to avoid muscle symptoms experienced by patients treated with statins. Early clinical studies demonstrated that administration of bempedoic acid resulted in lowering of LDL-C by 20–30% as monotherapy and by 40–50% when combined with ezetimibe, in addition to lowering of high sensitivity C-reactive protein by 20–30%. The CLEAR Outcomes trial of high cardiovascular risk patients, with elevated LDL-C levels and either unable or unwilling to take statins demonstrated that bempedoic acid reduced the rate of major adverse cardiovascular events. A greater incidence of elevation of hepatic transaminase and creatinine, gout, and cholelithiasis were consistently observed in bempedoic acid–treated patients.
Summary
Bempedoic acid presents an additional therapeutic option to achieve more effective lowering of LDL-C levels and reduction in cardiovascular risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical trials have consistently demonstrated that lowering levels of low-density lipoprotein cholesterol (LDL-C) produces reductions in cardiovascular event rates in high risk patients [1,2,3,4,5,6,7,8,9, 10••]. The results of these trials have transformed approaches to the prevention of cardiovascular disease [11, 12]. However, the finding that many individuals fail to achieve the degree of LDL-C lowering required for effective risk reduction suggests that additional approaches are required. This has led to the development of a range of new therapies for use in clinical practice.
LDL and Cardiovascular Risk
An abundant body of evidence has established that LDL-C plays a causal role in atherosclerotic disease. Early observations from cohort studies have been supported by the findings of both preclinical investigation and Mendelian randomization to demonstrate that higher LDL-C levels associate with a greater risk of atherosclerotic disease, supporting the need for therapeutic interventions to lower circulating levels of atherogenic lipid parameters [13]. Clinical trials of statin therapy demonstrated that lowering LDL-C levels associated with reductions in cardiovascular event rates in the primary and secondary prevention settings [1,2,3,4,5,6] and slow progression of coronary atherosclerosis [14,15,16]. More recent studies that compared the effects of more and less intensive statin therapy resulted in greater reduction in cardiovascular risk, associated with achieving lower LDL-C levels [6]. On the basis of these findings, prevention guidelines have been successively updated to highlight the importance of intensive lipid lowering, with statin therapy as the cornerstone, to achieve more effective prevention of cardiovascular events [11, 12].
The finding that many patients continue to experience cardiovascular events, despite statin treatment, supports the need for additional lipid-lowering agents in clinical practice [17]. Clinical trials have subsequently demonstrated the clinical benefit of adding either ezetimibe [7] or proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors [8, 9] to statin therapy. This supports the benefits of combination therapy to produce more effective lipid lowering in a greater proportion of patients, resulting in less clinical events. Ongoing investigation has been undertaken to develop novel lipid-lowering agents that can be employed as either monotherapy or in addition to statin therapy.
Bempedoic Acid
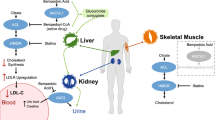
Bempedoic acid is an oral inhibitor of ATP citrate lyase (ACL), a factor involved in the same cholesterol synthesis pathway within the liver as hydroxy methyl glutaryl coenzyme A (HMGCoA) reductase, the target of statins [18]. Accordingly, inhibition of ACL should similarly result in reductions in hepatic cholesterol synthesis and an increase in hepatocyte surface expression of the LDL receptor, resulting in greater removal of LDL-C from the circulation (Figure 1). Mendelian randomization studies have established that polymorphisms resulting in less ACL associate with a reduction in cardiovascular risk, directly proportional to the degree of lowering of either LDL-C or apolipoprotein (apo) B [19]. This would suggest that ACL inhibition has the potential to be a promising agent for both lowering LDL-C levels and the prevention of cardiovascular disease.
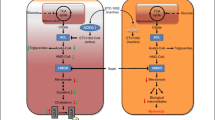
Mechanism of bempedoic acid and statins in lipid lowering. Mechanism of bempedoic acid and statins in lowering levels of low-density lipoprotein cholesterol (LDL-C). Both agents lead to a reduction in hepatic intracellular cholesterol levels, with consequent upregulation of surface LDL receptor expression, greater LDL clearance, and a reduction in plasma LDL-C levels. ATP, adenosine triphosphate; HMG-CoA, hydroxy methyl glutaryl coenzyme A
Bempedoic acid is an oral, small molecule inhibitor of ACL. It is ingested in an inactive form and requires activation by the isozyme ACSVL1 which is present in the liver, but not skeletal muscle [18, 20, 21]. In theory, this provides the potential for lipid lowering, without the myalgia that is experienced by many patients treated with a statin. Early clinical studies demonstrated that treatment with bempedoic acid produced reductions in LDL-C by 20–30% as monotherapy [22,23,24,25,26,27,28,29] and by 40–50% [30] when used in combination with ezetimibe, in patients with and without statin associated muscle symptoms. This led to a large phase 3 clinical development program: Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) to comprehensively evaluate the impact of bempedoic acid on lipid levels and cardiovascular outcomes.
Monotherapy studies of bempedoic acid have been conducted across the spectrum of statin tolerance. The CLEAR Harmony [27] and Wisdom [28] studies evaluated the impact of bempedoic acid in patients treated with either high intensity (51%) or low to moderate intensity (49%) statin therapy. These studies demonstrated placebo-corrected reductions in LDL-C with bempedoic acid by 18.1% and 17.4%, respectively. CLEAR Serenity [29] evaluated the impact of bempedoic acid in patients meeting a traditional definition for statin intolerance, requiring documented failure of two statins, one at the lowest available dose. Placebo-corrected reductions in LDL-C by bempedoic acid by 21.4% were reported. CLEAR Tranquility also involved patients with statin intolerance, but employed a more pragmatic definition for statin intolerance, permitting recruitment of patients who had failed only one statin at any dose. In this study, placebo-corrected reductions in LDL-C of 28.5% were observed with bempedoic acid [26]. Reductions in high sensitivity C-reactive protein (hsCRP) by 18.7–32.5% were also observed in these studies. These early studies demonstrated that treatment with bempedoic acid was associated with a greater incidence of liver transaminase and creatinine elevations, gout and cholelithiasis. The CLEAR Harmony and Wisdom studies reported the incidence of tendon rupture in 10 patients treated with bempedoic acid compared with 0 in the placebo groups.
A combination study of 382 patients with atherosclerotic cardiovascular disease, heterozygous familial hypercholesterolemia, or the presence of multiple cardiovascular risk factors, treated with maximally tolerated statin therapy, compared the effects of treatment with placebo, ezetimibe, and bempedoic acid 180 mg as monotherapy or in combination with ezetimibe for 12 weeks. Placebo-corrected reductions in LDL-C were observed with ezetimibe by 25%, bempedoic acid by 19% and the combination by 38% [30]. Reductions in hsCRP levels by 35.1% were observed with the combination of bempedoic acid and ezetimibe [30]. An additional study of the combination of bempedoic acid, ezetimibe, and atorvastatin 20 mg daily produced reductions in LDL-C by 63.6% and hsCRP by 47.7% [31].
CLEAR Outcomes
The CLEAR Outcomes study was conducted in 13,970 high cardiovascular risk patients with elevated LDL-C levels and documented statin intolerance. Patients were required to have clinically manifest atherosclerotic cardiovascular disease or be deemed to be at high risk of having a cardiovascular event with a LDL-C at least 100 mg/dL (2.6 mmol/L). All patients were required to have statin intolerance, defined as having experienced an adverse effect that started or increased during statin therapy and resolved or improved after therapy was discontinued. Patients were required to have experienced intolerance to at least two statins or one if they were unwilling to attempt a second statin or they were advised by a physician to not attempt a second statin. All patients and investigators were required to document that they were unable to tolerate medications that have been documented to reduce the risk of heart attack and stroke. Patients treated with very low doses of statins, less than the daily approved doses, were permitted to participate in the study. Patients were treated with bempedoic acid 180 mg or matching placebo daily and followed for at least 24 months and until at least 1620 primary endpoints (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and coronary revascularization) and 810 key secondary endpoints (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke) occurred [10••, 32].
Patients were recruited at 1250 sites in 32 countries from December 2016 to August 2019 and were followed for a median duration of 40.6 months, experiencing 1746 primary endpoints and 1238 key secondary endpoints. Baseline demographics included mean age 65 years, 48% female sex, 70% secondary prevention, 45% diabetes, LDL-C 139 mg/dL (3.5 mmol/L), and hsCRP 2.3 mg/L. Low-dose statins were used in 22% of patients. At 6 months, bempedoic acid produced greater lowering of LDL-C (− 21.7 vs − 0.6%) and hsCRP (− 22.2 vs + 2.4%) compared with placebo. Over the course of the study, the LDL-C levels between groups narrowed, as a result of disproportionate cross in of lipid-lowering agents in the placebo group [10••].
For the primary composite endpoint, a hazard ratio in favor of benefit with bempedoic acid was observed (HR 0.87, 95% CI 0.79–0.96, P = 0.004) was observed. This translated to an absolute risk reduction of 1.6% and number needed to treat to prevent one event of 63. For the key secondary composite endpoint a similar benefit was observed in favor of benefit with bempedoic acid (HR 0.85, 95% CI 0.76–0.96, P = 0.006). For both of these endpoints, event curves began to separate after 9 months, consistent with prior lipid-lowering trials. Similarly, reductions in the incidence of fatal and nonfatal myocardial infarction (HR 0.77, 95% CI 0.66–0.91, P = 0.002) and coronary revascularization (HR 0.81, 95% CI 0.72–0.92, P = 0.001) were also observed with bempedoic acid. In contrast, no significant reduction in stroke, all cause or cardiovascular mortality was observed [10••].
No difference was observed between the groups with regard to the incidence of adverse events, discontinuation of study drug or muscle events. Patients treated with bempedoic acid experienced a greater incidence of elevation of liver transaminases or creatinine, gout or cholelithiasis. These events were mild and rapidly resolved with discontinuation of study drug. On the basis of the finding from early studies, CLEAR Outcomes prespecified formal adjudication of tendon rupture events and found no difference in its incidence between treatment groups [10••].
Primary Prevention, Total Events, Diabetes, CTT
Subgroup analysis of the primary composite endpoint revealed no evidence of treatment heterogeneity. However, it did suggest potentially greater cardiovascular benefit with bempedoic acid in patients without clinically manifest atherosclerotic cardiovascular disease at study entry [10••]. These patients were recruited on the basis of having a Reynolds Risk score > 30%, SCORE risk > 7.5% over 10 years, diabetes with age > 65 years in women or > 60 years in men or a coronary calcium score > 400 Hounsfield units. The primary prevention cohort were more likely to be female (59%) and have diabetes (66%) with similar reductions in LDL-C and hsCRP with bempedoic acid. Greater reductions in the primary endpoint (HR 0.70, 95% CI 0.55–0.89, P = 0.002), key secondary endpoint (HR 0.64, 95% CI 0.48–0.84, P < 0.001) and myocardial infarction (HR 0.61, 95% CI 0.39–0.98) were observed with bempedoic acid. In contrast to the overall findings of the study, the primary prevention cohort demonstrated significant reductions in cardiovascular (HR 0.61, 95% CI 0.41–0.92) and all-cause (HR 0.73, 95% CI 0.54–0.98) mortality with bempedoic acid [33••].
Additional analyses of CLEAR Outcomes have demonstrated that the extent of cardiovascular risk reduction for the degree of LDL-C lowering fit on the Cholesterol Treatment Trialist’s curve for the secondary prevention cohort and was greater for those without clinically manifest disease at baseline (https://www.hcplive.com/view/clear-outcomes-data-at-endo-2023-provides-further-insight-into-effects-of-bempedoic-acid [https://www.hcplive.com/view/clear-outcomes-data-at-endo-2023-provides-further-insight-into-effects-of-bempedoic-acid]). Greater clinical benefit was observed for bempedoic acid on total events analysis and in patients with diabetes at baseline [34, 35]. No adverse effect was observed on measures of glycemic control and there was no greater likelihood of developing new onset diabetes during the study [35]. These findings extend the benefits of bempedoic acid on LDL-C and hsCRP to reductions in the risk of cardiovascular events.
Implications for Statin Intolerance
CLEAR Outcomes represents the largest clinical trial conducted in patients who were unable or unwilling to take approved doses of statins. Clinical registries of high cardiovascular risk patients demonstrate that at least 50% of patients fail to be treated with intensive statin therapy [36] and that 50% of patients prescribed a statin will cease therapy within the next 18 months [37, 38]. Predictably, reduced adherence to statin therapy will have implications for suboptimal reductions in LDL-C and cardiovascular risk [39]. A major factor contributing to either discontinuation or use of appropriate doses of statin therapy is the incidence of symptoms of intolerance, with an incidence of at least 10% in cohort studies [40]. The true mechanism underlying the experience of statin associated muscle symptoms remains uncertain, with some evidence that the nocebo effect may play an important role [41]. Nevertheless, patients unable to tolerate appropriate doses of statin therapy fail to achieve the degree of lipid lowering required to reduce their cardiovascular risk.
A number of therapeutic options currently exist for the management of these patients. Attempts at using lower doses of statins, at less than daily frequency of administration, has been reported to produce LDL-C lowering by more than 20% in patients [42]. Similarly, use of either ezetimibe or PCSK9 inhibitors [43] has been demonstrated to produce effective LDL-C lowering in patients with statin intolerance and both have been reported to produce reductions in cardiovascular risk in large outcomes trials [7,8,9]. The finding of CLEAR Outcomes provides definitive evidence that use of bempedoic acid effectively lowers both LDL-C and hsCRP, in addition to reducing cardiovascular risk in patients who are unable or unwilling to take a statin. This provides important evidence of a clinical pathway that could be effectively used for these patients.
Implications for Combination Therapy
The studies within the CLEAR development program establish the efficacy, safety, and tolerability data to guide use of bempedoic acid in clinical practice. While the utility in patients with statin intolerance is supported by the benefits of the cardiovascular outcomes trial, the potential to incorporate bempedoic acid within combination lipid lowering algorithms may provide a much greater therapeutic opportunity. Cohort studies of high cardiovascular risk patients consistently report a low rate of use of combination therapy, albeit with a trend toward increasing utilization [36, 44]. However, with progressive updates to treatment guidelines, advocating lower LDL-C goals, a greater proportion of patients will require use of combination therapy to reach these targets. This will require treatment of dyslipidemia to become similar to that of hypertension and type 2 diabetes, in which use of combination therapy has become commonplace.
Implications for Primary Prevention and High Risk
The additional analyses of the CLEAR Outcomes trial provide further information regarding the modifiability of cardiovascular risk. The finding of greater benefit in the primary prevention patients highlights the potential to substantially lower the risk of cardiovascular events in patients who are deemed to be at high risk, yet have not experienced an adverse clinical outcome. This supports similar findings of benefit of statins in high risk primary prevention cohorts and highlights the potential modifiability in these patients [45, 46]. Similarly, the finding of greater absolute benefit from the perspective of total events and in patients with diabetes further identifies the ability to target more intensive therapy to patients at greater risk, which has important health and economic implications.
Future Directions
The benefits of bempedoic acid further highlight the benefits of lipid lowering in reducing cardiovascular risk. Additional work is required to implement the findings of these studies into clinical practice. In addition, ongoing development programs are evaluating the therapeutic potential of novel LDL-C lowering agents including both oral [47] and new injectable [48] PCSK9 inhibitors, ongoing attempts to determine if cholesteryl ester transfer protein inhibitors reduce cardiovascular risk [49] and the emergence of gene editing interventions [50] that have the potential to produce once in a lifetime approaches to lipid lowering. With an increasing toolbox of therapeutic strategies to lower LDL-C there is a greater potential to achieve effective lipid lowering in the majority of high risk patients. The ongoing challenge to maintain long term adherence with therapy requires development of novel models of care focusing on both patients and their treating health care professionals.
Conclusions
Bempedoic acid provides an additional approach to lipid lowering with unequivocal data from clinical studies demonstrating its efficacy in improving lipid profiles and reducing cardiovascular risk. Whether administered as monotherapy in patients who are either unwilling or unable to take statins or in combination with other lipid-lowering agents, it provides another option in clinical practice to achieve more effective control of LDL-C and enhance our ability to prevent cardiovascular events.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389.
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7.
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9.
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–1357.
MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22.
Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–97.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–107.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
•• Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388(15):1353–64. Total results of the CLEAR Outcomes study.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–143.
Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease 1 Evidence from genetic, epidemiologic, and clinical studies A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart. 2017;38(32):2459–72.
Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87.
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65.
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291(9):1071–80.
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8.
Bilen O, Ballantyne CM. Bempedoic acid (ETC-1002): an investigational inhibitor of ATP citrate lyase. Curr Atheroscler Rep. 2016;18(10):61.
Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380(11):1033–42.
Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54(1):134–51.
Cramer CT, Goetz B, Hopson KL, et al. Effects of a novel dual lipid synthesis inhibitor and its potential utility in treating dyslipidemia and metabolic syndrome. J Lipid Res. 2004;45(7):1289–301.
Ballantyne CM, Davidson MH, Macdougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62(13):1154–62.
Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34(3):676–83.
Thompson PD, Rubino J, Janik MJ, et al. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015;9(3):295–304.
Ballantyne CM, McKenney JM, MacDougall DE, et al. Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol. 2016;117(12):1928–33.
Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203.
Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–32.
Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322(18):1780–8.
Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662.
Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603.
Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis. 2021;320:122–8.
Nicholls S, Lincoff AM, Bays HE, et al. Rationale and design of the CLEAR-outcomes trial: Evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–12.
•• Nissen SE, Menon V, Nicholls SJ, et al. Bempedoic acid for primary prevention of cardiovascular events in statin-intolerant patients. JAMA. 2023;330(2):131–40. Impact of bempedoic acid in high risk primary prevention patients.
Nicholls SJ, Nelson AJ, Lincoff AM, et al. Impact of bempedoic acid on total cardiovascular events. A prespecified analysis of the CLEAR Outcomes randomized clinical trial. JAMA Cardiol. 2024. https://doi.org/10.1001/jamacardio.2023.5155.
Ray KK, Nicholls SJ, Li N, et al. Efficacy and safety of bempedoic acid among patients with and without diabetes: prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diab Endocrin. 2024;12(1):19–28.
Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279–89.
Lin I, Sung J, Sanchez RJ, et al. Patterns of statin use in a real-world population of patients at high cardiovascular risk. J Manag Care Spec Pharm. 2016;22(6):685–98.
De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78(4):684–98.
Khunti K, Danese MD, Kutikova L, et al. Association of a combined measure of adherence and treatment intensity with cardiovascular outcomes in patients with atherosclerosis or other cardiovascular risk factors treated with statins and/or ezetimibe. JAMA Netw Open. 2018;1(8):e185554.
Bytyci I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43(34):3213–23.
Wood FA, Howard JP, Finegold JA, et al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med. 2020;383(22):2182–4.
Mampuya WM, Frid D, Rocco M, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166(3):597–603.
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580–90.
Ray KK, Haq I, Bilitou A, et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: the multinational observational SANTORINI study. Lancet Reg Health Eur. 2023;29:100624.
Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2021–31.
Ballantyne CM, Banka P, Mendez G, et al. Phase 2b randomized trial of the oral PCSK9 inhibitor MK-0616. J Am Coll Cardiol. 2023;81(16):1553–64.
Raal F, Fourie N, Scott R, et al. Long-term efficacy and safety of lerodalcibep in heterozygous familial hypercholesterolaemia: the LIBerate-HeFH trial. Eur Heart J 2023.
Nicholls SJ, Ditmarsch M, Kastelein JJ, et al. Lipid lowering effects of the CETP inhibitor obicetrapib in combination with high-intensity statins: a randomized phase 2 trial. Nat Med. 2022;28(8):1672–8.
Musunuru K, Chadwick AC, Mizoguchi T, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;593(7859):429–34.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
S.J.N. and T.A. drafted the manuscript. A.J.N. provided critical review.
Corresponding author
Ethics declarations
Conflict of Interest
S.J.N. has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Resverlogix, New Amsterdam Pharma, Novartis, InfraReDx and Sanofi-Regeneron. S.J.N. is also a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi- Regeneron, Vaxxinity, CSL Sequiris, and Novo Nordisk. S.J.N. also reports grants and personal fees from Esperion. A.J.N. has received research support and consulting fees from Amgen and Novartis. T.A. has no relationships to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abrahams, T., Nelson, A.J. & Nicholls, S.J. How Will Our Practice Change After the CLEAR Outcomes Trial?. Curr Atheroscler Rep 26, 83–89 (2024). https://doi.org/10.1007/s11883-024-01188-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-024-01188-5