Abstract
Nematode functional traits and community structure approaches are receiving attention and being debated regarding the similarity in gained information. This work applied two nematode methods (i.e. functional traits, community structure) and sex ratios to investigate sandy beaches experiencing physical disturbances and anthropogenic stressors along the Alexandrian coast of Egypt. It is the first study to identify the Egyptian marine benthic nematodes to genus level yielding fourteen genera. This study addressed the following questions. (A) Which analyses of functional traits, community structure, or sex ratio could distinguish beaches experiencing natural disturbances from anthropogenic stressors? (B) What are the driving forces that govern nematode assemblages? Designated beaches were high pollution and energy (HPHE), high pollution but low energy (HPLE), and clean high energy (CHE). The study was conducted in March 2014, applying a stratified random sampling design and advanced multivariate analyses. Results revealed significant discrimination among and within beaches based on functional traits, community structure, and sex ratio. The functional traits (FBM) grouped data into two main groupings, predators and scavengers. Predators and cylindrical shape-tail types were found in high salinity moderately-sorted fine-grained sand dominant at HPHE and CHE. Scavengers and a variety of tail-shape types inhabited low saline water with well-sorted coarse sediment at HPLE. The community structure (CSM) revealed three groupings, as each beach had a specific assemblage based on its response to the measured physiochemical factors (salinity, pH, and temperature). Three genera were responsible for the high contribution across each beach Graphonema sp (HPHE), Enoplolaimus sp. (HPLE), and Oncholaimus sp. (CHE), as well as the co-sharing of Axnolaimus sp at HPLE. However, Axnolaimus sp and Oncholaimus sp showed the highest dissimilarity among beaches. The contribution of the measured factors was 53.8% (FBM) and 35.7% (CSM). Trophic guilds were anthropogenic stressors dependent and tail-shape type physically disturbing-related. However, nematode genera were species-specific to habitat type. The sex ratio was male-biased, and Juvenile-stages seemed to be temperature-dependent. The dissimilarity in the functional trait and community structure data recommend their application in biomonitoring and coastal restoration programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessing ecosystem health is a tool for protecting coastal environments (Boesch and Paul 2001). Benthic community structure was used in the past, but it requires taxonomic expertise, is time-consuming (Losi et al. 2013), and has many drawbacks due to the differential species-specific ecological adaptations within the same community (Salmaso et al. 2015). Researchers hypothesize other ecological indicators including taxonomic distinction (Bevilacqua et al. 2012), higher taxa (Semprucci et al. 2015a), Alpha and Beta diversity components (Flach et al. 2012), faunal biomass, allometric attributes (Losi et al. 2013), functional guilds and trophic food (Bhusal et al. 2014), and functional biological traits (Schratzberger et al. 2007; Alves et al. 2014; Semprucci et al. 2018) as surrogates for taxonomic identification.
Nematodes are the most abundant and diverse meiofaunal taxa among marine habitats (Kreuzinger-Janik et al. 2019), survive under extreme conditions (Coull and Chandler 1992), are easy to collect in large numbers providing a highly reliable statistical database regardless of sample size volume (Platt and Warwick 1980) and can be classified by a number of biological traits (Schratzberger et al. 2007). Buccal morphology is a proxy for trophic relations (Jensen 1987; Vanaverbeke et al. 2004). Tail-shape type (Thistle et al. 1995; Fleeger et al. 2006) is another descriptor important in locomotion, reproduction, and retention in sediments. Each biological trait responds differently to the surrounding environmental condition (Soetaert et al. 2009; Fleeger et al. 2010; Mitwally and Fleeger 2015). Recently, these traits have been used to reflect the human impact on the ecosystems (Alves et al. 2013; Kalogeropoulou et al. 2015; Mitwally and Fleeger 2016).
Under natural conditions, the nematode sex ratio is 1:1 (Tietjen and Lee 1972), or it may be higher in favor of females (Sahraeian et al. 2020). The life cycle comprises six stages (Boufahja et al. 2011). Different juvenile stages and gravid females indicate continuous reproduction and sufficient food for developmental rates (Hodda and Nicholas 1990).
A quantitative study of meiofauna, which ranges from ~ 44 to 63 μm up to 500 up to 1000 μm (Giere 2009), started in the last century on Egyptian Mediterranean shores (Mitwally et al. 2004). However, biomonitoring and coastal restoration programs in Egypt do not account for meiofauna. Many studies summarize the advantages and disadvantages of using meiofauna in monitoring programs (Danovaro et al. 1995; Moreno et al. 2011; Semprucci et al. 2015a, b). Mitwally and Hamdan (2021) assess the ecological status of Alexandrian sandy beaches, Egypt. They found that meiofaunal natural variability was driven by salinity and sand grain sizes.
Egyptian Mediterranean coastal areas are threatened due to increasing natural disturbances and anthropogenic stressors (Frihy 2001). The Alexandrian coast is highly vulnerable to beach erosion, sea-level rise, rip currents, and anthropogenic activities (Soliman et al. 2014; Masria et al. 2015). The Egyptian Environmental Affairs Agency (EEAA 2015) declares two marine pollution hotspots, Abo-Qir Bay and El-Mex Bay, along the northern coast of Egypt due to their receiving mixtures of variable pollutants (Shreadah et al. 2014, 2019). In these bays, the high levels of chlorinated organic compounds, hydrocarbon oil, and heavy metal pollution can be found (Khairy et al. 2012; El Nemr et al. 2013).
This study aims to examine nematode assemblages and gives the first taxonomic identification of meiobenthic nematode taxa to genus levels at the Alexandrian coast of Egypt to address the following questions. (A) Which analyses of functional traits, community structure, or sex ratio can be used to distinguish beaches experiencing natural disturbances from anthropogenic stressors? (B) What are the driving forces that govern community structure of nematode assemblages? Our null hypothesis is that examined approaches will not reveal significant variations between beaches under natural disturbances or anthropogenic stressors. The data from each bay will mirror each other.
Materials and methods
Sampling design
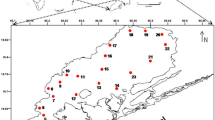
The current study applied the same stratified random sampling design as Mitwally and Hamdan (2021) to examine three sandy beaches; Abo-Qir Bay, highly polluted with high energy (HPHE), El-Mex Bay, highly polluted beach with low energy (HPLE), and the North West Coast, a clean beach with high energy (CHE). Our design is three sandy beaches, each composed of four profiles and five stations nested in with two replicates equal to 120 observations during March 2014.
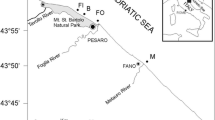
Study area(Fig. 1 )
Abo-Qir bay (HPHE)
Abo-Qir Bay is a shallow semicircular bay with ~ 360 km2 surface area and a maximum depth of ~ 16 m. The slope of the beach is gentle, backed by dunes. The Bay is bordered from the west by Abo-Qir Peninsula and from the east by the Rosetta Peninsula, where the Rosetta branch of the River Nile flows into the sea (Hamouda et al. 2015). Medium sandy sediment is dominant (Frihy 2003) except for the eastern side composed of muddy sediment due to discharge associated with the Rosetta estuary. The northwestern part has many rocky ridges, which cause some limitations in water exchange with the open sea (Elshanawany 2010). Abo-Qir Bay receives brackish and polluted waters via the El-Madyia outlet and El-Tabia pump station, respectively (Shreadah et al. 2019). High levels of chlorinated organic compounds and heavy metals enrichment were recorded at Abo-Qir Bay (Khairy et al. 2012; Abdel Ghani et al. 2013). It is a fully dissipative, eroding, beach that experiences dramatic erosion and sea-level rise (Frihy et al. 1996).
El-Mex bay (HPLE)
This elliptical-shaped bay has an area of ~ 20 Km2 and an average depth of 10 m, a rocky shoreline with narrow sandy beaches (Aboul Ezz et al. 2014) covered by coarse-grain particles, characterized by eddy currents, classified as a micro-tidal estuary, and water masses move eastward (Shreadah et al. 2014). It has two different water salinity masses: a surface water mass with salinity values less than 10 PSU and a bottom mass with salinity higher than 25 PSU (Nessim et al. 2010). It is highly eutrophic (Aboul Ezz et al. 2014), receiving polluted effluents via Lake Maruit and the El-Mex Pump station. Other pollutants include high concentrations of heavy metals and trace elements (Abdallah 2007), and different concentrations of polychlorinated biphenyl (PCBs) and organochlorine pesticides (Said et al. 2017).
The North West Coast (CHE)
This beach lies 100 km west of Alexandria, covered by white fine-carbonate oolitic sand grains (Nafaa and Frihy 1993; Iskander et al. 2007). This area is highly dynamic (Zaki et al. 2009), lacks civilization, has high water clarity, a moderately wide dissipative beach, a steep-slope shore, and hazardous rip currents (Frihy 2009). Coastal engineering projects such as sea walls, touristic villages, and others are dominant in this area of the northwestern coast of Alexandria (Iskander et al. 2007).
Field sampling
At each station, two replicate sediment samples were collected to a depth of 11 cm with a hand-held corer (4.8 cm2 surface area) for meiofaunal analysis. Sediment samples were preserved with 4% formalin containing Rose Bengal dye. Water temperature (T°C) was measured in situ using a 0.1 graduated mercury thermometer, water salinity samples were collected using standard glass salinity bottles, and the pH was measured by a pH meter (HANNA HI98107). For sedimentological analysis, sediment was scooped to a depth of 11 cm at each station.
Laboratory analysis
A Huys et al. (1996) technique was applied to extract meiofauna from sediment using a sieve of 63 μm mesh size, organisms were sorted, counted under a dissecting stereomicroscope, and abundance was standardized as the number of individuals per unit area of sediment (individuals 10 cm−2). Nematode specimens were picked out, processed with pure glycerin, and mounted on permanent slides. Jensen (1987) buccal cavity identification was used to describe the mouthparts of nematodes in general and that of predators and scavengers individually. Four trophic groups were assigned, deposit and epistrate feeders, predators and scavengers, using oil immersion (100X). The tail-shape type of each nematode was identified, according to Thistle and Sherman (1985), and each nematode was assigned to one of four tail-shape types; round shape (RT), elongate shape (ET), clavate conico-cylindrical shape (CCT), and conical shape (CT) types, using 20X and 40X compound microscope. The relative abundance of each feeding group and tail-shape type was calculated for each sample. Nematode taxonomic identification to genus level using the pictorial key of Platt and Warwick (1983), and life-history stage (i.e., male, female, and juvenile), were made and counted. Salinity (PSU) was determined, according to Strickland and Parsons (1972). We analyzed the organic carbon according to El Wakeel and Riley (1957), then the TOM percentage (%TOM) was calculated using Olausson (1975). Mean grain size and sediment sorting coefficient (sorting) were analyzed and calculated using Folk and Ward (1957).
Data analysis
All multivariate analyses were done using the Bray–Curtis similarity measure of the square root transformed data. PERMANOVA analysis (Anderson 2005) was conducted to test for variations in response variables among and within categorical factors (i.e., beaches, profiles, and stations) and was run (A) on the relative abundance functional biological matrix (FBM) of four feeding groups and four tail-shape types, and (B) on nematode community structure matrix (CSM) that consisted of 14 nematode taxa identified to genus. The sample design consisted of two fixed factors (beach and profile) and a random factor station. The beach factor has three levels (HPHE, HPLE, and CHE), and the factor profile is nested in the beach and has four levels (profiles 1 to 4). The station factor has five levels (stations 1 to 5) and is nested in beach and profile factors. Pair-wise comparisons were performed to detect posterior differences among/within all pairs of categorical factor levels. The permutation number of residuals is 9999 under a reduced model. The Monte Carlo p-values were calculated when a few unique values of permutation statistical (unique-perm) tests were obtained (Anderson et al. 2008), and all statistical results were described based on a significant α level ≤ 0.05.
The nMDS analysis (Clark and Warwick 2001) was applied using FBM and CSM matrices. The categorical factors were used as samples to test which matrix could discriminate among sandy beaches. The ANOSIM (Anderson et al. 2008) was run to test the null hypothesis that data within the same grouping are not clumped together as those among different groups. The ANOSIM is more sensitive to heterogeneity and dispersion within samples of the same group (Anderson and Walsh 2013) than PERMANOVA. The two-way partially hierarchical design was applied using the matrices of FBM and CSM. The sample statistic (R) and the significant statistic at the 0.1% level were calculated at all possible permutations 9999. To test for the contribution (%) of each functional trait and nematode genus to the average similarity within each site and average dissimilarity between every beach pairs, we applied the simple percentage of contribution (SIMPER) analysis using dissimilarity matrices of FBM and CSM at a cut of 70% of the low impact (Clarke and Gorley 2015).
Principal Coordinates analysis (PCO), an unconstrained ordination, was conducted to visualize the percentage of the total variation in the original resemblance data matrix of FBM and CSM alone without any specific model or hypothesis (Anderson et al. 2008) to test for clear separation among data from beaches. Distance-based redundancy analysis (dbRDA) (Legendre and Anderson 1999), a constrained model seeking linear combinations of response criterion and predictor variables, was conducted to visualize the percentage of variability in the original resemblance data matrix of FBM and CSM fitted the model and explained by dbRDA axes. The relative contribution, strength, and direction of each explanatory predictor variable (temperature, salinity, pH, TOM%, grain size, and sorting) in driving the variation along dbRDA axes were examined with one vector per predictor variable. The same response matrices of other analyses were used. All response data were square-root transformed based on Bray–Curtis similarity, whereas the environmental resemblance matrix was built on Euclidean distance after data normalizing. Ordination plots were visualized among beaches. All statistical analyses were performed by PRIMER 7 with PERMANOVA + .
Results
Nematode abundance
Nematode abundance was higher at HPLE beach (346 ± 360 individuals per 10cm2) than at HPHE (106 ± 264 individuals per 10cm2) and CHE (148 ± 198 individuals per 10cm2, Fig. 2A). Mean data was higher at profiles 2, 3, and 4 at HPLE than at the opposite profiles, at HPHE and CHE (Fig. 2B), and increased seaward direction towards station 5 (Fig. 2C).
Functional biological analysis
The relative abundance results of feeding groups (FTM %) revealed that the predator was the dominant group at HPHE and CHE, and scavenger was the dominant group at HPLE (Fig. 3A). The CT nematodes dominated the three beaches, followed by the CCT shape-type (Fig. 3B). The relative abundance of ET nematodes was relatively high at HPLE, whereas RT nematodes ranked third at HPHE. PERMANOVA (Table 1) revealed significant variations in the mean FBM among beaches and within profiles (Table 1). The highest significant differences in FBM were detected between HPLE and CHE (Table 2), whereas the lowest variation was between HPHE and CHE (Table 2). Analysis within profile levels showed significant variations in mean FBM at HPHE profile 1 compared to HPHE profiles 2, 3, and 4 (Table 2). Significant variation was also found between profiles 1 and 4 at HPLE. At CHE, variations within each profile couple were ranked descendingly based on the t-test values (Table 2). Pair-wise comparisons within nested stations in profiles and beaches revealed non-significant variations. Therefore, we showed these results in Appendix 1.
Nematode community structure
The nematode community structure consisted of eleven families; according to their dominance Oncholaimidae, Thoracostomospidae, Axonolaimidae, Chromadoridae, Xyalidae, Enoplidae, Monhysteridae, Epsilonematidae, Cyatholaimidae, Cephalobidae, and Ironidae (Fig. 4). Fourteen nematode taxa belonging to nine families were identified at the genus level. Axnolaimus sp., Graphonema sp., Oncholaimus sp.1, Oncholaimus sp.2, Theristus sp., Enoploides sp., Enoplus sp., Enoplolaimus sp.1, Enoplolaimus sp. 2, Epsilonema sp., Halomonhystera sp., Daptonema sp., Paracanthonchus sp., and Prochromadorella sp. Specimens of families Cephalobidae and Ironidae are assigned as unknown genera. The community structure consisted of 13 (HPHE), 14 (HPLE), and 9 (CHE) species (Fig. 4). PERMANOVA analysis results (Table 3) revealed significant variations among and within beaches (Table 3). The variation in community structure between HPHE and CHE was relatively higher than between HPLE and CHE, whereas the lowest variation was between HPHE and HPLE (Table 4). Analysis of profile levels (Pr.) revealed the most significant variations confined to Prs. 1 and 4 against each other, Prs. 1 and 2 (HPHE), Pr. 1 and 3, and Pr. 2 vs. Pr. 4 at CHE (Table 4). The station-level analysis detected scattered variations within the stations, but their permutations were quite a few, and data is shown in Appendix 2.
Environmental predictor parameters
The Temperature variation range was tight among beaches; the lowest salinity and pH were recorded at HPLE and HPHE, respectively (Table 5). Moderately-sorted, fine-grained sand dominated HPHE and CHE versus well-sorted coarse sand particles at HPLE. The highest values of mean sedimentary % TOM were detected at HPHE and the lowest at HPLE.
Analysis of criterion biota data
Ordinations of nMDS (Fig. 5) revealed variation among-within categorical factors following PERMANOVA analyses. The FBM ordinations revealed two main data groupings HPLE data vs. HPHE and CHE grouping (Fig. 5A). Ordination nMDS based on CSM (Fig. 5B) revealed three groupings, a left-hand side that consisted of most HPLE data and a few data of HPHE, a right-hand side grouping composed of CHE data, and the third grouping of HPHE data and the remaining data, at HPLE. The R statistics were 0.66 (FBM) and 0.81 (CSM), respectively. The highest significant differences for FBM were between HPHE vs. HPLE and HPLE vs. CHE. The ANOSIM analysis of CSM revealed significant differences between each pair of beaches (Table 6). A relatively small and modest global R statistic between profile groupings across all beaches was 0.07 and 0.26, respectively, for FBM and CSM (P = 0.007 and 0.0001).
The average similarity of FBM within each beach (Table 7) was the lowest at HPHE (52.14%) and the highest at CHE (65.63%). Predatory and CT nematodes contributed 75.32% of the similarity across the HPHE, whereas the contribution of the same traits (predators and CT) at CHE was 74.60%, and the CCT type shared the similarity with 15%. Scavengers were responsible for the highest contribution of similarity across HPLE (~ 50%), followed by CT and ET nematodes. The average dissimilarity between each pair of beaches ranked descending: HPHE vs. HPLE (68.68%), HPLE vs. CHE (65.45%), and HPHE vs. CHE (41.75%, Table 7). Five functional groups were responsible for the dissimilarity between each pair, and their cumulative contribution ranged from 78.08% (HPHE vs. HPLE) to 79.28% (HPLE vs. CHE). Scavengers and predators were responsible for the dissimilarity between HPHE vs. HPLE and HPLE vs. CHE, and cumulative contribution accounted for 40.52% and 43.17%, respectively (Table 7). The CCT and CT nematodes led the distinction between HPHE vs. CHE (41.40%), and Epistrate feeders accounted for 16.75% of the average dissimilarity between beaches. The contributions (%) of tail types were relatively equal between HPHE vs. HPLE and HPLE vs. CHE. However, they have different rankings (Table 7).
The average similarity of the CSM matrix at HPHE and CHE consisted of two data groupings each comprising five nematode species (Table 8). Graphonema sp. and one species of Oncholaimus sp. contributions to the average similarity were the highest at HPHE and CHE, respectively. At the HPLE, four species were responsible for the similarity across the beach Enoplolaimus sp.2 (23.45%) and Axnolaimus sp. (21.62%) were dominant. The highest average dissimilarity was between HPLE vs. CHE (64.86%), HPHE vs. CHE (59.95%), and HPHE vs. HPLE (58.13% Table 8). Six nematode species were responsible for the dissimilarity between HPHE vs. HPLE, and Axnolaimus sp. was the key species (16.54%). Two groupings consisted of seven nematode species, each differentiated between HPHE vs. CHE and HPLE vs. CHE. The Oncholaimus sp.1 (15.70%) was responsible for the dissimilarity between HPHE vs. CHE, whereas Axnolaimus sp. and Oncholaimus sp. 1 co-shared the contribution (~ 15%) to differentiate between HPLE vs. CHE.
The percentage of variation of the original unconstrained FBM resemblance matrix was explained by the first and second PCOs ordinations accounted for 81.4% among beaches (Fig. 6A). PCOs grouped data into two main groupings. Most of the HPLE data were located on the left-hand side of the plot, whereas HPHE and CHE data were grouped on the right-hand side. The first and second PCO coordinates accounted for 52.7% of the total variations, CSM matrix, among beaches (Fig. 6B). PCO analysis revealed two main groupings of CSM data; HPHE and HPLE data were gathered against CHE data.
Environmental parameters versus biota data
The FBM analysis revealed that the first dbRDA axis was responsible for 92.0% of the fitted model, 51.6% of the total variation, and was strongly related to salinity and grain size (Fig. 7A). The second axis was responsible for 3.9% of the fitted model, 2.2% of the total variation, and linked to pH. The dbRDA ordinations visualized two main groupings. The first grouping consisted of HPHE and CHE data clustered within the vector overlay circumference on the left-hand side and associated with salinity, grain size, and sorting coefficient vectors. The second grouping consisted of HPLE data and was located on the right-hand side away from the vector overlay circumference. The CSM model explained that the 1st dbRDA was responsible for 48.5% and 20.4% of variations in the fitted model and the total variation, respectively (Fig. 7B), and was related strongly to salinity, pH, and temperature. The 2nd axis explained 36.4% of variations in the fitted model and 15.3% of the total variation. It was related to pH. The dbRDAs ordination diagrams visualized three clear groupings, one grouping for each beach. The HPHE and CHE groupings were associated with salinity, grain size, sorting, and %TOM vectors, whereas HPLE was closely associated with temperature, and data groupings of CHE and HPLE were linked to the pH vector.
Sex ratio
Results of sex ratio (Fig. 8) revealed that the number of male individuals was approximately 1.5 times higher than that of female and ovigerous female individuals combined among beaches. Three stages of juveniles were accounted for (J2-J4). Juvenile Stage 3 dominated the HPHE and CHE beaches, whereas J4 dominated the HPLE beach.
Discussion
This work detailed a study of free-living benthic nematodes, functional traits, community structure, and sex ratio for the first time at the Alexandrian coast of Egypt. However, earlier studies dealt with nematode abundance, trophic guilds, and tail types (Mitwally et al. 2004). The aim of applying different approaches to nematode analysis was to discriminate between three beaches experiencing physical disturbances and anthropogenic stressors. Two were high-energy salinity (HPHE, CHE) and consist of moderately fine-grained sand, where the HPHE is highly contaminated, having the lowest pH. The third is energy, salinity low, and the well-sorted coarse sediment was dominant. Multivariate analyses rejected the null hypothesis of two methodologies, differentiated significantly among beaches, revealed different gained information from functional biological (FBM) and community structure (CSM) analyses, and sex-ratios data added some evidence. The FBM analysis revealed two data groupings (HPHE and CHE vs. HPLE), whereas CSM showed three assemblages at beaches. Salinity and sediment structure drove the functional traits among beaches. Evidence that anthropogenic stressors impacted nematodes trophic guilds, whereas tail-shape nematodes' types were physically disturbed. Species responded differently to the environmental variables and were physicochemical dependent. Graphonema sp., Enoplolaimus sp. 2, and Oncholaimus sp., respectively, dominated HPHE, HPLE, and CHE. However, two key genera Axnolaimus, and Oncholaimus, contributed highly to the average dissimilarity among beaches. Results of sex ratios were male-biased. Juvenile stages seemed to be temperature-dependent. A thorough discussion will achieve the aim of the study.
The use of functional traits is promising in the applied analyses of ecosystem assessments (Martini et al. 2020). Trait analysis, herein, discriminated between the high salinity and moderately-sorted fine-grained sand (HPHE and CHE) vs. low water salinity and well-sorted coarse particles (HPLE, Tables 1, 2), revealing two data groupings (Figs. 5A, 6A, 7A), indicating the potential of water salinity and sand particles driving the traits, consistent with Mitwally and Hamdan (2021). Despite the explained proportion (53.8%, Fig. 7A) of the total matrix variation confirming that sand particles and salinity were functional trait drivers, ~ 46% of the total variation in functional traits data did not account for the measured environmental factors (Fig. 7A), suggesting that other non-quantified variables could affect the response of nematode functional traits.
High-saline water with moderately-sorted fine sediment were predators and CT nematode dominant. The low-saline water with well-sorted coarse sand particles were dominated by scavengers, CT, and ET nematode inhabitants. The potential of sediment grains (Semprucci et al. 2014) and salinity (Hourston et al. 2009; Alves et al. 2014) being principal environmental factors affecting biological traits is well documented. The contribution of predators and CT nematodes across HPHE in conjunction with the lowest pH values (Tables 7 and 5) suggests the sensitivity of many species to acidified seawater excepting those exhibiting opportunistic behavior. Sarmento et al. (2015) commented that opportunistic behavior compensates for the density reduction in sensitive nematode species to low pH. The synergetic effect of waves and contaminations could explain predators and CT nematodes' dominance at HPHE (75.32%, Table 7). Most predatory nematodes have opportunistic characteristics (Moens et al. 2013), switch their feeding habit to tolerate the surrounding environmental condition (Mitwally and Fleeger 2015), endure or avoid pollution (Hedfi et al. 2021), and survive at high energy (Mitwally et al. 2004; Kovalenko et al. 2011). Nematodes in the hydrodynamic areas are biased to the CT (Semprucci et al. 2018). The CT tail type provides more efficient use of caudal glands for substratum adherence (Pérez-García et al. 2019). Most predatory nematodes have a CT type (Mitwally and Fleeger 2016). Despite the high contribution of predators and CT nematodes at CHE, the CCT, CT, and epistrate nematodes contributed more than 50% to the discrimination between the energetic beaches, suggesting their sensitivity to low pH values and contamination. This finding is consistent with Semprucci et al. (2015b), who reviewed the high contribution of epistrate feeders as a healthy ecosystem. A close association between epistrate feeders and CCT in a non-stressful ecosystem was documented (Mitwally and Fleeger 2015, 2016). The high contribution of scavengers (~ 50%) across HPLE could indicate their feeding on dead bodies in a highly polluted wave-less energy beach. Low energy causes pollution retention versus dispersion at high-energy beaches (Defeo et al. 2009). The high eutrophication at HPLE (Aboul Ezz et al. 2014) and pollution retention could inhibit primary production, increase mortality rates, and give a suitable substrate for scavengers to feed on decaying biomass. This interpretation is consistent with other studies that found scavengers and epigrowth feeders will colonize highly polluted environments (Thiermann et al. 1997; Hedfi et al. 2021; Nasri et al. 2021). Others found that Epsilonematidae were epigrowth feeders nematodes (Semprucci et al. 2013) which are recorded extensively in coarse-grain sediments (Pérez-García et al. 2019), and found, here, at the high pollution coarse sand particles (HPLE). The contribution of ET nematodes (Table 7, 12.45%) indicate low energy at HPLE. The ET nematodes are typical of a low hydrodynamic environment regime (Armenteros et al. 2009). Our results suggest that functional traits could be used to distinguish between sites experiencing physical disturbances and anthropogenic stressors, where trophic traits could indicate anthropogenic stressors, and the tail-shape type designate sites with physical turbulence. Our results recommend applying the functional traits methodology in biomonitoring and coastal restoration programs.
Nematode community structure is a traditional analysis applied as a potential biological indicator for monitoring different habitats (Stone et al. 2016). This study is the first identification of marine nematodes to families and genera at Alexandrian beaches. However, Abada et al. (2017) investigated freshwater nematodes, and the Oncholaimus genus is common in both studies. Besides, the current results are comparable with North-Western Mediterranean data (Moreno et al. 2006), where seven typical sandy shared genera Axnolaimus sp., Oncholaimus sps, Theristus sp., Enoploides sp., Enoplolaimus sps, and Epsilonema sp. In contrast to Gheskiere et al. (2005), nematode richness herein is low, probably due to long-term beach deterioration (Mitwally and Hamdan 2021) and the low productivity dominating eastern Mediterranean basins in general (Pruskia et al. 2021).
The CSM analysis revealed different data groupings at each beach (Tables 3, 4, 5, Figs. 6B, 7B). The contributing genera to the total nematode assemblages were five across HPHE and CHE versus four at HPLE, regardless of the total inhabitants (Fig. 4). The close associations between vector salinity (Fig. 7B) and nematode data at HPHE and CHE indicate that the genera occupied by these beaches are typical marine habitat water (Table 8). Whereas genera that inhabited the HPLE could be more brackish water endurance. Several studies documented a close correlation between nematode abundance, composition, and salinity (Adao et al. 2009; Hourston et al. 2009, 2011; Alves et al. 2013). However, others recorded an inverse relationship with high saline waters (Baia et al. 2021). Another key is the pH loaded negatively on dbRDA axes, indicating the species sensitivity at HPLE and CHE to minor changes in pH, whereas those genera that inhabited HPHE were more pH tolerant (Table 5). Zeppilli et al. (2015) reviewed variable consequences of low pH on meiofaunal assemblages that were study-type dependent. The temperature also had a vital role in affecting the nematode density and composition at HPLE (Fig. 7B). The highest temperature values at HPLE (22.0 ± 0.4 °C, Table 5) were probably due to the shallowness, low hydrodynamic, and sampling timing. However, the T°C variations were 0.5 °C among beaches. Temperature elevation affects reproduction success, population dynamics, species interaction, and community structure (Vafeiadou et al. 2018). Surprisingly, a noticeable shift in the role of sand grains and sorting affecting nematode genera could relate to Oncholaimus sp having relatively the same contribution across three beaches (Table 8) and nematodes' species-specific response. Nematode-inhabited coarse sediments differed in abundance and diversity from those occupied by fine-grained sand (Vanaverbeke et al. 2011), consistent with our results (Table 8). Nevertheless, the measured environmental factors accounted for 35.7% of the total variation in the CSM (Fig. 7B), indicating that other non-quantified factors contributed to ~ 65% of the nematode species-specific masked response to the measured variables. Therefore, nematode community structure is recommended as a potential assessment tool in biomonitoring and coastal restoration programs.
Surprisingly, the Graphonema sp. dominance at HPHE (24.42% Table 8) could highlight nematodes' opportunistic behavior and sediment quality at Abo-Qir beach. Members of this genus are typical marine warm-water organisms (Kito 1982; Hourston et al. 2005), and their occurrence was frequent or rare, assigned as epistrate (2A) feeders (Mirtoa et al. 2002; Moreno et al. 2009), classified as colonizers-persisters C-P3, and recorded at high-energy beaches (Hourston et al. 2005). These criteria fit HPHE environmental conditions, but predator nematodes dominated HPHE, and Graphonema sp was an epistrate feeder (Mirtoa et al. 2002). Sediment quality at HPHE probably was a good source of phytodetritus for Graphonema sp as epistrate feeders due to its proximity to Rosetta Promontory and the Nile River. This genus is tolerant to high-energy levels and could be sensitive to dispersed pollution. It is possible it could switch feeding habits to predators. Meiofauna showed rapid response and resilience to phytodetritus sedimentation after flooding disturbance (Pruskia et al. 2021), and the oligotrophic Mediterranean sea is very sensitive to riverine nutrient fluxes (Ait Ballagh et al. 2021). The more resilient the nematode genus to pollution and other disturbance, the more likely to be predatory colonizers (Bongers et al. 1991).
Conversely, at El-Mex Bay, Enoplolaimus sp., a typical predatory nematode (Baia et al. 2021), dominated at the low salinity coarse-grained sand HPLE beach (Table 8, 23.45%), where scavengers occupied this beach, suggesting this genus could shift feeding habits from predatory to scavengers to feed on decaying biomass, as was explained earlier. Axnolaimus sp. can tolerate metal pollution, as Gyedu-Ababio and Baird (2006) stated, and its contribution at HPLE was 21.63%. High levels of heavy metals have been docmented along the Alexandrian coast (El Nemr et al. 2007). Besides, Axnolaimus sp. are herbivorous nematodes (Moens et al. 2002) and could feed on detached plants at HPLE.
The widespread Oncholaimus sp. dominated the clean high-energy beach, the Northwest Coast (CHE, 26.26%, Table 8). Bhadury et al. (2015) and all references therein stated that the Oncholaimus genus is dominant in a sandy environment, probably due to its large body size and feeding habits (omnivores/predators). Sahraeian et al. (2020) documented its sensitivity to pollution. As the predators were more resilient to physical disturbances (Table 7), the Oncholaimus genus occupied the CHE.
The highest average dissimilarities in the contribution of nematode sets (Table 8) were between HPLE and CHE (~ 65%), probably due to the influence of Axnolaimus sp. (15.15%), which could be more tolerant to prevailing conditions at HPLE. It discriminated against the two highly polluted beaches (Table 8, 16.54%), indicating it's sensitivity to high salinity, fine-grained sand, and high energy. The genus Oncholaimus distinguished between HPHE and CHE, highlighting the genus's low pH sensitivity at HPHE as a proxy for pollution. The variable response of dominant genera to the measured or non-measured environmental conditions at each beach indicate that nematode is species-specific and agreed with De Meester et al. (2018). The relatively equal nematodes contribution among and within beaches (Table 8) could suggest nematodes' spatial turnover and the tolerant species replacement of the more sensitive individuals, keeping the ecosystem balance (Baselga 2010). However, further studies are necessary for a better understanding of our findings.
Male-biased sex ratios (Fig. 8) is consistent with many studies (Heip et al. 1978; Warwick 1981; Wall et al. 2002; Gambi et al. 2003) and could be an indicator of a physically disturbed environment, resource-limited environment, male higher metabolic index (Kim and Shirayama 2001), and (or) lower expenses to the population than females. However, Sahraeian et al. (2020) found that the ratios were female-biased in anthropogenic-stressed habitats. Our beaches experience physical disturbance and anthropogenic stressors, and the individuals were pooled per genera to obtain the overall sex ratios combined at each beach. Therefore, a further study on the sex per genus is recommended. The Juveniles stage 3 inhabited HPHE and CHE vs. stage 4 at HPLE could relate to the higher temperature at HPLE (Table 5) that could accelerate the development rates and agreed with Moens and Vincx (2000). However, we assume that a high energy environment could delay the rate of maturation, an assumption that requires further laboratory investigations.
The information gained from the functional analysis is not a simple reflection of the community structure analysis. It captures additional ecological facts and reveals variable spatial patterns (Singh and Ingole 2016; Jansen et al. 2018). The two FBM data groupings (Fig. 5A, 6A) were salinity and sand-grains-dependent (Fig. 7A). Conversely, three gained groupings from CSM analysis (Fig. 5B, 6B) were physicochemical dependent (salinity, pH, and temperature, Fig. 7B) with a species-specific response (Table 8). Functional data fitted the examined matrices better than that of community structure. The stress value (Fig. 5), total variations of PCO analysis (Fig. 6), and dbRDA (Fig. 7) highlighted the role of measured variables, whereas community structure emphasized the effect of the non-measured factors. The lower the stress value at nMDS and the higher the contribution of measured factors to total variations indicate the higher capture of all information into examined models (Anderson et al. 2008). Although our methods and data are different from Semprucci et al. (2018), our study suggests using functional traits, community structure, and sex ratio may be legitimate analyses to determine the disturbance of ecosystems. However, we recommend applying traits analysis in monitoring and coastal restoration programs, giving a generalization about ecosystem challenges but with caution.
References
Abada AEA, Ghanim NF, Sherif AH, Salama NA (2017) Benthic freshwater nematode community dynamics under conditions of Tilapia aquaculture in Egypt. Afr J Aquat Sci 42:381–387. https://doi.org/10.2989/16085914.2017.1410464
Abdallah MA (2007) Speciation of trace metals in coastal sediments of El-Mex Bay south Mediterranean Sea-west of Alexandria (Egypt). Environ Monit Assess 132:111–123. https://doi.org/10.1007/s10661-006-9507-z
Abdel Ghani S, El Zokm G, Shobier A, Othman T, Shreadah M (2013) Metal pollution in surface sediments of Abu-Qir Bay and Eastern Harbour of Alexandria, Egypt. Egypt J Aquat Res 39:1–12. https://doi.org/10.1016/j.ejar.2013.03.001
Aboul Ezz SM, Abdel Aziz NE, Abou Zaid MM, El Raey M, Abo-Taleb H (2014) Environmental assessment of El-Mex Bay, Southeastern Mediterranean by using Rotifera as a plankton bio-indicator. Egypt J Aquat Res 40:43–57. https://doi.org/10.1016/j.ejar.2014.03.005
Adao H, Alves AS, Patrıacio J, Neto JM, Costa MJ, Marques JC (2009) Spatial distribution of subtidal Nematoda communities along the salinity gradient in southern European estuaries. Acta Oecol 3(5):287–300. https://doi.org/10.1016/j.actao.2008.11.007
Ait Ballagh FE, Rabouille C, Andrieux-Loyer F, Soetaert K, Lansard B, Bombled B, Monvoisin G, Elkalay K, Khalil K (2021) Spatial Variability of Organic Matter and Phosphorus Cycling in Rhône River Prodelta Sediments (NW Mediterranean Sea, France): a Model-Data Approach Estuaries and Coasts. https://doi.org/10.1007/s12237-020-00889-9
Alves AS, Adão H, Ferrero TJ, Marques JC, Costa MJ, Patrício J (2013) Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecol Ind 24:462–475. https://doi.org/10.1016/j.ecolind.2012.07.013
Alves AS, Veríssimo H, Costa MJ, Marques JC (2014) Taxonomic resolution and Biological Traits Analysis (BTA) approaches in estuarine free-living nematodes. Estuar Coast Shelf Sci 138:69–78. https://doi.org/10.1016/j.ecss.2013.12.014
Anderson MJ (2005) PERMANOVA, Permutational multivariate analysis of variance, A computer program. Department of Statistics, University of Auckland, New Zealand:1–23.
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+for PRIMER: Guide to Software and Statistical Methods PRIMER-e (Quest Research Limited): Plymouth, Uk:1–214.
Anderson MJ, Walsh DCI (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol Monogr 83:557–574.
Armenteros M, Ruiz-Abierno A, Fernández-Garcés R, Pérez-García JA, Díaz-Asencio L, Vincx M, Decraemer W (2009) Biodiversity patterns of free-living marine nematodes in a tropical bay: Cienfuegos, Carribean Sea. Estuar Coast Shelf Sci 85:179–189. https://doi.org/10.1016/j.ecss.2009.08.002
Ait Ballagh FE et al. (2021) Spatial Variability of Organic Matter and Phosphorus Cycling in Rhône River Prodelta Sediments (NW Mediterranean Sea, France): a Model-Data Approach Estuaries and Coasts, © Coastal and Estuarine Research Federation 2021https://doi.org/10.1007/s12237-020-00889-9
Baia E, Rollnic M, Venekey V (2021) Seasonality of pluviosity and saline intrusion drive meiofauna and nematodes on an Amazon freshwater-oligohaline beach. J Sea Res 170. https://doi.org/10.1016/j.seares.2021.102022
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Bevilacqua S, Sandulli R, Plicanti A, Terlizzi A (2012) Taxonomic distinctness in Mediterranean marine nematodes and its relevance for environmental impact assessment. Mar Pollut Bull 64:1409–1416. https://doi.org/10.1016/j.marpolbul.2012.04.016
Bhadury P, Mondal N, Ansari KTP, Pitale R, Prasade A, Nagale P, Apte D (2015) Checklist of free-living marine nematodes from intertidal sites along the central west coast of India Check List the journal of biodiversity data 11. https://doi.org/10.15560/11.2.1605
Bhusal DR, Kallimanis AS, Tsiafouli MA, Sgardelis SP (2014) Higher taxa vs. functional guilds vs. trophic groups as indicators of soil nematode diversity and community structure. Ecol Indic 41:25–29. https://doi.org/10.1016/j.ecolind.2014.01.019
Boesch DF, Paul JF (2001) An Overview of Coastal Environmental Health Indicators. Hum Ecol Risk Assess 7(5):000–000.
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Mar Ecol Prog Ser 76:135–142.
Boufahja F, Hedfi A, Amorri J, Aïssa P, Beyrem H, Mahmoudi E (2011) Examination of the bioindicator potential of Oncholaimus campylocercoides (Oncholaimidae, Nematoda) from Bizerte bay (Tunisia). Ecol Indic 11:1139–1148. https://doi.org/10.1016/j.ecolind.2010.12.014
Clarke KR, Gorley RN (2015) Getting started with PRIMER 7. PRIMER-E Ltd, Plymouth 1st edition:1–18.
Coull B, Chandler G (1992) Pollution and meiofauna: field, laboratory, and mesocosm studies. Oceanography and Marine Biology: An Annual Review.
Clarke KR, Warwick RM (2001) Changes in Marine Communities An approach to statistical analysis and interpretation Priemer E 2nd Edition, 1:176.
Danovaro R, Fabiano M, Vincx M (1995) Meiofauna Response to the Agip Abruzzo Oil Spill in Subtidai Sediments of the Ligurian Sea. Mar Pollut Bull 30:133–145.
De Meester N, Van Daele T, Van Malderen J, Monteiro L, Van Colen C, Derycke S, Moens T (2018) Active and species-specific dispersal behaviour in a marine nematode cryptic species complex. Mar Ecol Prog Ser 600:71–83.
Defeo O et al (2009) Threats to sandy beach ecosystems: A review. Estuar Coast Shelf Sci 81:1–12. https://doi.org/10.1016/j.ecss.2008.09.022
EEAA (2015) Alexandria Coastal Zone Management Project (ACZMP) Ministry of Environmental Affairs & Egyptian Environmental Affairs Agency SFG1484V2:1–15.
El Nemr A, El-Sadaawy MM, Khaled A, Draz SO (2013) Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments of the Mediterranean: assessment and source recognition of petroleum hydrocarbons. Environ Monit Assess 185:4571–4589. https://doi.org/10.1007/s10661-012-2889-1
El Nemr A, Said TO, Khaled A, El-Sikaily A, Abd-Allah AM (2007) The distribution and sources of polycyclic aromatic hydrocarbons in surface sediments along the Egyptian Mediterranean coast. Environ Monit Assess 124:343–359. https://doi.org/10.1007/s10661-006-9231-8
El Wakeel S, Riley J (1957) The Determination of Organic Carbon in Marine Muds. ICES J Mar Sci 22:180–183
Elshanawany R (2010) Microfossil assemblages as proxies to reconstruct anthropogenic induced eutrophication of two marginal Eastern Mediterranean Basins Ph DThesis Department of Geosciences/Marum, Universität Bremen, Klagenfurter Strasse, D-28359 Bremen, Germany:1–225.
Flach PZS, Ozorio CP, Melo AS (2012) Alpha and beta components of diversity of freshwater nematodes at different spatial scales in subtropical coastal lakes. Fundam Appl Limnol / Arch Hydrobiol 180:249–258. https://doi.org/10.1127/1863-9135/2012/0182.
Fleeger JW, Carman KR, Weisenhorn PB, Sofranko H, Marshall T, Thistle D, Barry JP (2006) Simulated sequestration of anthropogenic carbon dioxide at a deep-sea site: Effects on nematode abundance and biovolume. Deep Sea Res Part I 53:1135–1147. https://doi.org/10.1016/j.dsr.2006.05.007
Fleeger JW, Johnson DS, Carman KR, Weisenhorn PB, Gabriele A, Thistle D, Barry JP (2010) The response of nematodes to deep-sea CO2 sequestration: A quantile regression approach. Deep Sea Res Part I 57:696–707 https://doi.org/10.1016/j.dsr.2010.03.003
Folk RL, Ward WC (1957) Brazos River Bar: A Study In The Significance Of Grain Size Parameters. J Sedimentol 27:3–26
Frihy OE (2001) The necessity of environmental impact assessment (EIA) in implementing coastal projects: lessons learned from the Egyptian Mediterranean Coast Ocean & Coastal Management 44:489–516
Frihy OE (2003) The Nile Delta-Alexandria coast: Vulnerability to sea-level rise, consequences and adaptation. Mitig Adapt Strateg Glob Chang 8:115–138
Frihy OE (2009) Morphodynamic implications for shoreline management of the western-Mediterranean sector of Egypt. Environ Geol 58:1177–1189. https://doi.org/10.1007/s00254-008-1595-3
Frihy OE, Dewidar KM, E1 Raey MM (1996) Evaluation of coastal problems at Alexandria, Egypt. Ocean Coast Manage 30:281–295
Gambi C, Vanreusel A, Danovaro R (2003) Biodiversity of nematode assemblages from deep-sea sediments of the Atacama Slope and Trench (South Pacific Ocean). Deep-Sea Res I 50:103–117.
Gheskiere T, Vincx M, Weslawski JM, Scapini F, Degraer S (2005) Meiofauna as descriptor of tourism-induced changes at sandy beaches. Mar Environ Res 60:245–265. https://doi.org/10.1016/j.marenvres.2004.10.006
Giere O (2009) Meiobenthology:The Microscopic Motile Fauna of Aquatic Sediments. Springer-Verlag Berlin Heidelberg 2nd edition:1–537.
Gyedu-Ababio TK, Baird D (2006) Response of meiofauna and nematode communities to increased levels of contaminants in a laboratory microcosm experiment. Ecotoxicol Environ Saf 63:443–450. https://doi.org/10.1016/j.ecoenv.2005.01.010.
Hamouda A, El Gendy N, Salah M, S. EG (2015) Acoustic Survey along Heraklieon and East Canopus Ancient Greek Cities, Abu Quir Bay, Alexandria, Egypt. J Earth Sci Clim Change 06. https://doi.org/10.4172/2157-7617.1000289
Hedfi A et al. (2021) Nematode traits after separate and simultaneous exposure to Polycyclic Aromatic Hydrocarbons (anthracene, pyrene and benzo[a]pyrene) in closed and open microcosms. Environ Pollut 116759 https://doi.org/10.1016/j.envpol.2021.116759
Heip CHR, Smol N, Absillis V (1978) Influence of Temperature on the Reproductive Potential of Oncholaimus oxyuris (Nematoda: Oncholaimidae). Mar Biol 45:255–260.
Hodda M, Nicholas W (1990) Production of meiofauna in an Australian Estuary. Wetlands (Australia) 9:41–47.
Hourston M, Potter IC, Warwick RM, Valesini FJ (2011) The characteristics of the nematode faunas in subtidal sediments of a large microtidal estuary and nearshore coastal waters differ markedly. Estuar Coast Shelf Sci 94:68–76. https://doi.org/10.1016/j.ecss.2011.05.022
Hourston M, Potter IC, Warwick RM, Valesini FJ, Clarke KR (2009) Spatial and seasonal variations in the ecological characteristics of the free-living nematode assemblages in a large microtidal estuary. Estuar Coast Shelf Sci 82:309–322. https://doi.org/10.1016/j.ecss.2009.01.018
Hourston M, Warwick RM, Valesini FJ, Potter IC (2005) To what extent are the characteristics of nematode assemblages in nearshore sediments on the west Australian coast related to habitat type, season and zone? Estuar Coast Shelf Sci 64:601–612. https://doi.org/10.1016/j.ecss.2005.04.001
Huys R, Gee JM, Moore C, Hamond R (1996) Marine and brackish water harpacticoid copepods Part I Synopses of the British Fauna (New Series) Book Kermack, DM, Barnes R.S.K and Crothers (eds) London:1–352.
Iskander MM, Frihy OE, El Ansary AE, El Mooty MM, Nagy HM (2007) Beach impacts of shore-parallel breakwaters backing offshore submerged ridges, Western Mediterranean Coast of Egypt. J Environ Manage 85:1109–1119. https://doi.org/10.1016/j.jenvman.2006.11.018
Jansen J, Hill NA, Dunstan PK, Eléaume MP, Johnson CR (2018) Taxonomic Resolution, Functional Traits, and the Influence of Species Groupings on Mapping Antarctic Seafloor Biodiversity. Front Ecol Evol 6. https://doi.org/10.3389/fevo.2018.00081
Jensen P (1987) Feeding ecology of free-living aquatic nematodes. Mar Ecol Prog Ser 35:187–196
Kalogeropoulou V, Keklikoglou K, Lampadariou N (2015) Functional diversity patterns of abyssal nematodes in the Eastern Mediterranean: A comparison between cold seeps and typical deep sea sediments. J Sea Res 98:57–72. https://doi.org/10.1016/j.seares.2014.11.003.
Khairy MA, Kolb M, Mostafa AR, El-Fiky A, Bahadir M (2012) Risk posed by chlorinated organic compounds in Abu Qir Bay, East Alexandria, Egypt. Environ Sci Pollut Res Int 19:794–811. https://doi.org/10.1007/s11356-011-0605-2
Kim D, Shirayama Y (2001) Respiration Rates of Free-Living Marine Nematodes in the Subtidal Coarse-Sand Habitat of Otsuchi Bay, Northeastern Honshu, Japan. Zool Sci 18(969):973.
Kito K (1982) Phytal Marine Nematode Assemblage on Sargassum confusum Agardh, with Reference to the Structure and Seasonal Fluctuations JourFacSci Hokkadio Univ sev, VI, Zool:143–160.
Kovalenko KE, Thomaz SM, Warfe DM (2011) Habitat complexity: approaches and future directions. Hydrobiologia 685:1–17https://doi.org/10.1007/s10750-011-0974-z.
Kreuzinger-Janik B, Bruchner-Huttemann H, Traunspurger W (2019) Effect of prey size and structural complexity on the functional response in a nematode- nematode system. Sci Rep 9:5696 5691-5698. https://doi.org/10.1038/s41598-019-42213-x
Legendre P, Anderson MJ (1999) Distance-Based Redundancy Analysis: Testing Multispecies Responses In Multifactorial Ecological Experiments. Ecol Monogr 69:1–24.
Losi V, Moreno M, Gaozza L, Vezzulli L, Fabiano M, Albertelli G (2013) Nematode biomass and allometric attributes as indicators of environmental quality in a Mediterranean harbour (Ligurian Sea, Italy). Ecol Indic 30:80–89 https://doi.org/10.1016/j.ecolind.2013.01.034
Martini S et al (2020) Functional trait-based approaches as a common framework for aquatic ecologists. Limnol Oceanogr. https://doi.org/10.1002/lno.11655.
Masria A, Iskander M, Negm A (2015) Coastal protection measures, case study (Mediterranean zone, Egypt). J Coast Conserv 19:281–294 https://doi.org/10.1007/s11852-015-0389-5
Mirtoa S, La Rosaa T, Gambi C, Danovarob R, Mazzolaa A (2002) Nematode community response to fish-farm impact in the western Mediterranean. Environ Pollut 116:203–214.
Mitwally H, Fleeger J (2015) Long-term nutrient enrichment alters nematode trophic structure and body size in a Spartina alterniflora salt marsh. Mar Ecol 36:910–925. https://doi.org/10.1111/maec.12181
Mitwally H, Fleeger J (2016) A test of biological trait analysis with nematodes and an anthropogenic stressor. Environ Monit Assess 188:140. https://doi.org/10.1007/s10661-016-5128-3
Mitwally H, Montagna P, Halim Y, Khalil A, Dorgham M, Atta M (2004) Egyptian sandy beach meiofauna and benthic diatoms. RappCommIntMer Medit 37:537
Mitwally HM, Hamdan AM (2021) Environmental drivers of meiofaunal natural variability, Egypt, Southeastern Mediterranean. Environ Monit Assess 193:185. https://doi.org/10.1007/s10661-021-08927-0
Moens T et al. (2013) Ecology of free-living marine nematodes. Handbook of Zoology De Gruyter, Berlin
Moens T, Luyten C, Middelburg JJ, Herman P, Vincx M (2002) Tracing organic matter sources of estuarine tidal flat nematodes with stable carbon isotopes. Mar Ecol Prog Ser 234:127–137.
Moens T, Vincx M (2000) Temperature and salinity constraints on the life cycle of two brackish-water nematode species. J Exp Mar Biol Ecol 243:115–135.
Moreno M, Albertelli G, Fabiano M (2009) Nematode response to metal, PAHs and organic enrichment in tourist marinas of the Mediterranean Sea. Mar Pollut Bull 58:1192–1201. https://doi.org/10.1016/j.marpolbul.2009.03.016
Moreno M, Ferrero TJ, Granelli V, Marin V, Albertelli G, Fabiano M (2006) Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuar Coast Shelf Sci 66:357–367. https://doi.org/10.1016/j.ecss.2005.08.016
Moreno M, Semprucci F, Vezzulli L, Balsamo M, Fabiano M, Albertelli G (2011) The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol Ind 11:328–336. https://doi.org/10.1016/j.ecolind.2010.05.011
Nafaa MG, Frihy OE (1993) Beach and Nearshore Features Along the Dissipative Coastline of the Nile Delta, Egypt. J Coast Res 9:423
Nasri A et al. (2021) Ecotoxicity of polybrominated diphenyl ether (BDE-47) on a meiobenthic community with special emphasis on nematodes. Environ Pollut 116727. https://doi.org/10.1016/j.envpol.2021.116727
Nessim R, Bassiouny A, Zaki H, Moawad M, Kandeel K (2010) Environmental studies at El-Mex region (Alexandria-Egypt) during 2007–2008. World Appl Sci J 9:779–787.
Olausson E (1975) Methods for the chemical analysis of sediments FAO Fisheries Technical Papers (FAO)
Pérez-García JA, Marzo-Pérez D, Armenteros M (2019) Spatial scale influences diversity patterns of free-living nematode assemblages in coral degradation zones from the Caribbean Sea. Mar Biodivers. https://doi.org/10.1007/s12526-019-00945-z
Platt H, Warwick R (1980) The significance of free-living nematodes to the littoral ecosystem. Ecosystems 2:729–759
Platt HM, Warwick RM (1983) Freeliving marine nematodes. Part 1: British enoplids. Pictorial key to world genera and notes for the identification of British species. Cambridge University press, for the Linnean Society of London.
Pruskia AM, Rzeznik-Orignac J, Kerhervé P, Vétion G, Bourgeois S, Péru E, Brosset P, Toussaint F, Rabouille C (2021) Dynamic of organic matter and meiofaunal community on a river-dominated shelf (Rhône prodelta, NW Mediterranean Sea): Responses to river regime. Estuar Coast Shelf Sci On line. https://doi.org/10.1016/j.ecss.2021.107274.
Sahraeian N, Sahafi HH, Mosallanejad H, Ingels J, Semprucci F (2020) Temporal and spatial variability of free-living nematodes in a beach system characterized by domestic and industrial impacts (Bandar Abbas, Persian Gulf, Iran). Ecol Indic 118:106697 https://doi.org/10.1016/j.ecolind.2020.106697.
Said TO, El Zokm GM, Mohamed LA, Okbah MA, Soliman NF (2017) Distribution of organochlorines and ecological risks in surface sediments from El-Mex Bay, Mediterranean Sea. Hum Ecol Risk Assess 23:1539–1552https://doi.org/10.1080/10807039.2017.1316184.
Salmaso N, Naselli-Flores L, Padisák J (2015) Functional classifications and their application in phytoplankton ecology. Freshw Biol 60:603–619. https://doi.org/10.1111/fwb.12520.
Sarmento VC, Souza TP, Esteves AM, Santos PJP (2015) Effects of seawater acidification on a coral reef meiofauna community. Coral Reefs 34:955–966. https://doi.org/10.1007/s00338-015-1299-6.
Schratzberger M, Warr K, Rogers SI (2007) Functional diversity of nematode communities in the southwestern North Sea. Mar Environ Res 63:368–389. https://doi.org/10.1016/j.marenvres.2006.10.006.
Semprucci F, Balsamo M, Frontalini F (2014) The nematode assemblage of a coastal lagoon (Lake Varano, southern Italy): ecology and biodiversity patterns. Sci Mar 78:579–588 https://doi.org/10.3989/scimar.04018.02A.
Semprucci F, Cesaroni L, Guidi L, Balsamo M (2018) Do the morphological and functional traits of free-living marine nematodes mirror taxonomical diversity? Mar Environ Res 135:114–122. https://doi.org/10.1016/j.marenvres.2018.02.001.
Semprucci F, Colantoni P, Baldelli G, Sbrocca C, Rocchi M, Balsamo M (2013) Meiofauna associated with coral sediments in the Maldivian subtidal habitats (Indian Ocean). Mar Biodivers 43:189–198 https://doi.org/10.1007/s12526-013-0146-7)
Semprucci F, Frontalini F, Sbrocca C, du Chatelet EA, Bout-Roumazeilles V, Coccioni R, Balsamo M (2015a) Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environ Monit Assess 187:187–251https://doi.org/10.1007/s10661-015-4493-7
Semprucci F, Losi V, Moreno M (2015b) A review of Italian research on free-living marine nematodes and the future perspectives on their use as Ecological Indicators (EcoInds) Mediterranean. Mar Sci 352–365 https://doi.org/10.12681/mms.1072
Shreadah AM, Abdel-Mohsen M El-Sayed A, A AST, Mohamed A A, Hamam AR H (2019) Evaluation of Different Anthropogenic Effluents Impacts on the Water Quality Using Principal Component Analysis: A Case Study of Abu-Qir Bay-Alexandria-Egypt. Int J Environ Monit Anal 7:56 https://doi.org/10.11648/j.ijema.20190703.11
Shreadah M, Masoud M, Khattab A, El Zokm G (2014) Impacts of different drains on the seawater quality of El-Mex bay (Alexandria, Egypt). J Ecol Nat Environ 6:287–303 https://doi.org/10.5897/jene2014.0465
Singh R, Ingole BS (2016) Structure and function of nematode communities across the Indian western continental margin and its oxygen minimum zone. Biogeosciences 13:191–209. https://doi.org/10.5194/bg-13-191-2016
Soetaert K et al. (2009) Factors affecting nematode biomass, length and width from the shelf to the deep sea. Mar Ecol Prog Ser 392:123–132 https://doi.org/10.3354/meps08202
Soliman A, Elsharnouby B, Elkamhawy H (2014 ) Shoreline Changes Due to Construction of Alexandria Submerged Breakwater, Egypt ICHE Hamburg - Lehfeldt & Kopmann (eds) Bundesanstalt für Wasserbau ISBN 978–3–939230–32–8:675–684
Stone D, Costa D, Daniell TJ, Mitchell SM, Topp CFE, Griffiths BS (2016) Using nematode communities to test a European scale soil biological monitoring programme for policy development. Appl Soil Ecol 97:78–85. https://doi.org/10.1016/j.apsoil.2015.08.017
Strickland JDH, Parsons TR (1972) A Practical Handbook of Seawater Analysis. The Journal of the Fisheries Research Board of Canada Ottawa, Ont'ario, Canada 310.
Thiermann F, Akoumianaki I, Hughes JA, Giere O (1997) Benthic fauna of a shallow-water gaseohydrothermal vent area in the Aegean Sea (Milos, Greece). Mar Biol 128: 149±159 128:149–159.
Thistle D, Lambshead J, Sherman K (1995) Nematode tail-shape groups respond to environmental differences in the deep sea. Vie Milieu 45:107–115.
Thistle D, Sherman KM (1985) The nematode fauna of a deep-sea site exposed to strong near-bottom currents. Deep-Sea Res 32:11177–11188.
Tietjen JH, Lee JJ (1972) Life Cycles of Marine Nematodes Influence of Temperature and Salinity on the Development. Oecologia (Berl) 10:167–176.
Vafeiadou A-M, Chintiroglou C, Moens T (2018) Effects of an increased temperature regime on the population dynamics and species interactions of marine nematodes. J Exp Mar Biol Ecol 502:142–152. https://doi.org/10.1016/j.jembe.2017.02.008
Vanaverbeke J, Merckx B, Degraer S, Vincx M (2011) Sediment-related distribution patterns of nematodes and macrofauna: two sides of the benthic coin? Mar Environ Res 71:31–40. https://doi.org/10.1016/j.marenvres.2010.09.006
Vanaverbeke J, Soetaert K, Vincx M (2004) Changes in morphometric characteristics of nematode communities during a spring phytoplankton bloom deposition. Mar Ecol Prog Ser 273:139–146
Wall J, Skene K, Neilson R (2002) Nematode community and trophic structure along a sand dune succession. Biol Fertil Soils 35:293–301. https://doi.org/10.1007/s00374-002-0478-0
Warwick RM (1981) The Influence of Temperature and Salinity on Energy Partitioning in the Marine Nematode Diplolaimeiloides bruciei. Oecologia (Berl) 51:318-325.
Zaki H, Goma R, Tadros A, Mahmoud M (2009) Environmental parameters of Alexnadria Inshore North western Coastall Area, Alexandria. World Appl Sci 7:715–725
Zeppilli D et al (2015) Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar Biodivers 45:505–535. https://doi.org/10.1007/s12526-015-0359-z
Acknowledgements
The author is deeply thankful to Prof. Dr. Alexei V. Tchesunov, Department of Invertebrate Zoology, Moscow University, Russia, for nematode species identification. She is also grateful to Prof Dr. John W Fleeger, Louisiana State University, the USA, for reading the manuscript and for valuable suggestions. She is honored to Dr. Christine Ritter, Rockport, Texas, USA for her help checking English grammar and editing. This research did not have any specific grant from the public, commercial, or not-for-profit sectors.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitwally, H.M. A comparison of physical disturbance and pollution stressors in sandy beaches using nematode functional biological traits. J Coast Conserv 26, 39 (2022). https://doi.org/10.1007/s11852-022-00884-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11852-022-00884-1