Abstract
Purpose of Review
The purpose of this literature review was to review the latest advancements with biologics in rapid drug desensitization. Our methodology was to highlight both desensitization to biologics themselves and the use of biologics in desensitization to both biologic and nonbiologic drugs.
Recent Findings
Biologics are a vast category of drugs that include monoclonal antibodies, nanobodies, modern vaccinations, and even hormones. Desensitization to biologics can be safely performed through standardized procedure. Biomarkers are used both in vitro and in vivo to help identify and classify hypersensitivity reactions. Hypersensitivity reactions to the mRNA vaccinations against SARS-CoV-2 present their own unique challenges to management. There are specific excipients in monoclonal antibodies that are thought to be responsible for many of their hypersensitivity reactions. Certain biologics can even be used to assist in desensitization to other drugs.
Summary
Rapid drug desensitization is a standardized procedure that may be able to help many patients who have experienced hypersensitivity reactions to biologics and would best be treated with them to continue to receive them. Biologic drugs have opened a new era in medicine for the prevention and treatment of infectious diseases, cancer, and inflammatory diseases. Hypersensitivity reactions to biologics are quite common. This literature review presents the latest advancements in our understanding of hypersensitivity reactions to biologics, how rapid drug desensitization can be used to continue therapy despite history of hypersensitivity, and how biologics themselves can be used to aid in desensitization itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biologics are drugs comprised of organic molecules that only living systems can produce and always have either a gene or a protein as their therapeutic target [1]. They comprise a diverse class of medications that includes monoclonal antibodies, nanobodies, modern vaccinations, and hormones. Monoclonal antibodies were once categorized and named based on the degree of immunogenicity of their Fab fragments (e.g., having the suffix “-mab” with infixes of “-o-” for murine, “-xi-” for chimeric, “-zu-” for humanized, “-u-” for fully human, or the suffix “-cept” for receptor fusion) but those made after November 2021 are now categorized and named differently—with suffixes “-tug” for unmodified immunoglobulins, “-bart” for monospecific antibodies with artificially engineered constant domains, “-mig” for multispecific antibodies, and “-ment” for fragments without an Fc domain [2,3,4] (Fig. 1). Nanobodies are specifically just the antigen-binding fragment of what could be recognized as a monoclonal antibody with no Fc portion or corresponding Fab fragment, making them the smallest known natural molecules that can bind a target protein epitope [5]. There have been numerous advances in the therapy of specific cancers and neurodegenerative diseases with the use of nanobodies without a single reported case of hypersensitivity to date. Modern vaccinations that use an mRNA platform, first used clinically with vaccinations against SARS-CoV-2, are by their very design biologic drugs and have shown tremendous efficacy [6,7,8]. Exogenous hormones used for the management of various endocrine diseases are also of biologic origin.
International Nonproprietary Names (INN) nomenclature for monoclonal antibodies. A The former classification (before November 2021) first required all monoclonal antibodies (MAbs) to carry the suffix -mab unless they were formed by the fusion of a receptor ligand and an Fc segment, where it would carry the suffix -cept. An infix would be included in the middle of the term (-o-, -xi-, -zu-, or -u-) based on how the MAb was produced (murine, chimeric, humanized, or human). A chimeric antibody has foreign amino acids making up the entire V heavy and light chains and is linked to heavy and light C regions of human origin. A humanized antibody has segments of foreign amino acids interspersed with V segments of human amino acids and the V heavy and light domains are linked to heavy and light C regions of human origin. Other infixes exist in the naming convention but have never been used (-e- for hamster, -i- for primate, etc.). B The current classification applies to all MAbs made after November 2021 and classifies them by their complementarity-determining regions (CDRs) and C regions. MAbs with unmodified C regions and identical sets of CDRs that recognize the same epitope of their target have the suffix -tug. This applies no matter the source of the rest of the MAb, even if chimeric or humanized. Full-sized MAbs with engineered amino acid changes in C regions (even the hinge region) and identical sets of CDRs recognizing the same epitope have the suffix -bart. Immunoglobulins that are bispecific and multispecific (having two different sets of CDRs) regardless of shape have the suffix -mig. These MAbs need not be full-sized immunoglobulins; they only need to have CDRs for two different epitopes, including for example BiTEs (bispecific T-engaging antibodies) and BiKEs (bispecific killer cell engagers) shown here, which are sets of V light and heavy chain CDRs directly linked together without an Fc region. Lastly, monospecific fragments of a full-sized immunoglobulin that are lacking a partial or entire C region have the suffix -ment. Examples shown here are a Fab fragment (fragment antigen-binding), a Fab(‘ab’)2 fragment (immunoglobulin cleaved by pepsin below the hinge region), and a scFv (single-chain variable fragment). The infixes used in the previous classification are no longer a part of the new classification. C Shown for comparison are a nanobody (a.k.a. single domain antibody) and a nanobody linked to a single-chain variable fragment. Abbr: V, variable; C, constant (created, in part, with BioRender.com)
Desensitization to Biologics
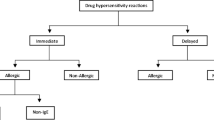
A major concern of the use of biologics in everyday clinical practice is that, when compared to other drugs, biologics have a relatively greater rate of hypersensitivity reactions, with rates reported upwards of a quarter of all patients, depending on the drug [9]. Even the first exposure to a biologic can lead to a hypersensitivity reaction [10]. There are seven types of reactions defined by their clinical presentation (phenotype), pathophysiology (endotype), and biomarkers: infusion reaction, type I IgE/non-IgE, cytokine release, mixed, type II, type III, and type IV (Fig. 2) [11••]. Rapid drug desensitization is a standardized procedure where successively increasing doses of the causative medication are rapidly administered that induce specific tolerance, allowing for continued administration of the medication [12, 13]. To determine eligibility for desensitization, patients are risk stratified by the severity of their reaction and by the results of skin testing (Fig. 3) [14, 15]. Sometimes, drug provocation testing is performed to further determine those who would most benefit from desensitization [16, 17]. Patients are premedicated (Fig. 4) and then reintroduced to the medication with rapidly increasing doses [11••]. Reactions that may occur during desensitization are managed as they arise (Fig. 5). Resulting desensitization to the medication is only maintained through continually repeated exposure to the specific drug. The patient will become sensitized again if there is any prolonged lack of exposure to the drug. With rapid drug desensitization, most hypersensitivity reactions to biologics can be safely overcome [18,19,20,21,22,23].
The seven types of drug reactions and whether rapid drug desensitization is indicated (created, in part, with BioRender.com. From Yang BC, Castells MC. The Who, What, Where, When, Why, and How of Drug Desensitization. Immunology and Allergy Clinics of North America. 2022. https://doi.org/10.1016/j.iac.2021.12.004; with permission)
Algorithm for risk stratification for intravenous rapid drug desensitization. SCARs, severe cutaneous adverse reactions (toxic epidermal necrolysis, Stevens-Johnson syndrome, drug eruption with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis) (from Yang BC, Castells MC. Rituximab hypersensitivity and desensitization: a personalized approach to treat cancer and connective tissue diseases. Ann Allergy Asthma Immunol. 2019;123(1):11–5. https://doi.org/10.1016/j.anai.2019.03.008; with permission)
Premedication for rapid drug desensitization is personalized for each patient and is based on the symptoms of the original reaction (from Yang BC, Castells M. Diagnosis and Treatment of Drug Hypersensitivity Reactions to Biologicals: Medical Algorithm. Allergy. 2020. https://doi.org/10.1111/all.14432; with permission)
Algorithm for the management of breakthrough reactions during intravenous rapid drug desensitization (from Yang BC, Castells MC. Rituximab hypersensitivity and desensitization: a personalized approach to treat cancer and connective tissue diseases. Ann Allergy Asthma Immunol. 2019;123(1):11–5. https://doi.org/10.1016/j.anai.2019.03.008; with permission)
Advancements in Biomarkers
Serum tryptase is a helpful biomarker to be measured during a hypersensitivity reaction and helps to identify the type of reaction [24]. Given its short half-life, it should be initially measured between 30 and 120 min of the start of the hypersensitivity reaction. Baseline tryptase should also be measured 2 to 3 weeks after the event [11••]. An elevation of 2 + (1.2 × the baseline tryptase level) would be considered elevated. The acute serum tryptase level is difficult to interpret without the baseline serum tryptase measurement. When elevated, it is highly suggestive of a type I reaction, possibly IgE-mediated, as it is released when there is systemic mast cell degranulation [25]. In addition, the measurement of serum tryptase 24 h prior to surgery and again during episodes of possible anaphylaxis as manifested by hypotension and/or cutaneous, gastrointestinal, and respiratory changes during surgery has been found to be useful in identifying perioperative hypersensitivity and should be accompanied by allergist referral for further workup [26].
Hereditary alpha tryptasemia (HAT) is an autosomal dominant genetic disorder from having extra copies of the TPSAB1 allele and leads to a far increased risk of anaphylaxis [27]. In a study at our institution of 101 patients with genetically confirmed HAT, 58 of those patients (57.4%) had experienced at least one episode of anaphylaxis [28•]. Baseline serum tryptase levels of these patients averaged 17.2 ng/mL and ranged from 6.2 to 51.3 ng/mL. Data from this study suggests that any patient with even a single tryptase measurement of > 8.0 ng/mL has a significant possibility of having hereditary alpha tryptasemia and should be genotyped.
Cetuximab is a monoclonal antibody against EGFR with uses in the management of metastatic colorectal cancer and head and neck squamous cell cancers. The first biomarker of potential hypersensitivity is the presence of IgE against alpha-1,3-galactose (a.k.a. alpha-Gal) in patients who are to undergo treatment with cetuximab [29]. It was discovered that those who lived in the southeastern USA and Stockholm area of Sweden were more likely to carry such specific IgE. It was found in those who had hypersensitivity to consuming beef in these areas, the cross-reactivity in sensitization presumed to be from tick bites. Prior to testing for alpha-Gal, 80% of patients would experience hypersensitivity reaction to cetuximab, 3% of them severe [30]. It is now standard of care to test for IgE to alpha-Gal before beginning treatment with cetuximab to prevent this type of hypersensitivity reaction.
The activation of basophils also occurs in type I hypersensitivity reactions when IgE is crosslinked. There are two markers of basophil activation: CD203c and CD63 [31]. CD203c has a very low expression in resting cells but is upregulated after basophil activation. CD63 is normally expressed on the inner side of secretory granules and become exposed to the cytoplasmic membrane upon degranulation. The basophil activation test (BAT) is an in vitro assay that measures the degree of basophil activation by measuring CD63 and/or CD203c by flow cytometry in the hopes of detecting type I hypersensitivity. In a case series of 18 patients receiving rituximab, 5 patients who experienced hypersensitivity reaction had significantly elevated CD63 + basophils compared to the others [32]. Gradual decrease of BAT positivity to adalimumab has been demonstrated in 4 patients with each successive injection of the drug during rapid desensitization, showing a higher activation threshold in basophils because of desensitization [33].
BAT may be positive even when skin testing may be negative. In one case study, two patients with localized hypersensitivity reaction etanercept had negative skin tests but positive BAT and underwent successful rapid desensitization [34]. Another case study shows a patient with severe anaphylaxis to pertuzumab with negative skin prick and intradermal tests with a positive BAT for both markers who then underwent successful rapid desensitization [35]. This shows that BAT may be a useful adjunct to skin testing in the detection of type I hypersensitivity.
There are currently limitations to the use of BAT. Although its specificity is high, sensitivity ranges from 42 to 70%; a positive test is suggestive of type I hypersensitivity, but a negative test cannot yet rule it out [31]. BAT requires dedicated laboratory expertise and cannot detect non-IgE-mediated hypersensitivity reactions.
Another in vitro test that has the potential to identify multiple types of hypersensitivity reactions is the lymphocyte transformation test (LTT). Since T cells are involved in all types of hypersensitivity reactions, the increased activity of drug-specific T cells can be a sign of drug-specific hypersensitivity. The LTT measures the proliferation of T cells in response to a drug in vitro [36]. For example, in a patient with delayed cutaneous hypersensitivity reaction (type IV) to atezolizumab, both skin prick and intradermal tests were negative [37]. Drug provocation test reproduced the same delayed symptoms and LTT was positive; rapid desensitization was thereafter performed successfully. The LTT is a very technically demanding test and not commercially available as a ready-to-use assy. It is important to remember that a negative test cannot rule out hypersensitivity.
The clinical phenotype of a cytokine release hypersensitivity reaction (fever, chills, pain, nausea, vomiting, headache) is driven by IL-6. In a study from our institution, 21 patients who experienced a combined 38 breakthrough reactions during rapid desensitization to either chemotherapy or monoclonal antibodies had their serum IL-6 levels drawn at the time of their reactions [38]. In those 29 reactions where IL-6 levels were elevated, their complaints were primarily fever and neuromuscular in nature. A comparable rise in serum tryptase was not seen. IL-6 is a useful biomarker for the detection of cytokine release reactions. The fact that these reactions occurred during rapid desensitization also suggests that desensitization itself can control some but not all pathways of hypersensitivity reactions.
Vaccines
When the mRNA vaccinations against SARS-CoV-2 were given emergency use authorization by the FDA in December 2020, surveillance data was carefully collected by the CDC about adverse events [39]. Keeping in mind that this data is self-reported and thus may be over-estimated, anaphylaxis from the vaccine is rare, occurring in 2.8 to 5 per million people [40]. Thirty-one percent of those with anaphylaxis to the vaccine had a prior history of anaphylaxis. Sixty percent of those who reacted had previous history of allergic reactions (e.g., food allergy, drug allergy, Hymenoptera allergy, reaction to allergen immunotherapy, and/or carry an epinephrine autoinjector) [41]. Over 90% of patients who reported a reaction to the vaccination are female. To put these numbers into perspective, when compared to common vaccines available for other diseases ranging from 0.5 to 1 in 1,000,000, the rate of anaphylaxis to the mRNA vaccinations is thus approximated to be 10 × higher [42]. This was somewhat unexpected as there were no cases of anaphylaxis in the large phase 3 clinical trials for either mRNA vaccination, but patients who had a history of severe allergy to any component of the vaccines were excluded [7, 8]. The reported reactions are mostly vasovagal in nature (i.e., diaphoresis, bradycardia, nausea, vomiting) rather than anaphylaxis (i.e., urticaria, angioedema, hypotension) [43].
The exact cause of hypersensitivity reaction in the mRNA vaccinations is not yet fully known. It has been conjectured that “a certain portion” of patients with anaphylaxis to these vaccines may have underlying undiagnosed hereditary alpha tryptasemia [44]. These vaccinations have none of the excipients that are found to be culprits of anaphylaxis in other vaccines, such as gelatin, latex, egg protein, or surfactant (polysorbate 80) [45•]. The mRNA vaccinations package the mRNA in lipid nanoparticles as naked mRNA is directly immunogenic and would be broken down by the host immune system before it reaches its destination. The negative charge of the mRNA is counteracted by the positive charge of the lipid nanoparticle “sheath” that envelops it at low pH. There is some evidence in vitro that mast cells may possibly take up the sheathed vaccine particles and that there may be disruption of the phagosome caused by the lipid nanoparticle that leads to mast cell degranulation [46]. It is also not known if the lipid nanoparticle sheath can lead to direct mast cell degranulation by activation of other receptors, such as MRGPRX2 [47]. Even though the exact lipid component of the Pfizer-BioNTech vaccine differs from that of the Moderna vaccine, they are both made more water soluble with polyethylene glycol (PEG 2000). If there is existence of anti-PEG IgG or IgM, this may cause direct compliment activation, leading to anaphylaxis [48]. However, this very same compliment activation may lead to greater efficacy of the vaccine by increasing dendritic cell uptake of the vaccine particles and increased spike protein expression and more efficient antigen presentation to T cells [45•]. No other vaccination has PEG as an excipient, so there is no prior data available for comparison from other vaccinations. As PEG is a likely source of anaphylaxis, it is not yet proven, and more investigation is necessary.
In adults, the COVID-19 mRNA vaccinations require 2 primary doses (or 3 primary doses in specific at-risk populations) as well as 1 to 2 boosters based on risk to complete the series [49]. If one experiences a reaction on the first dose, the risk of reaction increases drastically on any subsequent dose. Assuming PEG is the most likely culprit excipient in the mRNA vaccines, then skin testing can be performed to determine sensitivity to PEG or even to related polysorbate in those who experienced hypersensitivity reaction, although the clinical utility of such testing remains under debate as it has not been determined if the mechanism of reactions are IgE-mediated [50]. In a case series of six patients who had an episode of anaphylaxis to their first dose of mRNA COVID-19 vaccine, two of the six patients were positive on skin prick test for PEG or polysorbate [51]. All had successful desensitization to the Moderna COVID-19 vaccination. In another study, 159 patients with first-dose reactions (19 of whom had anaphylaxis) were given premedication with antihistamines and given the second dose and tolerated it with either no reaction or, at most, a mild reaction that was self-limited [52]. Skin testing to PEG was performed in 80 patients that were considered to have the highest risk, none of whom were positive. Given this study, subsequent doses of vaccination should not be avoided; it may be safely given with premedication and desensitization may not be required as the mechanism of reaction is still not known.
Immune Checkpoint Inhibitors
Newer monoclonal antibody therapies are available, used in the management of multiple types of cancer, collectively called immune checkpoint inhibitors (CPIs). They directly target T cell ligands, preventing T cell silencing. These include monoclonal antibodies against programmed cell death protein 1 (PD-1), its ligand (PD-L1), cutaneous T lymphocyte antigen 4 (CTLA-4 a.k.a. CD152), and lymphocyte activation gene 3 (LAG-3) [53]. Between 50 and 75% of all patients treated with CPIs experience an adverse event [54]. Hypersensitivity is mostly reported as skin reactions, ranging from drug rashes to severe cutaneous adverse reactions (e.g., toxic epidermal necrolysis or Stevens-Johnson syndrome); the degree of type I hypersensitivity is not known. However, multiple case studies have reported success with rapid desensitization to CPIs [55].
Excipients in Biologics
Polysorbate (PS-20 and PS-80) is a surfactant that is commonly used as an FDA-approved excipient in many monoclonal antibody drugs (as well as being the active ingredient in many lubricant eye drops). High-dose systemic administration of polysorbates leads to hypotension and tachycardia [56]. In studies both in animals and in humans, blood was incubated with polysorbate demonstrating in vitro generation of anaphylatoxins C3a and C5a, suggesting that the immunogenicity of these excipients is complement-driven [57, 58]. In one case study where two patients were treated with omalizumab, one patient experienced ocular angioedema from lubricant eye drops; the other patient had a positive intradermal test to polysorbate [59]. In another study, a patient had anaphylaxis after their first injection of omalizumab, and when skin testing was performed to other monoclonal antibodies, reaction to certolizumab was positive (the only common excipient being PEG/macrogol in certolizumab, which is cross-reactive with polysorbate) [60]. In another patient being treated for psoriasis, adalimumab caused generalized wheals and pruritus and switching to ustekinumab caused severe urticaria [61]. In this patient, skin prick test to polysorbate was positive; the only common excipient between the two monoclonal antibodies was polysorbate. Another patient on infliximab therapy experienced breathing difficulty and facial flushing on their 4th infliximab injection, switched then experienced urticaria on their 9th adalimumab injection, and even switched to ustekinumab only to experience urticaria and breathing difficulties on their 3rd injection [62]. Skin prick test was positive to the only common excipient among all three medications: polysorbate. When evaluating patients who have experienced hypersensitivity reactions to monoclonal antibodies, skin prick testing to polysorbate may yield useful in identifying type I hypersensitivity and risk stratification for rapid desensitization.
Nanobodies
Nanobodies have several possible clinical applications, ranging from their use in molecular imaging (nuclear and optical imaging) and therapy (drug delivery systems, agonist, and antagonist capabilities) [63]. To date, there are no known hypersensitivity reactions to nanobodies, and the expected potential immunogenicity is extremely low as there is an absence of any significant activation of dendritic cells [64].
Hormones
Diabetes mellitus is a disorder of insulin: caused either by the lack of production of enough insulin or peripheral tissue resistance to insulin. Essential to the overall management of diabetes is administration of exogenous insulin. The incidence of hypersensitivity reactions to insulin has vastly decreased over the past 100 years as it was purified from animal origin to what we now use as human insulin, the current incidence being < 1 to 2.4% [65]. The most common excipient that contributes to insulin allergy is metacresol, a preservative used in every commercial preparation of insulin [66]. Other possible excipients that could cause reactions include protamine, zinc, or even glycerol [67]. Those who experience immediate local hypersensitivity to multiple preparations of subcutaneous insulin commonly experience the same such reactions when exposed to an insulin “saline” training pen that includes all the excipients without the insulin itself [68]. Rapid desensitization to insulin with premedication and administration of progressively increasing doses of the desired type of insulin has been successfully performed [67, 69]. Even a patient who presented with diabetic ketoacidosis requiring urgent hemodialysis to correct severe metabolic acidosis experienced a successful rapid desensitization to insulin, transitioning from intravenous insulin to subcutaneous insulin over a course of days [70].
The mainstay of treatment for hypothyroidism is replacement thyroid hormone in the form of levothyroxine. With its predictable pharmacodynamics, it is the drug of choice over liothyronine or desiccated thyroid extracts [71]. For those who experience hypersensitivity reaction to levothyroxine, since the first-published oral rapid desensitization in 2013 [72], it has been repeatedly replicated successfully by others [73, 74].
Progesterone hypersensitivity has also been reported when used for in vitro fertilization (IVF) when women are exposed to high doses of progesterone [75]. Symptoms can extend from a rash to anaphylaxis. In this case series of 6 patients who reacted to progesterone for IVF, they all underwent rapid drug desensitization with vaginal suppositories to achieve successful tolerance of the high doses required for IVF.
Desensitization Using Biologics
When a patient’s primary drug hypersensitivity reaction presents as having a high probability of being IgE-mediated (i.e., immediate reaction phenotype, positive skin testing, measured serum anti-drug IgE), premedication that directly controls IgE levels before rapid desensitization may be useful. First, there is the biologic omalizumab, a humanized IgG1 monoclonal antibody against free IgE at the site of the FcεRI receptor, preventing IgE crosslinking and mast cell degranulation [76]. It is currently FDA approved for both the management of poorly controlled severe allergic asthma, chronic idiopathic urticaria, and nasal polyps [77]. It has also been successfully used as an adjunctive treatment in patients with mastocytosis to dampen the effects of venom immunotherapy [76, 78,79,80]. Research into its utility as premedication in aspirin desensitization has yielded conflicting results. In a series of 275 patients who underwent successful standardized rapid desensitization to aspirin, 8 of 9 patients who were on omalizumab during desensitization reacted, suggesting that omalizumab does not prevent reactions during desensitization specifically to aspirin [81]. In another study, 5 of 7 patients on omalizumab undergoing rapid desensitization to aspirin did not experience respiratory symptoms during the desensitization; the other two experienced aspirin-provoked bronchospasm [82]. In a case report of a patient with insulin allergy who failed traditional rapid desensitization in the past, a combination of biologics was given: first rituximab to reduce IgE production from active plasma cells, then omalizumab to prevent IgE crosslinking [83]. The patient remained asymptomatic while also receiving mycophenolate and only 2 mg of prednisone daily. In a patient receiving chemotherapy with FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin) who had an episode of anaphylaxis during third desensitization, there were no further episodes of anaphylaxis during 13 additional desensitizations after starting the patient on omalizumab [84]. Omalizumab has even been used successfully for desensitization to rituximab in children [85].
Ligelizumab is a monoclonal antibody against IgE with a manifold greater affinity for IgE in vitro and greater potency in vivo than omalizumab that is currently in phase III clinical trials [86,87,88,89,90,91,92]. Their latest published information, however, does not show superiority in efficacy for the indication of chronic idiopathic urticaria when compared to omalizumab, even though the studies met their clinical endpoints. Quilizumab is a humanized IgG1 monoclonal antibody that binds membrane IgE, not soluble IgE, and phase II trials performed thus far do not show significant clinical improvements in chronic idiopathic urticaria [93, 94]. Lastly, MEDI4212 is a monoclonal antibody against IgE that just published its phase I study, showing increased rapid decrease of total IgE but no sustained decrease of total IgE, suggesting limited potential over omalizumab [95]. Further investigation is necessary to determine if any of these drugs can be used in premedication to control reactions during rapid desensitization.
Conclusion
Biologics, whether chimeric, humanized, and human antibodies, targeted mRNA, or nanobodies have opened a new era in medicine for the prevention and treatment of infectious diseases, cancer, and inflammatory diseases. While their safety has been largely proven, hypersensitivity reactions are rare, but remain the most important factor preventing the use of first-line therapies for reactive and allergic patients. Understanding the underlying mechanisms of hypersensitivity reactions requires the wide utilization of biomarkers such as skin testing, currently cost limited, and others such as tryptase and now IL-6, which permit risk stratification of patients and their qualification for desensitization. Desensitization is primarily limited to type I, including anaphylaxis, and type IV reactions and is a highly effective and safe procedure for re-exposure to biologics in severely allergic patients. While desensitization requires a multidisciplinary approach between pharmacy, nursing, specialists, and allergists, its implementation should be the standard of care to provide all patients their best medication.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this article.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Morrow T, Felcone LH. Defining the difference: what makes biologics unique. Biotechnol Healthc. 2004;1(4):24–9.
Balocco R, De Sousa Guimaraes Koch S, Thorpe R, Weisser K, Malan S. New INN nomenclature for monoclonal antibodies. Lancet. 2022;399(10319):24. https://doi.org/10.1016/S0140-6736(21)02732-X.
WHO. New INN nomenclature scheme for monoclonal antibodies. Geneva: World Heal Org. 2022.
WHO. Nomenclature for monoclonal antibodies. Geneva: World Heal Org. 2009.
Awad RM, Meeus F, Ceuppens H, Ertveldt T, Hanssens H, Lecocq Q, et al. Emerging applications of nanobodies in cancer therapy. Int Rev Cell Mol Biol. 2022;369:143–99. https://doi.org/10.1016/bs.ircmb.2022.03.010.
Xu S, Yang K, Li R, Zhang L. mRNA Vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21(18). https://doi.org/10.3390/ijms21186582.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389.
Maggi E, Vultaggio A, Matucci A. Acute infusion reactions induced by monoclonal antibody therapy. Expert Rev Clin Immunol. 2011;7(1):55–63. https://doi.org/10.1586/eci.10.90.
Caiado J, Castells MC. Drug desensitizations for chemotherapy: safety and efficacy in preventing anaphylaxis. Curr Allergy Asthma Rep. 2021;21(6):37. https://doi.org/10.1007/s11882-021-01014-x.
•• Yang BC, Castells MC. The who, what, where, when, why, and how of drug desensitization. immunology and allergy clinics of North America. 2022. https://doi.org/10.1016/j.iac.2021.12.004. Thorough guide on the pathophysiologic mechanisms of rapid drug desensitization and how to perform desensitization.
Yang BC, Castells MC. Rituximab hypersensitivity and desensitization: a personalized approach to treat cancer and connective tissue diseases. Ann Allergy Asthma Immunol. 2019;123(1):11–5. https://doi.org/10.1016/j.anai.2019.03.008.
Yang BC, Castells M. Diagnosis and treatment of drug hypersensitivity reactions to biologicals: medical algorithm. Allergy. 2020. https://doi.org/10.1111/all.14432.
Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–6. https://doi.org/10.1016/j.jaci.2004.04.029.
Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvao VR, Berlin ST, et al. Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. 2016;137(4):1154–64 e12. https://doi.org/10.1016/j.jaci.2015.10.039.
Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7(2):618–32. https://doi.org/10.1016/j.jaip.2018.07.031.
Madrigal-Burgaleta R, Vazquez-Revuelta P, Marti-Garrido J, Lleonart R, Ali FR, Alvarez-Cuesta E. Importance of diagnostics prior to desensitization in new drug hypersensitivity: chemotherapeutics and biologicals. Current Treatment Options in Allergy. 2020;7(1):1–13. https://doi.org/10.1007/s40521-020-00238-y.
Castells M. Drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: the role of desensitizations. Front Immunol. 2017;8:1472. https://doi.org/10.3389/fimmu.2017.01472.
Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122(3):574–80. https://doi.org/10.1016/j.jaci.2008.02.044.
Hsu Blatman KS, Castells MC. Desensitizations for chemotherapy and monoclonal antibodies: indications and outcomes. Curr Allergy Asthma Rep. 2014;14(8):453. https://doi.org/10.1007/s11882-014-0453-5.
Hong DI, Dioun AF. Indications, protocols, and outcomes of drug desensitizations for chemotherapy and monoclonal antibodies in adults and children. J Allergy Clin Immunol Pract. 2014;2(1):13–9; quiz 20. https://doi.org/10.1016/j.jaip.2013.11.007.
Brennan PJ, Rodriguez Bouza T, Hsu FI, Sloane DE, Castells MC. Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol. 2009;124(6):1259–66. https://doi.org/10.1016/j.jaci.2009.09.009.
Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140(2):321–33. https://doi.org/10.1016/j.jaci.2017.06.012.
Picard M, Galvao VR. Current knowledge and management of hypersensitivity reactions to monoclonal antibodies. J Allergy Clin Immunol Pract. 2017;5(3):600–9. https://doi.org/10.1016/j.jaip.2016.12.001.
Isabwe GAC, Garcia Neuer M, de Las Vecillas Sanchez L, Lynch DM, Marquis K, Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol. 2018;142(1):159–70 e2. https://doi.org/10.1016/j.jaci.2018.02.018.
Vitte J, Sabato V, Tacquard C, Garvey LH, Michel M, Mertes PM, et al. Use and interpretation of acute and baseline tryptase in perioperative hypersensitivity and anaphylaxis. J Allergy Clin Immunol Pract. 2021;9(8):2994–3005. https://doi.org/10.1016/j.jaip.2021.03.011.
Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48(12):1564–9. https://doi.org/10.1038/ng.3696.
• Giannetti MP, Weller E, Bormans C, Novak P, Hamilton MJ, Castells M. Hereditary alpha-tryptasemia in 101 patients with mast cell activation-related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126(6):655–60. https://doi.org/10.1016/j.anai.2021.01.016. Excellent evidence for maintaining suspicion for hereditary alpha-tryptasemia in patients with anaphylaxis.
Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. https://doi.org/10.1056/NEJMoa074943.
Chinuki Y, Morita E. Alpha-Gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296–300. https://doi.org/10.1016/j.alit.2019.04.001.
Campos L, Galvao VR, Kalil J, Castells M, Giavina-Bianchi P. BAT in the diagnosis of drug allergy: a novel tool in clinical daily practice? Curr Allergy Asthma Rep. 2019;19(4):20. https://doi.org/10.1007/s11882-019-0852-8.
Piva E, Chieco-Bianchi F, Krajcar V, Aversa S, Plebani M. Adverse reactions in patients with B-cell lymphomas during combined treatment with rituximab: in vitro evaluation of rituximab hypersensitivity by basophil activation test. Am J Hematol. 2012;87(11):E130–1. https://doi.org/10.1002/ajh.23329.
Thevenot J, Ferrier le Bouedec MC, Buisson A, Bommelaer G, D'Incan M, Rouzaire P. Rapid desensitization to adalimumab is associated with decreased basophil sensitivity. J Investig Allergol Clin Immunol. 2019;29(2):141–3. https://doi.org/10.18176/jiaci.0350.
de la Varga MR, Gutierrez Fernandez D, Foncubierta Fernandez A, Andres Garcia JA, Medina VF. Rapid subcutaneous desensitization for treatment of hypersensitivity reactions to etanercept in two patients with positive basophil activation test. Allergol Int. 2017;66(2):357–9. https://doi.org/10.1016/j.alit.2016.09.002.
Gonzalez-de-Olano D, Morgado JM, Juarez-Guerrero R, Sanchez-Munoz L, Letellez-Fernandez J, Malon-Gimenez D, et al. Positive basophil activation test following anaphylaxis to pertuzumab and successful treatment with rapid desensitization. J Allergy Clin Immunol Pract. 2016;4(2):338–40. https://doi.org/10.1016/j.jaip.2015.10.007.
Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59(8):809–20. https://doi.org/10.1111/j.1398-9995.2004.00547.x.
Giraldo-Tugores M, Fernandez-Lozano C, Carron-Herrero A, Gajate P, Martinez-Botas J, Pueyo-Lopez C, et al. Successful rapid desensitization to atezolizumab in delayed hypersensitivity confirmed with lymphocyte transformation test. J Allergy Clin Immunol Pract. 2022. https://doi.org/10.1016/j.jaip.2021.12.041.
Jakubovic BD, Sanchez-Sanchez S, Hamadi S, Lynch DM, Castells M. Interleukin-6: a novel biomarker for monoclonal antibody and chemotherapy-associated hypersensitivity confirms a cytokine release syndrome phenotype-endotype association. Allergy. 2020. https://doi.org/10.1111/all.14644.
Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405. https://doi.org/10.1016/j.vaccine.2015.07.035.
Blumenthal KG, Robinson LB, Camargo CA Jr, Shenoy ES, Banerji A, Landman AB, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325(15):1562–5. https://doi.org/10.1001/jama.2021.3976.
Tanno LK, Castells M, Caminati M, Senna G, Demoly P. Anaphylaxis and coronavirus disease 2019 vaccine: a danger relationship? Curr Opin Allergy Clin Immunol. 2021;21(5):411–7. https://doi.org/10.1097/ACI.0000000000000778.
Dreskin SC, Halsey NA, Kelso JM, Wood RA, Hummell DS, Edwards KM, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9(1):32. https://doi.org/10.1186/s40413-016-0120-5.
Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–9. https://doi.org/10.1056/NEJMra2035343.
Castells M, Demoly P, Tanno LK. [Anaphylaxis and COVID-19 vaccines]. Rev Fr Allergol (2009). 2021;61(8):8S30–8S5. https://doi.org/10.1016/S1877-0320(21)00439-5.
• Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147(6):2075–82 e2. https://doi.org/10.1016/j.jaci.2021.04.002. Good review of theoretical mechanisms of COVID-19 vaccine hypersensitivity.
Duguay BA, Huang KW, Kulka M. Lipofection of plasmid DNA into human mast cell lines using lipid nanoparticles generated by microfluidic mixing. J Leukoc Biol. 2018;104(3):587–96. https://doi.org/10.1002/JLB.3TA0517-192R.
McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–41. https://doi.org/10.1038/nature14022.
Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–77. https://doi.org/10.1002/wnan.1339.
Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. 2020. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html . Accessed July 20, 2022.
Greenhawt M, Shaker M, Golden DBK. PEG/polysorbate skin testing has no utility in the assessment of suspected allergic reactions to SARS-CoV-2 vaccines. J Allergy Clin Immunol Pract. 2021;9(9):3321–2. https://doi.org/10.1016/j.jaip.2021.06.025.
AlMuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. 2022;3: 825164. https://doi.org/10.3389/falgy.2022.825164.
Krantz MS, Kwah JH, Stone CA Jr, Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530–3. https://doi.org/10.1001/jamainternmed.2021.3779.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. https://doi.org/10.3389/fonc.2018.00086.
Ibraheim H, Perucha E, Powell N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58(Suppl 7):vii17-vii28. https://doi.org/10.1093/rheumatology/kez465.
Labella M, Castells M. Hypersensitivity reactions and anaphylaxis to checkpoint inhibitor-monoclonal antibodies and desensitization. Ann Allergy Asthma Immunol. 2021;126(6):623–9. https://doi.org/10.1016/j.anai.2021.03.008.
Singh SK, Mahler HC, Hartman C, Stark CA. Are injection site reactions in monoclonal antibody therapies caused by polysorbate excipient degradants? J Pharm Sci. 2018;107(11):2735–41. https://doi.org/10.1016/j.xphs.2018.07.016.
Weiszhar Z, Czucz J, Revesz C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012;45(4):492–8. https://doi.org/10.1016/j.ejps.2011.09.016.
Qiu S, Liu Z, Hou L, Li Y, Wang J, Wang H, et al. Complement activation associated with polysorbate 80 in beagle dogs. Int Immunopharmacol. 2013;15(1):144–9. https://doi.org/10.1016/j.intimp.2012.10.021.
Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. 2007;28(3):313–9. https://doi.org/10.2500/aap.2007.28.3003.
Perino E, Freymond N, Devouassoux G, Nicolas JF, Berard F. Xolair-induced recurrent anaphylaxis through sensitization to the excipient polysorbate. Ann Allergy Asthma Immunol. 2018;120(6):664–6. https://doi.org/10.1016/j.anai.2018.02.018.
Perez-Perez L, Garcia-Gavin J, Pineiro B, Zulaica A. Biologic-induced urticaria due to polysorbate 80: usefulness of prick test. Br J Dermatol. 2011;164(5):1119–20. https://doi.org/10.1111/j.1365-2133.2011.10220.x.
Kato M, Oiso N, Uchida S, Yanagihara S, Sano H, Tohda Y, et al. Biologic-induced urticaria due to polysorbate 20. J Dermatol. 2019;46(7):e230–2. https://doi.org/10.1111/1346-8138.14808.
Oliveira S, Heukers R, Sornkom J, Kok RJ, van Bergen En Henegouwen PM. Targeting tumors with nanobodies for cancer imaging and therapy. J Control Release. 2013;172(3):607–17. https://doi.org/10.1016/j.jconrel.2013.08.298.
Ackaert C, Smiejkowska N, Xavier C, Sterckx YGJ, Denies S, Stijlemans B, et al. Immunogenicity risk profile of nanobodies. Front Immunol. 2021;12: 632687. https://doi.org/10.3389/fimmu.2021.632687.
Schernthaner G. Immunogenicity and allergenic potential of animal and human insulins. Diabetes Care. 1993;16(Suppl 3):155–65. https://doi.org/10.2337/diacare.16.3.155.
Kim D, Baraniuk J. Delayed-type hypersensitivity reaction to the meta-cresol component of insulin. Ann Allergy Asthma Immunol. 2007;99(2):194–5. https://doi.org/10.1016/S1081-1206(10)60645-X.
Elfekih H, Hadjkacem F, Elleuch M, Ghorbel D, Charfi N, Mnif F, et al. Successful treatment of insulin allergy with desensitization therapy: a case report and literature review. Iran J Allergy Asthma Immunol. 2019;18(5):572–83. https://doi.org/10.18502/ijaai.v18i5.1927.
Heinzerling L, Raile K, Rochlitz H, Zuberbier T, Worm M. Insulin allergy: clinical manifestations and management strategies. Allergy. 2008;63(2):148–55. https://doi.org/10.1111/j.1398-9995.2007.01567.x.
Wheeler BJ, Taylor BJ. Successful management of allergy to the insulin excipient metacresol in a child with type 1 diabetes: a case report. J Med Case Rep. 2012;6:263. https://doi.org/10.1186/1752-1947-6-263.
Shuster S, Borici-Mazi R, Awad S, Houlden RL. Rapid desensitization with intravenous insulin in a patient with diabetic ketoacidosis and insulin allergy. AACE Clin Case Rep. 2020;6(4):e147–50. https://doi.org/10.4158/ACCR-2019-0562.
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–751. https://doi.org/10.1089/thy.2014.0028.
Fevzi D, Mustafa G, Ozgur K, Cetin T, Abdullah B, Sait Y, et al. Successful oral desensitization to levothyroxine. Ann Allergy Asthma Immunol. 2013;111(2):146–7. https://doi.org/10.1016/j.anai.2013.06.003.
Bahloul N, Ben Mahmoud L, Ghozzi H, Hadjkacem F, Zeghal K, Abid M, et al. Hypersensitivity to levothyroxine: a case report of a successful oral desensitization. Therapie. 2018;73(4):349–50. https://doi.org/10.1016/j.therap.2017.10.009.
Jiang CY, Shen BS, Yun TZ, Singarayar C, Hui FS. Successful oral levothyroxine desensitization in a patient with severe hypothyroidism post radioactive iodine therapy: a case report. J ASEAN Fed Endocr Soc. 2021;36(2):213–5. https://doi.org/10.15605/jafes.036.02.10.
Prieto-Garcia A, Sloane DE, Gargiulo AR, Feldweg AM, Castells M. Autoimmune progesterone dermatitis: clinical presentation and management with progesterone desensitization for successful in vitro fertilization. Fertil Steril. 2011;95(3):1121 e9–13. https://doi.org/10.1016/j.fertnstert.2010.10.038.
Carter MC, Metcalfe DD, Matito A, Escribano L, Butterfield JH, Schwartz LB, et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: a work group report of the Mast Cells Disorder Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2019;143(3):880–93. https://doi.org/10.1016/j.jaci.2018.10.063.
Novartis/Genentech: Xolair Prescribing Information (Package Insert). https://www.gene.com/download/pdf/xolair_prescribing.pdf . Accessed.
Kontou-Fili K. High omalizumab dose controls recurrent reactions to venom immunotherapy in indolent systemic mastocytosis. Allergy. 2008;63(3):376–8. https://doi.org/10.1111/j.1398-9995.2007.01604.x.
Kontou-Fili K, Filis CI. Prolonged high-dose omalizumab is required to control reactions to venom immunotherapy in mastocytosis. Allergy. 2009;64(9):1384–5. https://doi.org/10.1111/j.1398-9995.2009.02045.x.
Kontou-Fili K, Filis CI, Voulgari C, Panayiotidis PG. Omalizumab monotherapy for bee sting and unprovoked “anaphylaxis” in a patient with systemic mastocytosis and undetectable specific IgE. Ann Allergy Asthma Immunol. 2010;104(6):537–9. https://doi.org/10.1016/j.anai.2010.04.011.
Waldram J, Walters K, Simon R, Woessner K, Waalen J, White A. Safety and outcomes of aspirin desensitization for aspirin-exacerbated respiratory disease: a single-center study. J Allergy Clin Immunol. 2018;141(1):250–6. https://doi.org/10.1016/j.jaci.2017.05.006.
Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann Allergy Asthma Immunol. 2018;121(1):98–104. https://doi.org/10.1016/j.anai.2018.05.007.
Yong PF, Malik R, Arif S, Peakman M, Amiel S, Ibrahim MA, et al. Rituximab and omalizumab in severe, refractory insulin allergy. N Engl J Med. 2009;360(10):1045–7. https://doi.org/10.1056/NEJMc0808282.
Prieto-Garcia A, Noguerado B, Rojas P, Torrado I, Rodriguez-Fernandez A, Tornero P. Unexpected anaphylaxis after completing a desensitization protocol to oxaliplatin: successful adjuvant use of omalizumab. J Investig Allergol Clin Immunol. 2019;29(1):53–5. https://doi.org/10.18176/jiaci.0326.
Gemici Karaaslan HB, Karabag Yilmaz E, Gulmez R, Canpolat N, Kiykim A, Cokugras HC. Omalizumab may facilitate drug desensitization in patients failing standard protocols. Pediatr Allergy Immunol. 2022;33(5): e13783. https://doi.org/10.1111/pai.13783.
Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–85. https://doi.org/10.1111/cea.12400.
Gauvreau GM, Arm JP, Boulet LP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol. 2016;138(4):1051–9. https://doi.org/10.1016/j.jaci.2016.02.027.
Maurer M, Gimenez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for Chronic Spontaneous Urticaria. N Engl J Med. 2019;381(14):1321–32. https://doi.org/10.1056/NEJMoa1900408.
Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbaren N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun. 2020;11(1):165. https://doi.org/10.1038/s41467-019-13815-w.
Jensen RK, Jabs F, Miehe M, Molgaard B, Pfutzner W, Mobs C, et al. Structure of intact IgE and the mechanism of ligelizumab revealed by electron microscopy. Allergy. 2020;75(8):1956–65. https://doi.org/10.1111/all.14222.
Maurer M, Gimenez-Arnau A, Bernstein JA, Chu CY, Danilycheva I, Hide M, et al. Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: A one-year extension study. Allergy. 2021. https://doi.org/10.1111/all.15175.
Turk M, Yilmaz I. High-Dose Omalizumab versus Ligelizumab for the Treatment of Chronic Spontaneous Urticaria: Do Not We Need a Head-To-Head Comparison? Int Arch Allergy Immunol. 2021;182(5):461–2. https://doi.org/10.1159/000512373.
Harris JM, Cabanski CR, Scheerens H, Samineni D, Bradley MS, Cochran C, et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol. 2016;138(6):1730–2. https://doi.org/10.1016/j.jaci.2016.06.023.
Harris JM, Maciuca R, Bradley MS, Cabanski CR, Scheerens H, Lim J, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res. 2016;17:29. https://doi.org/10.1186/s12931-016-0347-2.
Sheldon E, Schwickart M, Li J, Kim K, Crouch S, Parveen S, et al. Pharmacokinetics, pharmacodynamics, and safety of MEDI4212, an anti-IgE monoclonal antibody, in subjects with atopy: a phase I study. Adv Ther. 2016;33(2):225–51. https://doi.org/10.1007/s12325-016-0287-8.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on this submission made substantial contributions to the conception and design of the work, drafted the work, revised it critically for important intellectual content, approve this version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Castells is the Anaphylaxis and Drug Allergy Section Editor for the Journal. Dr. Yang is currently employed at Ribon Therapeutics. The authors did not receive support from any organization for the submitted work. The authors have no financial or proprietary interests in any material discussed in this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Anaphylaxis and Drug Allergy
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, B.C., Castells, M.C. Utilizing Biologics in Drug Desensitization. Curr Allergy Asthma Rep 23, 1–11 (2023). https://doi.org/10.1007/s11882-022-01052-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-022-01052-z