Abstract
Dyslexia is one of the most studied learning disorders. Despite this, its biological basis and main causes are still not fully understood. Electroencephalography (EEG) could be a powerful tool in identifying the underlying mechanisms, but knowledge of the EEG correlates of developmental dyslexia (DD) remains elusive. We aimed to systematically review the evidence on EEG correlates of DD and establish their quality. In July 2021, we carried out an online search of the PubMed and Scopus databases to identify published articles on EEG correlates in children with dyslexia aged 6 to 12 years without comorbidities. We follow the PRISMA guidelines and assess the quality using the Appraisal Tool questionnaire. Our final analysis included 49 studies (14% high quality, 63% medium, 20% low, and 2% very low). Studies differed greatly in methodology, making a summary of their results challenging. However, some points came to light. Even at rest, children with dyslexia and children in the control group exhibited differences in several EEG measures, particularly in theta and alpha frequencies; these frequencies appear to be associated with learning performance. During reading-related tasks, the differences between dyslexic and control children seem more localized in the left temporoparietal sites. The EEG activity of children with dyslexia and children in the control group differed in many aspects, both at rest and during reading-related tasks. Our data are compatible with neuroimaging studies in the same diagnostic group and expand the literature by offering new insights into functional significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Definition and cognitive mechanisms underlying developmental dyslexia
DD manifests as an unexpected difficulty in acquiring reading skills despite adequate education, intelligence, and sociocultural opportunities and without obvious sensory deficits. Depending on the characteristics of the language, accuracy and/or fluency can be affected (Diamanti et al., 2018). According to the Diagnostic and Statistical Manual for Mental Disorders (5th ed.; DSM-5; American Psychiatric Association 2013), the incidence of specific learning disorders, including DD, ranges from 5 to 12%. Following DSM-5, the main four criteria for diagnosing DD include the presence of difficulties in learning to read that have persisted for at least 6 months despite additional help or targeted instruction being provided. These difficulties interfere with everyday activities (such as academic achievement) and are well below the age-expected level (defined as performance below 1.5 average SD). Reading problems manifest upon admission into the school system and are not explained by other impairments such as intellectual disabilities, sensory, or neurological problems. By involving reading acquisition, a central skill in most school systems, DD is associated with many negative school outcomes, including reduced educational attainment and academic self-efficacy (Elgendi et al., 2021). Due to its developmental nature, DD persists until adulthood, with consequences also in the work context (Nalavany et al., 2018).
As largely demonstrated, learning to read involves multiple processes ranging from cognitive and linguistic abilities to visual and attentional processes. Although, in the past, the effort was to identify the single causal mechanism of dyslexia, more recently, it has been recognized that variable patterns of weakness can contribute to reading difficulty in children (O’Brien & Yeatman, 2021). Research on developmental dyslexia has indeed documented deficits in vision (e.g., Stein & Walsh, 1997), attention (e.g., Vidyasagar & Pammer, 2010), auditory and temporal processes (e.g., Vandermosten et al., 2010), and phonology and language (e.g., Hulme et al., 2015). In addition, weaknesses in executive functions, particularly in working memory, have been reported (e.g., Lonergan et al., 2019).
Using neuroimaging techniques such as functional magnetic resonance imaging (fMRI), researchers have identified brain circuits crucially involved in typical and dyslexic reading. A coarse neuroanatomical model of reading and DD has proposed abnormal brain activation occurs in dyslexic readers in the left posterior temporoparietal cortex (middle temporal gyrus, superior temporal gyrus, supramarginal gyrus, and angular gyrus), the left occipitotemporal cortex (inferior temporal gyrus and fusiform gyrus), and the left frontal cortex (inferior frontal gyrus and precentral gyrusHancock et al., 2017; Martin et al., 2016; Richlan, 2020; Richlan et al., 2009).
However, although there are great improvements in comprehending the involved neuroanatomical circuits, little evidence exists to show that fundamental brain processes are affected and how the brain compensates for those disruptions.
Although the spatial resolution is lower compared to fMRI, electrical signals allow for exploring networks with temporal dynamics that functionally do not completely overlap with their fMRI counterparts. Many electrophysiological studies have provided evidence for basic perceptual deficits in DD. Abnormal event-related potentials (ERPs) for auditory and visual processing of speech and non-speech stimuli were found in both children and adults with dyslexia (for example, Bishop, 2007; Hämäläinen et al., 2013; Heim & Keil, 2004; Schulte-Körne & Bruder, 2010). ERPs are measures of electrical activity driven by changes in cognitive processing that are usually time locked to stimuli and could be defined as a measure of the flow of sensory-related and action-related information in neuronal networks of the brain (even if some evidence suggests that some ERP components might be generated by stimulus-induced changes in ongoing brain dynamics (Penny et al., 2002). ERPs are extrapolated from the electroencephalogram (EEG), which, as a whole, provides insight into functional brain organization through the patterns of different brain oscillations. EEG shows overlapping electrical oscillation rhythms representing spontaneous activities in resting states with eyes open and closed. In response to stimuli, EEG rhythms react by synchronizing and desynchronizing, which does not represent signal processing per se, but rather a modulation of the information flow in the brain following stimulation.
Although EEG rhythms have been discarded and ignored for years, considered a noisy background activity, the appearance of new methods in recent years has allowed the latter to face its renascence. The spectral power in the different frequency bands is the first and simpler source of information we can obtain from quantitative analysis of EEG, despite the different analysis techniques. It is determined by the synchronous activity of oscillating networks of neurons, and it reflects crucial aspects of processing information in the brain (Buzsáki & Draguhn, 2004). Phase synchronization of brain oscillations across spatially distinct brain regions has been suggested to be an important neuronal communication mechanism by dynamically linking neurons into functional networks (Womelsdorf et al., 2007). Under stimulation, endogenous oscillations phase reset their activity to the rhythmic information in the input, synchronizing cell activity so that peaks in excitation co-occur with stimulus delivery, thereby enhancing neural processing (Canolty et al., 2006; Lakatos et al., 2005). The different frequencies at which the networks oscillate have been divided into five groups—delta (0.5–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (13–30 Hz), and gamma (> 30 Hz)—with different functional meanings and involvement in a variety of perceptual, sensorimotor, and cognitive operations. Alpha-band oscillations are the dominant oscillations in the human brain with an active role in information processing and a possible inhibitory function (Klimesch, 2012). Abundant during sleep, in the awake state delta is associated with functional cortical deafferentation or inhibition of the sensory input that interferes with internal concentration (Harmony, 2013). The existence of several beta rhythms with different frequencies, topographies, and different functional properties presumes no single neuronal mechanism for their generation (Kropotov, 2009). Finally, it has been shown that gamma band activity plays a crucial role in several cognitive tasks; moreover, it seems to interact with the activity in other frequency bands: in speech tasks, gamma interacts with theta, which accounts for syllabic perception, becoming crucial in processing linguistic stimuli (Giraud & Poeppel, 2012). The current hypothesis is that alterations in the oscillatory patterns of EEG play a critical role in the maintenance of brain functions and, consequently, may offer crucial information about brain functions.
This work aims to systematically review the literature on the EEG correlates of DD. We will exclude the broad category of ERPs, given the different functional meanings and also considering the presence in the literature of many reviews about them (for example, Bishop, 2007; Hämäläinen et al., 2013; Heim & Keil, 2004; Schulte-Körne & Bruder, 2010). We focused on children who received the first diagnosis of dyslexia to analyze this problem. Several reports in the literature suggest that the first diagnosis of learning disabilities is more frequent during primary school (e.g., Arrhenius et al., 2021). For this reason, we focused on the age range of 6–12 years. We intended to identify and retrieve international evidence, establish the quality of that evidence, address any uncertainty, and evaluate and synthesize the results. We hope that conflicting evidence could lead to further research.
Methods
Protocol and registration
We performed a systematic review of published journal articles on the correlates of EEG in DD, following the PRISMA guidelines (Page et al., 2021). The study protocol has been registered and is publicly available at https://osf.io/4yz7j, where the resources obtained from this study are also available.
Eligibility criteria
Types of studies
Case series and case–control studies investigating the correlates of EEG in DD were included. Participants in each study had a diagnosis of dyslexia according to current diagnostic manuals (e.g., ICD, DSM) and/or national guidelines. No publication date or publication status restrictions were imposed. Only English studies were included.
Types of participants
Participants aged 6 to 12 years with DD (i.e., not acquired) were included. To limit the exclusion of works, we included those works with broader age ranges but in which the results differentiated for age. That means that works that include older children but allow for extrapolating specific results on 6–12 age ranges have been included. Comorbidities were considered exclusion criteria; in studies in which patients with comorbidities were also involved, but patients without were also present, only the results for the latter were considered.
Types of outcome measures
Except for ERPs, all EEG methodologies were included (in the supplementary materials, a description of EEG measures is reported).
Information sources
We conducted our search in July 2021 using PubMed and SCOPUS (Elsevier API) bibliographic databases, which include most of the EMBASE database (https://www.elsevier.com/solutions/embase-biomedical-research). The search was conducted using the following string: dyslexia AND (children OR developmental OR pediatric OR paediatric) AND EEG. This string returned 261 results in Scopus and 458 in PubMed. The final search results were exported to store and remove duplicates in the Mendeley bibliographic software package. There was only an internal duplicate within the PubMed database. Internal and external duplicates between the databases were removed from the list. The electronic database search was supplemented by screening the reference lists of each retrieved paper and scanning relevant reviews, obtaining two additional works. In total, 560 results were selected.
Study selection and data collection process
The eligibility assessment was performed independently and standardized in an unblinded manner by two reviewers (E. C. and L. V.). A third reviewer resolved disagreements between reviewers (P. B. or B. C.). We developed a data extraction sheet that captured relevant information on key study characteristics and all EEG techniques used to investigate DD. Studies have been double coded.
Data items
The following information was collected from the records: year of publication, groups (e.g., patients with DD, healthy controls, or controls with other clinical characteristics), sample sizes, age at testing, criteria for defining the diagnosis of DD, EEG methodology, experimental conditions, supplementary neuropsychological/cognitive/achievements measures, EEG results, and correlation between EEG findings and supplementary measures.
Risk of bias in individual studies
The risk of bias at the study level was assessed by two reviewers (E. C. and L. V.) using the Appraisal Tool for Cross‐Sectional Studies (AXIS; Downes et al., 2016). This 20‐item tool was developed in response to the increase in cross‐sectional studies that inform evidence-based medicine and the consequent importance of ensuring that these studies are of high quality and low bias. AXIS assesses the quality of cross‐sectional studies based on the following criteria: clarity of objectives/objectives and target population; appropriate study design and sampling framework; justification for sample size; measures taken to address non-respondents and the potential for response bias; risk factors and outcome variables measured in the study; clarity of methods and statistical approach; appropriate presentation of results, including internal consistency; justified discussion points and conclusion; discussion of limitations; and identification of ethical approval and conflicts of interest. The scoring system conforms to a “yes,” “no,” or “do not know/comment” design. We classified the studies into four quality categories based on the number of “yes” answers for each of the 20 questions included in the AXIS tool as follows (Bull et al., 2019): “high” (more than 15 positive answers), “medium” (between 10 and 15), “low” (between 5 and 9), and “very low” (equal or less than 4). The overall quality categories of the studies are reported in Table 1.
Results
Study selection
Figure 1 summarizes the following workflow (Haddaway & McGuinness, 2020). The 560 results were screened based on the title of the articles, and 133 were excluded for not being neurophysiological studies investigating DD, for not being original research (reviews, meta-analyses, abstracts, or proceedings), or for not being in English. The full texts of the remaining 427 articles were screened, and further exclusion criteria excluded 378 additional articles. Articles were excluded based on not being original quantitative research or case reports (n = 14), involving participants outside the age range selected without differentiation between ages (n = 85), diagnosing dyslexia in a way not defined according to inclusion criteria (n = 1), involving participants with comorbidities (n = 2), not focusing on EEG in DD (n = 265), and being irretrievable (n = 11). The final analysis included 49 studies.
Quality of studies
Individual scores for each included study are reported in Fig. 2.
The cumulative quality score of all studies relative to the AXIS questionnaire is reported in Table 1. Only 14% were classified as high-quality level (> 15 positive answers), while the majority (63%) fell into the medium level. Finally, the quality of 20% was considered low and, for 1 study, very low. The most common vulnerabilities are the sample size estimate, ethical approval information, and missing data management. The percentage of responses for each question is shown in Fig. 3.
Some elements of the AXIS on the study design are positive in all studies due to part of the inclusion criteria.
Typology of the studies
Although EEG is a well-known methodology, we lack normative data to interpret quantitative findings, particularly for relatively new methodologies such as connectivity. Therefore, all studies are case–control comparisons of children with dyslexia and control children without the disorder. Only three studies did not compare children with dyslexia with controls. Still, children with dyslexia with nonspecific reading delay (Bosch-Bayard, 2018 2020) and children with dyslexia who have poor reading ability were compared to children with dyslexia who have capable reading ability (Mahmoodin 2016).
The studies could be at rest (resting state) or during cognitive stimulation recorded by EEG: 12 out of the 49 studies used both approaches. Eighteen of the 49 studies were published before 2000. Table 2 reports all the included studies and their main characteristics (authors, year of publication, sample size, age of the children at the evaluation, language used, methodology, and study quality following the AXIS questionnaire).
Resting-state EEG
We found 24 studies investigating resting-state EEG (RS-EEG): 11 out of 24 studies focused solely on RS-EEG, while the other 13 out of 24 studies performed both an RS-EEG and an EEG during a task (in this section, only the results of the RS-EEG will be reported, whereas the results during a task will be described in the next section).
Spectral analysis of the RS-EEG
The methodology used most frequently is spectral analysis, which shows the spectral content in the different frequency bands (delta, 1.5–4 Hz; theta, 4–7 Hz; alpha, 8–12 Hz; beta, 13–40 Hz; gamma, > 40 Hz). The methodology has been used alone (Arns et al., 2007; Bruni et al., 2009; Colon et al., 1979; Fein et al., 1983, 1986; Galin et al., 1988, 1992; Harmony et al., 1990; Mahmoodin et al., 2016, 2019; Papagiannopoulou & Lagopoulos, 2016; Remschmidt & Warnke, 1992; Rippon and Brunswick 2000) or combined with other methods (Babiloni et al., 2012; Bosch-Bayard et al., 2018; Flynn & Deering, 1989a; Flynn et al., 1992; Fraga González et al., 2016; Leisman, 2002; Reda et al., 2021; Xue et al., 2020).
Figure 4 shows the results obtained by spectral analysis of RS-EEG. We also reported the non-significant results to render the data more readable. In general, it seems that DD is characterized by an increase in the delta frequency and theta and a reduction in alpha and beta.
Other methodologies
Results obtained from other methodologies are less homogeneous, and a summary is not possible; Table 3 reports the results of these studies (Arns et al., 2007; Ayers & Torres, 1967; Babiloni et al., 2012; Bosch-Bayard et al., 2020; Bruni et al., 2009; Duffy et al. 1980a; Eroğlu et al., 2022; Farrag & El-Behary, 1990; Fraga González et al., 2016; Gerald Leisman, 2002; Reda et al., 2021; Shiota et al., 2000; Xue et al., 2020).
Correlation between resting EEG and reading performance
Some rest studies correlate EEG activity with specific tests performed before or after recordings (Table 4).
EEG during a task
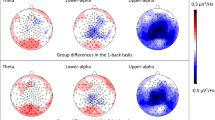
Thirty-two studies explored EEG brain activity during a task. Figure 5 reports the type of stimulation task and the methodology applied to the EEG. Most studies compared the EEG and the performance of DD and control children in linguistic, reading, or cognitive (Go-noGo, attention, reasoning, etc.) tasks (Dushanova & Tsokov, 2020, 2021; Dushanova et al., 2020; Flynn & Deering, 1989a, 1989b; Flynn et al., 1992; Galin et al., 1988, 1992; Jakovljević et al., 2021; Klimesch et al., 2001; Leisman, 2002; Mahmoodin et al., 2016; Ortiz et al., 1992; Penolazzi et al., 2008; Remschmidt & Warnke, 1992; Rippon & Brunswick, 2000; Seri & Cerquiglini, 1993; Spironelli et al., 2006, 2008; Taskov & Dushanova, 2020, 2021; Žarić et al., 2017). A smaller number of other studies evaluate several other tasks: writing, speech, spelling, music, during the vision of an audio story, listening, and tapping (Colling et al., 2017; Di Liberto et al., 2018; Duffy et al., 1980a, 1980b; Flynn et al., 1992; Galin et al., 1992; Haynes et al., 1989; Mahmoodin et al., 2019; Martinez-Murcia et al., 2020; Mattson et al., 1992).
The results are not comparable, given the different tasks and EEG analysis methodologies, but the differences between DD and control children appear mainly localized in the left temporoparietal sites.
Table 5 reports the task used, the differences found in children with dyslexia compared to controls, and the localization findings.
Comparing at-rest and during-task conditions
Twelve works were performed both at rest and during the task conditions. Some authors found differences in both conditions, but more pronounced in the task condition (Duffy et al. 1992) or the rest condition (Leisman, 2002), whereas others found differences only during the task condition (Flynn et al., 1992; Galin et al., 1988; Ortiz et al., 1992). Finally, some studies did not clearly report what happened during the at-rest condition (Duffy et al. 1980b; Flynn & Deering, 1989b; Galin et al., 1992; Mahmoodin et al., 2016, 2019; Zainuddin et al., 2018).
Other kinds of studies
Some studies of EEG in children with dyslexia are not possible to include in the previous paragraphs because of the different natures of the works. We will briefly describe them as follows.
Bosch-Bayard et al. (2018) wanted to find a classification equation that discriminates the two groups with high accuracy. They obtained a discrimination equation that did not participate in the Boder classification algorithm, with a specificity and sensitivity of 0.94 to discriminate DD from the nonspecific reading delay.
Using a statistically based technique, Duffy et al., (1980a, 1980b) searched for rules for the classification of children with dyslexia. They developed classification rules that successfully diagnosed 80 to 90% of the subjects.
Eroglu et al. (2022) investigated possible disturbances in the complexity of EEG signals (connectivity measures) on multiple time scales in people with dyslexia and the potential positive effects of special neurofeedback and multisensory learning treatment. After treatment, the lower complexity of the experimental group increased to the typically developing group on lower and medium temporal scales in all channels. Fein et al. (1983) assessed the test–retest reliability of both absolute and relative spectra. They found excellent absolute and relative power reliability under properly controlled conditions.
Finally, Zainuddin et al. (2018) used a support vector machine algorithm to classify EEG signals from typical, poor, and capable children with dyslexia while writing words and nonwords. Beta and theta-to-beta ratios formed the input features for the classifier. It was found that the best performance of the support vector machine was obtained with 91% overall accuracy when both kernel scale and box constraint were set to 1.
Differences between dyslexia subtypes
Only a few studies evaluated the differences between dyslexia subtypes. In their works, Bosch-Bayard et al. (2018) and Bosch-Bayard et al. (2020) focused on dyslexia with phonological deficits (dysphonetic) compared with children with nonspecific reading delays. By analyzing the power spectra, in 2018, they found that the DD group had significantly higher activity in the delta and theta bands than the nonspecific reading delays group in the frontal, central, and parietal areas bilaterally. Two years later, using measures of EEG connectivity, they found that the left calcarine sulcus was more active in the DD group, while the left rolandic operculum was more active in the nonspecific reading delays group. Instead, Flynn and Deering (1989a) and Flynn et al. (1992) compared two types of dyslexia: dysphonetic and dyseidetic (with orthographic deficits). They found left temporal differences in children with dyseidetic dyslexia and right parietal-occipital differences for those with dysphonetic dyslexia, supporting predictions derived from a compensation-from-strength model of dyslexia.
Discussion
We performed a systematic review of the evidence using EEG in DD. Finally, we selected 49 works, both EEG studies at rest and during a task. The articles differed greatly in methodology, which makes a summary of the results challenging. However, some points have come to light. Even at rest, children with dyslexia and children in the control group exhibited differences in several EEG measures, particularly an increase in delta and theta and a reduction in alpha frequencies, without a clear localization. The same frequencies recorded at rest appear to be associated with learning performances. During reading-related tasks, differences between children with dyslexia and children in the control group appear more localized at the left temporoparietal sites, and the spectral frequencies appear differently involved. Theta range remained the frequency band that hosts the main number of differences between children with dyslexia and children in the control group, but some work also found the involvement of the beta and gamma bands.
Current research on electrophysiological correlates of language acquisition could help interpret our data. For example, many studies have been done on speech processing, a cognitive ability strictly associated with reading. Delta, theta, and gamma oscillations have been shown to be specifically engaged by the quasirhythmic properties of speech (Giraud & Poeppel, 2012). Different frequencies account for different properties of the language: the transformation of the auditory signal input into lexical and phrasal units occurs at a very low modulation rate, roughly 1–2 Hz. Frequencies in a slightly higher range (1.5–4; i.e., delta) account for prosodic perception (Ghitza & Greenberg, 2009) and (4–7; i.e., theta) for syllabic perception (Luo & Poeppel, 2007; Poeppel et al., 2008). Higher frequencies (30–40 Hz, the high beta/low gamma band) process stimulus information concurrently with the theta band, lying in a nesting relation such that the phase of theta shapes the properties of gamma (Giraud & Poeppel, 2012). Frequency bands could have similar functions in the reading process. In fact, studies in children, adolescents, and adults with dyslexia converge to identify an atypical auditory neural synchronization of oscillations, suggesting deviant neural processing of both syllabic and phonemic rate information (De Vos et al., 2017; Di Liberto et al., 2018; Lehongre et al. 2011; Lizarazu et al., 2015; Molinaro et al., 2016). It has been proposed that if people with dyslexia parse speech at a frequency slightly higher or lower than the usual frequency rate, their phonemic representations could be abnormal (Ziegler et al., 2009). This anomaly would selectively complicate the grapheme-to-phoneme matching, leaving speech perception and production unaffected. These studies are compatible with the results from ERPs, which revealed altered processing of certain acoustic information relevant to speech perception in individuals with dyslexia, such as frequency changes and temporal patterns (Schulte-Körne & Bruder, 2010).
Competing neurobiological hypotheses alternatively assign a crucial role to higher versus lower frequency bands. It has been suggested that dyslexic people may be less responsive to modulations at specific frequencies that are optimal for phonemic analysis (30 Hz) (Lehongre et al., 2011) or that they may fail to reset gamma activity (Schroeder et al., 2010). Other authors, more in line with the results of our review, emphasized the role of lower frequencies and, in particular, theta oscillations. A deficit in theta is thought to alter low temporal modulation tracking syllable coding and even multisensory processing, with consequences for attention and auditory-visual integration (Goswami, 2011; Ziegler et al., 2009). De Vos and colleagues (De Vos et al., 2017) found in adolescents with DD atypical alpha (reduced) and beta (increased) synchronization. They advocated that the alpha reduction could be related to phonological processing problems. At the same time, the over-synchronization of beta range oscillations could be a compensatory mechanism to improve the processing of phonemic rate information. Although different methodologies and age ranges, we also found numerous abnormalities in alpha and beta frequencies (Duffy et al., 1980a; Dushanova & Tsokov, 2021; Flynn et al., 1992; Galin et al., 1988, 1992; Haynes et al., 1989; Zulkifli Mahmoodin et al., 2019; Ortiz et al., 1992; Rippon & Brunswick, 2000; Spironelli et al., 2008; Taskov & Dushanova, 2021). Furthermore, alpha appears globally reduced in at rest conditions, whereas beta offers more contradictory results. If the theory of the compensatory beta effect is correct, it is possible that our younger samples do not exhibit compensatory effects yet.
The relationship between the deficits in different band frequencies and the stages of learning to read could also explain the scarcity of results in the gamma band of our review: It may be that the 12-year filter has determined a specific trend in the type of deficit. Interestingly, in our review, the alterations in the frequency bands appear to be associated with learning performance, supporting the neurobiological meaning of these components. It is noteworthy that when considering EEG frequency bands, it is important to consider that the bands may not be a perfect match with those of the adult or older child. Particularly, a shift in frequency peaks with age has been shown (Campus et al., 2021; Clarke et al., 2001; Orekhova et al., 2006).
The most heterogeneity in our review is in studies using stimulation. Most compared the EEG and the performance of DD and control children’s performance in linguistic, reading, or cognitive tasks. A smaller number of other studies evaluate several other tasks: writing, speech, spelling, music, listening to an audio story or someone reading, and tapping. The majority of tasks explore functions directly involved in dyslexia or strictly connected. However, there are also studies exploring different cognitive functions in DD children. These are interesting because they explore new hypotheses on dyslexia and its association with not obvious cognitive functions, like vigilance and visuospatial abilities. Unfortunately, the results are not comparable, given the different tasks and EEG analysis methodologies, but the differences between DD and control children appear mainly localized in the left temporoparietal sites. Still, caution should thus be taken in interpreting power differences between groups in the context of neural tracking differences, as they rely not only on distinct analytical approaches but also on different experimental paradigms. Probably for that reason, the data coming from our review does not capture the complex picture that emerges from the most recent research. For example, intriguing insights came from studies on the hemispheric specialization of specific frequencies in DD.
In summary, the left hemisphere appears to specialize in local high-frequency verbal computations, while the right hemisphere codes low frequencies of the speech envelope and interhemispheric cognitive control (Giraud & Poeppel, 2012). Impairment of the right hemisphere circuitry of frontoparietal attention networks has been hypothesized to be the primary cause of dyslexia (Goswami, 2011; Lehongre et al., 2011; Lizarazu et al., 2015; Molinaro et al., 2016; Power et al., 2016). Such a dysfunction would have a cascading negative effect on phonemic processing in the dorsal reading network (Kershner, 2019, 2020).
Our evaluation of the quality of the studies highlights an overall weakness of the reported studies. Many studies are old, and the methodological sections do not follow current guidelines of transparency and reliability of methods. The greatest weakness appears to be the small sample sizes of most studies; furthermore, almost none reported the method for selecting the sample size. Methodological concerns, the intrinsic high interindividual variability of electrophysiological techniques and the developmental phase, the tendency to publish only positive findings, and the use of different methodologies render the possibility of synthesizing and drawing conclusions very challenging. We could have included the grey literature to overcome these limitations, but we initially decided to limit the search to peer-reviewed published works. All these concerns hinder the possibility of establishing clear markers in EEG correlates of DD. However, a trend emerges despite differences in experimental conditions and analysis methodology (at rest, differences and involvement of the theta during reading-related tasks). The trend may reflect processing vulnerability in children with dyslexia or compensatory processing strategies that inappropriately activate areas of the reading network in this specific age range.
Finally, only a few studies evaluated the differences between dyslexic subtypes. A wide range of literature highlights the presence of different subtypes of DD. The few existing studies support differences at rest and during reading tasks. These conditions have to be better addressed because of the possible different cores (endophenotype) involved and the consequent additional variability in the results if not considered.
Conclusion
This review seems to highlight some interesting insights: (a) there are abnormalities in spontaneous cerebral activity (“at rest”) of both temporal sites and more widerspread scalp placements in children with dyslexia, and (b) reading-related tasks elicited differences in frequencies considered crucial for speech processing, and the differences are localized in the temporoparietal sites. Although EEG localizations do not necessarily correspond to the underlying neuroanatomical regions, the finding of a left temporoparietal involvement is compatible with neuroimaging abnormalities, especially in the left and posterior regions. It should be noted that the current research on EEG correlates in DD is more advanced than is apparent from our review, which comprised a reduced number of works and some very old. This incongruency could denote a trend in research to select older participants, probably due to the greater simplicity of conducting studies with older and more collaborative children. Adolescents and adults are also suitable for more complex tasks. Furthermore, older age allows more certainty in diagnosis over time. However, we think that the range 6–12 is crucial because it represents the first appearance and diagnosis of the disorder and could offer important insights into the first phases of consolidation of both abilities and dysfunction. Therefore, we hope that future research addresses the functional role of atypical activation and involvement of specific frequencies in 6–12 years of DD to understand how fundamental brain processes are affected and how the brain compensates for those disruptions. Evaluation of the emergence and characterization of spectral EEG components and their deviation from the expected typical trajectory may be important to understanding early abnormalities of brain development, also in very early phases, as shown in the DD literature (Ozernov-Palchik & Gaab, 2016) and other research fields (Cainelli et al., 2021). This has the potential to lead to more effectiveness and could change the outcome trajectories for those with reading deficits.
References
Haddaway, N. R., & McGuinness, L. (2020). PRISMA2020: R package and ShinyApp for producing PRISMA 2020 compliant flow diagrams (Version 0.0.1). Zenodo. 10.5281/ZENODO.4287835
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596
Arns, M., Peters, S., Breteler, R., & Verhoeven, L. (2007). Different brain activation patterns in dyslexic children: Evidence from EEG power and coherence patterns for the double-deficit theory of dyslexia. Journal of Integrative Neuroscience, 6(1), 175–190. https://doi.org/10.1142/S0219635207001404
Arrhenius, B., Gyllenberg, D., Vuori, M., Tiiri, E., Lempinen, L., & Sourander, A. (2021). Relative age and specific learning disorder diagnoses: A Finnish population-based cohort study. JCPP Advances, 1(1), e12001. https://doi.org/10.1111/jcv2.12001
Ayers, F. W., & Torres, F. (1967). The incidence of eeg abnormalities in a dyslexic and a control group. Journal of Clinical Psychology, 23(3), 334–336. https://doi.org/10.1002/1097-4679(196707)23:3%3c334::AID-JCLP2270230313%3e3.0.CO;2-I
Babiloni, C., Stella, G., Buffo, P., Vecchio, F., Onorati, P., Muratori, C., Miano, S., Gheller, F., Antonaci, L., Albertini, G., & Rossini, P. M. (2012). Cortical sources of resting state EEG rhythms are abnormal in dyslexic children. Clinical Neurophysiology, 123(12), 2384–2391. https://doi.org/10.1016/j.clinph.2012.05.002
Bishop, D. V. M. (2007). Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: Where are we, and where should we be going? Psychological Bulletin, 133(4), 651–672. https://doi.org/10.1037/0033-2909.133.4.651
Bosch-Bayard, J., Peluso, V., Galan, L., Sosa, P. V, Chiarenza, G. A., Valdes Sosa, P., Chiarenza, G. A., Sosa, P. V, & Chiarenza, G. A. (2018). Clinical and electrophysiological differences between subjects with dysphonetic dyslexia and non-specific reading delay. Brain Sciences, 8(9). https://doi.org/10.3390/brainsci8090172
Bosch-Bayard, J., Girini, K., Biscay, R. J., Valdes-Sosa, P., Evans, A. C., & Chiarenza, G. A. (2020). Resting EEG effective connectivity at the sources in developmental dysphonetic dyslexia. Differences with nonspecific reading delay. International Journal of Psychophysiology, 153(April), 135–147. https://doi.org/10.1016/j.ijpsycho.2020.04.021
Bruni, O., Ferri, R., Novelli, L., Terribili, M., Troianiello, M., Finotti, E., Leuzzi, V., & Curatolo, P. (2009a). Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep, 32(10), 1333–1340. https://doi.org/10.1093/sleep/32.10.1333
Bruni, O., Ferri, R., Novelli, L., Finotti, E., Terribili, M., Troianiello, M., Valente, D., Sabatello, U., & Curatolo, P. (2009b). Slow EEG amplitude oscillations during NREM sleep and reading disabilities in children with dyslexia. Developmental Neuropsychology, 34(5), 539–551. https://doi.org/10.1080/87565640903133418
Bull, C., Byrnes, J., Hettiarachchi, R., & Downes, M. (2019). A systematic review of the validity and reliability of patient-reported experience measures. Health Services Research, 54(5), 1023–1035. https://doi.org/10.1111/1475-6773.13187
Buzsáki, G., & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science (New York, N.Y.), 304(5679), 1926–1929. https://doi.org/10.1126/SCIENCE.1099745
Cainelli, E., Vedovelli, L., Wigley, I. L. C. M., Bisiacchi, P. S., & Suppiej, A. (2021). Neonatal spectral EEG is prognostic of cognitive abilities at school age in premature infants without overt brain damage. European Journal of Pediatrics, 180(3), 909–918. https://doi.org/10.1007/s00431-020-03818-x
Campus, C., Signorini, S., Vitali, H., De Giorgis, V., Papalia, G., Morelli, F., & Gori, M. (2021). Sensitive period for the plasticity of alpha activity in humans. Developmental Cognitive Neuroscience, 49.https://doi.org/10.1016/j.dcn.2021.100965
Canolty, R. T., Edwards, E., Dalal, S. S., Soltani, M., Nagarajan, S. S., Kirsch, H. E., Berger, M. S., Barbare, N. M., & Knight, R. T. (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science, 313(5793), 1626–1628. https://doi.org/10.1126/science.1128115
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). Age and sex effects in the EEG: Development of the normal child. Clinical Neurophysiology, 112(5), 806–814. https://doi.org/10.1016/S1388-2457(01)00488-6
Colling, L. J., Noble, H. L., & Goswami, U. (2017). Neural entrainment and sensorimotor synchronization to the beat in children with developmental dyslexia: An EEG study. Frontiers in Neuroscience, 11(JUL). https://doi.org/10.3389/fnins.2017.00360
Colon, E. J., Notermans, S. L. H., de Weerd, J. P. C., & Kap, J. (1979). The discriminating role of EEG power spectra in dyslexic children. Journal of Neurology, 221(4), 257–262. https://doi.org/10.1007/BF00314642
De Vos, A., Vanvooren, S., Vanderauwera, J., Ghesquière, P., & Wouters, J. (2017). Atypical neural synchronization to speech envelope modulations in dyslexia. Brain and Language, 164, 106–117. https://doi.org/10.1016/J.BANDL.2016.10.002
Di Liberto, G. M., Peter, V., Kalashnikova, M., Goswami, U., Burnham, D., & Lalor, E. C. (2018). Atypical cortical entrainment to speech in the right hemisphere underpins phonemic deficits in dyslexia. NeuroImage, 175, 70–79. https://doi.org/10.1016/j.neuroimage.2018.03.072
Diamanti, V., Goulandris, N., Campbell, R., & Protopapas, A. (2018). Dyslexia profiles across orthographies differing in transparency: An evaluation of theoretical predictions contrasting English and Greek. Scientific Studies of Reading, 22(1), 55–69. https://doi.org/10.1080/10888438.2017.1338291
Downes, M. J., Brennan, M. L., Williams, H. C., & Dean, R. S. (2016). Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open, 6(12). https://doi.org/10.1136/bmjopen-2016-011458
Duffy, F. H., Denckla, M. B., Bartels, P. H., & Sandini, G. (1980a). Dyslexia: Regional differences in brain electrical activity by topographic mapping. Annals of Neurology, 7(5), 412–420. https://doi.org/10.1002/ana.410070505
Duffy, F. H., Denckla, M. B., Bartels, P. H., Sandini, G., & Kiessling, L. S. (1980b). Dyslexia: Automated diagnosis by computerized classification of brain electrical activity. Annals of Neurology, 7(5), 421–428. https://doi.org/10.1002/ana.410070506
Dushanova, J. A., & Tsokov, S. A. (2020). Small-world EEG network analysis of functional connectivity in developmental dyslexia after visual training intervention. Journal of Integrative Neuroscience, 19(4), 601–618. https://doi.org/10.31083/J.JIN.2020.04.193
Dushanova, J. A., & Tsokov, S. A. (2021). Altered electroencephalographic networks in developmental dyslexia after remedial training: A prospective case-control study. Neural Regeneration Research, 16(4), 734–743. https://doi.org/10.4103/1673-5374.295334
Dushanova, J., Lalova, Y., Kalonkina, A., & Tsokov, S. (2020). Speech–brain frequency entrainment of dyslexia with and without phonological deficits. Brain Sciences, 10(12), 1–23. https://doi.org/10.3390/brainsci10120920
Elgendi, M. M., Stewart, S. H., MacKay, E. J., & Deacon, S. H. (2021). Two aspects of psychological functioning in undergraduates with a history of reading difficulties: Anxiety and self-efficacy. Annals of Dyslexia, 71(1), 84–102. https://doi.org/10.1007/s11881-021-00223-3
Eroğlu, G., Gürkan, M., Teber, S., Ertürk, K., Kırmızı, M., Ekici, B., Arman, F., Balcisoy, S., Özgüz, V., & Çetin, M. (2022). Changes in EEG complexity with neurofeedback and multisensory learning in children with dyslexia: A multiscale entropy analysis. Applied Neuropsychology: Child, 11(2), 133–144. https://doi.org/10.1080/21622965.2020.1772794
Farrag, A.-K.F., & El-Behary, A.-R.A. (1990). Specific reading disability in Egyptian children: Clinical picture, diagnosis and prognosis. Neuroepidemiology, 9(1), 50–56. https://doi.org/10.1159/000110751
Fein, G., Galin, D., Yingling, C. D., Johnstone, J., Davenport, L., & Herron, J. (1986). EEG spectra in dyslexic and control boys during resting conditions. Electroencephalography and Clinical Neurophysiology, 63(2), 87–97. https://doi.org/10.1016/0013-4694(86)90001-5
Fein, G., Galin, D., Johnstone, J., Yingling, C. D., Marcus, M., & Kiersch, M. E. (1983). EEG power spectra in normal and dyslexic children. I. Reliability during passive conditions. Electroencephalography and Clinical Neurophysiology, 55(4), 399–405. https://doi.org/10.1016/0013-4694(83)90127-X
Flynn, J. M., & Deering, W. M. (1989a). Subtypes of dyslexia: Investigation of Boder’s system using quantitative neurophysiology. Developmental Medicine & Child Neurology, 31(2), 215–223. https://doi.org/10.1111/j.1469-8749.1989.tb03981.x
Flynn, J. M., & Deering, W. M. (1989b). Topographic brain mapping and evaluation of dyslexic children. Psychiatry Research, 29(3), 407–408. https://doi.org/10.1016/0165-1781(89)90103-0
Flynn, J. M., Deering, W., Goldstein, M., & Rahbar, M. H. (1992). Electrophysiological correlates of dyslexic subtypes. Journal of Learning Disabilities, 25(2), 133–141. https://doi.org/10.1177/002221949202500207
Fraga González, G., Van der Molen, M. J. W., Žarić, G., Bonte, M., Tijms, J., Blomert, L., Stam, C. J., & Van der Molen, M. W. (2016). Graph analysis of EEG resting state functional networks in dyslexic readers. Clinical Neurophysiology, 127(9), 3165–3175. https://doi.org/10.1016/j.clinph.2016.06.023
Galin, D., Herron, J., Johnstone, J., Fein, G., & Yingling, C. (1988). EEG alpha asymmetry in dyslexics during speaking and block design tasks. Brain and Language, 35(2), 241–253. https://doi.org/10.1016/0093-934X(88)90110-1
Galin, D., Raz, J., Fein, G., Johnstone, J., Herron, J., & Yingling, C. (1992). EEG spectra in dyslexic and normal readers during oral and silent reading. Electroencephalography and Clinical Neurophysiology, 82(2), 87–101. https://doi.org/10.1016/0013-4694(92)90151-7
Ghitza, O., & Greenberg, S. (2009). On the possible role of brain rhythms in speech perception: Intelligibility of time-compressed speech with periodic and aperiodic insertions of silence. Phonetica, 66(1–2), 113–126. https://doi.org/10.1159/000208934
Giraud, A., & Poeppel, D. (2012). Cortical oscillations and speech processing: Emerging computational principles and operations. Nature Neuroscience, 15(4), 511–517. https://doi.org/10.1038/NN.3063
Goswami, U. (2011). A temporal sampling framework for developmental dyslexia. Trends in Cognitive Sciences, 15(1), 3–10. https://doi.org/10.1016/j.tics.2010.10.001
Hämäläinen, J. A., Salminen, H. K., & Leppänen, P. H. T. (2013). Basic auditory processing deficits in dyslexia: Systematic review of the behavioral and event-related potential/ field evidence. Journal of Learning Disabilities, 46(5), 413–427. https://doi.org/10.1177/0022219411436213
Hancock, R., Richlan, F., & Hoeft, F. (2017). Possible roles for fronto-striatal circuits in reading disorder. Neuroscience and Biobehavioral Reviews, 72, 243–260. https://doi.org/10.1016/j.neubiorev.2016.10.025
Harmony, T., Hinojosa, G., Marosi, E., Becker, J., Rodriguez, M., Reyes, A., & Rocha, C. (1990). Correlation between eeg spectral parameters and an educational evaluation. International Journal of Neuroscience, 54(1–2), 147–155. https://doi.org/10.3109/00207459008986630
Harmony, T. (2013). The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience, 7(DEC), 83. https://doi.org/10.3389/FNINT.2013.00083/BIBTEX
Haynes, W. O., Haynes, M. D., & Strickland-Helms, D. F. (1989). Alpha hemispheric asymmetry in children with learning disabilities and normally achieving children during story comprehension and rehearsal prior to narrative production. Journal of Learning Disabilities, 22(6), 391–396,399. https://doi.org/10.1177/002221948902200612
Heim, S., & Keil, A. (2004). Large-scale neural correlates of developmental dyslexia. European Child and Adolescent Psychiatry, 13(3), 125–140. https://doi.org/10.1007/s00787-004-0361-7
Hulme, C., Nash, H. M., Gooch, D., Lervåg, A., & Snowling, M. J. (2015). The foundations of literacy development in children at familial risk of dyslexia. Psychological Science, 26(12), 1877–1886. https://doi.org/10.1177/0956797615603702
Jakovljević, T., Janković, M. M., Savić, A. M., Soldatović, I., Čolić, G., Jakulin, T. J., Papa, G., & Ković, V. (2021). The relation between physiological parameters and colour modifications in text background and overlay during reading in children with and without dyslexia. Brain Sciences, 11(5). https://doi.org/10.3390/brainsci11050539
Kershner, J. R. (2019). Neurobiological systems in dyslexia. Trends in Neuroscience and Education, 14, 11–24. https://doi.org/10.1016/j.tine.2018.12.001
Kershner, J. R. (2020). Neuroscience and education: Cerebral lateralization of networks and oscillations in dyslexia. Laterality, 25(1), 109–125. https://doi.org/10.1080/1357650X.2019.1606820
Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences, 16(12), 606–617. https://doi.org/10.1016/J.TICS.2012.10.007
Klimesch, W., Doppelmayr, M., Wimmer, H., Schwaiger, J., Röhm, D., Gruber, W., & Hutzler, F. (2001). Theta band power changes in normal and dyslexic children. Clinical Neurophysiology, 112(7), 1174–1185. https://doi.org/10.1016/S1388-2457(01)00545-4
Kropotov, J. D. (2009). Quantitative EEG, event-related potentials and neurotherapy. In Quantitative EEG, event-related potentials and neurotherapy (1st ed.). Academic Press - Elsevier. https://doi.org/10.1016/B978-0-12-374512-5.X0001-1
Lakatos, P., Shah, A. S., Knuth, K. H., Ulbert, I., Karmos, G., & Schroeder, C. E. (2005). An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of Neurophysiology, 94(3), 1904–1911. https://doi.org/10.1152/jn.00263.2005
Lehongre, K., Ramus, F., Villiermet, N., Schwartz, D., & Giraud, A. L. (2011). Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron, 72(6), 1080–1090. https://doi.org/10.1016/j.neuron.2011.11.002
Leisman, G. (2002). Coherence of hemispheric function in developmental dyslexia. Brain and Cognition, 48(2–3), 425–431. https://doi.org/10.1006/brcg.2001.1392
Lizarazu, M., Lallier, M., Molinaro, N., Bourguignon, M., Paz-Alonso, P. M., Lerma-Usabiaga, G., & Carreiras, M. (2015). Developmental evaluation of atypical auditory sampling in dyslexia: Functional and structural evidence. Human Brain Mapping, 36(12), 4986–5002. https://doi.org/10.1002/hbm.22986
Lonergan, A., Doyle, C., Cassidy, C., MacSweeney Mahon, S., Roche, R. A. P., Boran, L., & Bramham, J. (2019). A meta-analysis of executive functioning in dyslexia with consideration of the impact of comorbid ADHD. Journal of Cognitive Psychology, 31(7), 725–749. https://doi.org/10.1080/20445911.2019.1669609
Luo, H., & Poeppel, D. (2007). Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron, 54(6), 1001–1010. https://doi.org/10.1016/J.NEURON.2007.06.004
Mahmoodin, Z, Mansor, W., Khuan, L., Mohamad, N., & Amirin, S. (2016). Feature extraction of electroencephalogram signal generated from writing in dyslexic children using daubechies wavelet transform. Jurnal Teknologi, 78(6–8), 119–125. https://doi.org/10.11113/jt.v78.9071
Mahmoodin, Zulkifli, Lee, K. Y., Mansor, W., & Zainuddin, A. Z. A. (2019). Support vector machine with theta-beta band power features generated from writing of dyslexic children. International Journal of Integrated Engineering, 11(3), 42–50. https://doi.org/10.30880/ijie.2019.11.03.005
Martin, A., Kronbichler, M., & Richlan, F. (2016). Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Human Brain Mapping, 37(7), 2676–2699. https://doi.org/10.1002/HBM.23202
Martinez-Murcia, F. J., Ortiz, A., Gorriz, J. M., Ramirez, J., Lopez-Abarejo, P. J., Lopez-Zamora, M., & Luque, J. L. (2020). EEG connectivity analysis using denoising autoencoders for the detection of dyslexia. International Journal of Neural Systems, 30(7), 1–17. https://doi.org/10.1142/S0129065720500379
Mattson, A. J., Sheer, D. E., & Fletcher, J. M. (1992). Electrophysiological evidence of lateralized disturbances in children with learning disabilities. Journal of Clinical and Experimental Neuropsychology, 14(5), 707–716. https://doi.org/10.1080/01688639208402857
Molinaro, N., Lizarazu, M., Lallier, M., Bourguignon, M., & Carreiras, M. (2016). Out-of-synchrony speech entrainment in developmental dyslexia. Human Brain Mapping, 37(8), 2767–2783. https://doi.org/10.1002/hbm.23206
Nalavany, B. A., Logan, J. M., & Carawan, L. W. (2018). The relationship between emotional experience with dyslexia and work self-efficacy among adults with dyslexia. Dyslexia, 24(1), 17–32. https://doi.org/10.1002/dys.1575
O’Brien, G., & Yeatman, J. D. (2021). Bridging sensory and language theories of dyslexia: Toward a multifactorial model. Developmental Science, 24(3), e13039. https://doi.org/10.1111/desc.13039
Orekhova, E. V., Stroganova, T. A., Posikera, I. N., & Elam, M. (2006). EEG theta rhythm in infants and preschool children. Clinical Neurophysiology, 117(5), 1047–1062. https://doi.org/10.1016/j.clinph.2005.12.027
Ortiz, T., Exposito, F. J., Miguel, F., Martin-Loeches, M., & Rubia, F. J. (1992). Brain mapping in dysphonemic dyslexia: In resting and phonemic discrimination conditions. Brain and Language, 42(3), 270–285. https://doi.org/10.1016/0093-934X(92)90101-J
Ozernov-Palchik, O., & Gaab, N. (2016). Tackling the “dyslexia paradox”: Reading brain and behavior for early markers of developmental dyslexiax. Wiley Interdisciplinary Reviews: Cognitive Science, 7(2), 156–176. https://doi.org/10.1002/wcs.1383
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Medicine, 18(3), e1003583. https://doi.org/10.1371/JOURNAL.PMED.1003583
Papagiannopoulou, E. A., & Lagopoulos, J. (2016). Resting state EEG hemispheric power asymmetry in children with dyslexia. Frontiers in Pediatrics, 4(FEB), 11. https://doi.org/10.3389/fped.2016.00011
Penny, W. D., Kiebel, S. J., Kilner, J. M., & Rugg, M. D. (2002). Event-related brain dynamics. Trends in Neurosciences, 25(8), 387–389. https://doi.org/10.1016/S0166-2236(02)02202-6
Penolazzi, B., Spironelli, C., & Angrilli, A. (2008). Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology, 45(6), 1025–1033. https://doi.org/10.1111/j.1469-8986.2008.00709.x
Poeppel, D., Idsardi, W., & van Wassenhove, V. (2008). Speech perception at the interface of neurobiology and linguistics. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1493), 1071–1086. https://doi.org/10.1098/RSTB.2007.2160
Power, A. J., Colling, L. J., Mead, N., Barnes, L., & Goswami, U. (2016). Neural encoding of the speech envelope by children with developmental dyslexia. Brain and Language, 160, 1–10. https://doi.org/10.1016/j.bandl.2016.06.006
Reda, F., Gorgoni, M., D’Atri, A., Scarpelli, S., Carpi, M., Di Cola, E., Menghini, D., Vicari, S., Stella, G., De Gennaro, L., D’atri, A., Scarpelli, S., Carpi, M., Di Cola, E., Menghini, D., Vicari, S., Stella, G., De Gennaro, L., D’Atri, A., … De Gennaro, L. (2021). Sleep-related declarative memory consolidation in children and adolescents with developmental dyslexia. Brain Sciences, 11(1), 1–17.https://doi.org/10.3390/brainsci11010073
Remschmidt, H., & Warnke, A. (1992). Visual information processing and cerebral activation in dyslexic boys: Quantitative EEG analysis during discrimination reading tasks. European Child & Adolescent Psychiatry, 1(1), 42–53. https://doi.org/10.1007/BF02084433
Richlan, F., Kronbichler, M., & Wimmer, H. (2009). Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping, 30(10), 3299–3308. https://doi.org/10.1002/HBM.20752
Richlan, F. (2020). The functional neuroanatomy of developmental dyslexia across languages and writing systems. Frontiers in Psychology, 11.https://doi.org/10.3389/FPSYG.2020.00155
Rippon, G., & Brunswick, N. (2000). Trait and state EEG indices of information processing in developmental dyslexia. International Journal of Psychophysiology, 36(3), 251–265. https://doi.org/10.1016/S0167-8760(00)00075-1
Schroeder, C. E., Wilson, D. A., Radman, T., Scharfman, H., & Lakatos, P. (2010). Dynamics of active sensing and perceptual selection. Current Opinion in Neurobiology, 20(2), 172–176. https://doi.org/10.1016/j.conb.2010.02.010
Schulte-Körne, G., & Bruder, J. (2010). Clinical neurophysiology of visual and auditory processing in dyslexia: A review. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 121(11), 1794–1809. https://doi.org/10.1016/j.clinph.2010.04.028
Seri, S., & Cerquiglini, A. (1993). Dynamic changes of alpha power as a probe of linguistic processes in normal and dyslexic children. Brain Topography, 5(4), 395–398. https://doi.org/10.1007/BF01128697
Shiota, M., Koeda, T., & Takeshita, K. (2000). Cognitive and neurophysiological evaluation of Japanese dyslexia. Brain and Development, 22(7), 421–426. https://doi.org/10.1016/S0387-7604(00)00167-4
Spironelli, C., & B, P., C, V., & A, A. (2006). Inverted EEG theta lateralization in dyslexic children during phonological processing. Neuropsychologia, 44(14), 2814–2821. https://doi.org/10.1016/J.NEUROPSYCHOLOGIA.2006.06.009
Spironelli, C., Penolazzi, B., & Angrilli, A. (2008). Dysfunctional hemispheric asymmetry of theta and beta EEG activity during linguistic tasks in developmental dyslexia. Biological Psychology, 77(2), 123–131. https://doi.org/10.1016/j.biopsycho.2007.09.009
Stein, J., & Walsh, V. (1997). To see but not to read; the magnocellular theory of dyslexia. Trends in Neurosciences, 20(4), 147–152. https://doi.org/10.1016/S0166-2236(96)01005-3
Taskov, T, & Dushanova, J. (2021). Functional connectivity in developmental dyslexia during speed discrimination. Symmetry, 13(5). https://doi.org/10.3390/sym13050749
Taskov, T., & Dushanova, J. (2020). Reading-network in developmental dyslexia before and after visual training. Symmetry, 12(11), 1–18. https://doi.org/10.3390/sym12111842
Vandermosten, M., Boets, B., Luts, H., Poelmans, H., Golestani, N., Wouters, J., & Ghesquière, P. (2010). Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proceedings of the National Academy of Sciences of the United States of America, 107(23), 10389–10394. https://doi.org/10.1073/pnas.0912858107
Vidyasagar, T. R., & Pammer, K. (2010). Dyslexia: A deficit in visuo-spatial attention, not in phonological processing. Trends in Cognitive Sciences, 14(2), 57–63. https://doi.org/10.1016/j.tics.2009.12.003
Womelsdorf, T., Schoffelen, J. M., Oostenveld, R., Singer, W., Desimone, R., Engel, A. K., & Fries, P. (2007). Modulation of neuronal interactions through neuronal synchronization. Science, 316(5831), 1609–1612. https://doi.org/10.1126/science.1139597
Xue, H., Wang, Z., Tan, Y., Yang, H., Fu, W., Xue, L., & Zhao, J. (2020). Resting-state EEG reveals global network deficiency in dyslexic children. Neuropsychologia, 138, 107343. https://doi.org/10.1016/j.neuropsychologia.2020.107343
Zainuddin, A. Z. A., Mansor, W., Lee, K. Y., & Mahmoodin, Z. (2018). Performance of support vector machine in classifying EEG signal of dyslexic children using RBF kernel. Indonesian Journal of Electrical Engineering and Computer Science, 9(2), 403–409. https://doi.org/10.11591/ijeecs.v9.i2.pp403-409
Žarić, G., Correia, J. M., Fraga González, G., Tijms, J., van der Molen, M. W., Blomert, L., & Bonte, M. (2017). Altered patterns of directed connectivity within the reading network of dyslexic children and their relation to reading dysfluency. Developmental Cognitive Neuroscience, 23, 1–13. https://doi.org/10.1016/j.dcn.2016.11.003
Ziegler, J. C., Pech-Georgel, C., George, F., & Lorenzi, C. (2009). Speech-perception-in-noise deficits in dyslexia. Developmental Science, 12(5), 732–745. https://doi.org/10.1111/j.1467-7687.2009.00817.x
Acknowledgements
The present work was carried out within the scope of the research program Dipartimenti di Eccellenza (art.1, commi 314-337 legge 232/2016), which was supported by a grant from Ministero dell'Istruzione, dell’Università e della Ricerca (MIUR) to the Department of General Psychology, University of Padua.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cainelli, E., Vedovelli, L., Carretti, B. et al. EEG correlates of developmental dyslexia: a systematic review. Ann. of Dyslexia 73, 184–213 (2023). https://doi.org/10.1007/s11881-022-00273-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11881-022-00273-1