Abstract
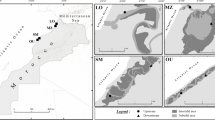
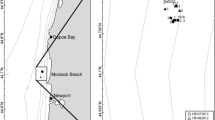
Sandy beaches are challenging ecosystems, in which biota experience extreme physical conditions. We sampled meiofauna in conjunction with environmental factors that are well-known to affect faunal associations to describe the ecological state of sandy beaches that experience natural and human-made disturbances. We applied a random stratified sampling design with monthly collections (1800 cores) at three beaches on the Alexandria, Egypt, coast during two sampling periods over 1 year from November to April and May to September. We used multivariate analyses to compare beaches for water quality, particle size, and meiofaunal assemblages. The environmental analysis explained 60% of the total variation of physical factors among beaches and grouped beaches that moderately sorted fine-grained sand and high water salinity vs. the beach with well-sorted, coarse-grain, and low salinity. Meiofaunal analyses revealed unexpected results. The abundance and temporal variation were low, and the explained proportion of natural variation by the putative environmental factors was small. The natural variation was an indicator of long-term beach ruin and oligotrophic conditions. Our results suggest that a large fraction of natural variation in beach meiofauna is stochastic or that other, non-measured, the natural forces (e.g., storm events) or human-made forces (e.g., tourism activities) are essential contributors to variation. Our best models indicate that meiofauna is more resilient to natural disturbances than to human-made stressors, and the higher the beach exposure to the synergetic effects of natural forces and anthropogenic stressors, the lower the ecological state is.




Similar content being viewed by others
References
Abdelhamid, M. A., El-Barbary, M. I., & El-Deweny, M. E. M. (2013). Bacteriological status of ashtoum El-Gamil protected area. Egyptian Journal of Aquatic Biology and Fisheries, 17, 11–23.
Abo-Taleb, H. A., El Raey, M., Abou Zaid, M. M., Ezz, S. A., & Aziz, N. A. (2015). Study of the physico-chemical conditions and evaluation of the changes in eutrophication-related problems in El- Mex Bay. African Journal of Environmental Science and Technology, 9, 354-364.
Adao, H., Alves, A. S., Patrıacio, J., Neto, J. M., Costa, M. J., & Marques, J. C. (2009). Spatial distribution of subtidal Nematoda communities along the salinity gradient in southern European estuaries. Acta Oecologica, 3(5), 287–300.
Alves, A. S., Adão, H., Ferrero, T. J., Marques, J. C., Costa, M. J., & Patrício, J. (2013). Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecological Indicators, 24, 462–475.
Anderson, M. J. (2003). DISTLM forward Distance-based multivariate analysis for a linear model using forward selection A computer program, Department of Statistics University of Auckland, pp. 1–10.
Anderson, M. J. (2005). PERMANOVA, Permutational multivariate analysis of variance (pp. 1–23). University of Auckland, New Zealand.
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA+for PRIMER: Guide to software and statistical methods (pp. 1–214). Plymouth, Uk.
Armenteros, M., Marti´N, I., Williams, J. P., Creagh, B., Gonza´Lez-Sanso´ N, G., & Capetillo, N. (2006). Spatial and temporal variations of meiofaunal communities from the Western Sector of the Gulf of Batabano. Cuba. I. Mangrove Systems. Estuaries and Coasts 29, 124-132.
Baldrighi, E., Semprucci, F., Franzo, A., Cvitkovic, I., Bogner, D., Despalatovic, M., Berto, D., Formalewicz, M. M., Scarpato, A., Frapiccini, E., Marini, M., & Grego, M. (2019). Meiofaunal communities in four Adriatic ports: Baseline data for risk assessment in ballast water management. Marine Pollution Bulletin, 147, 171–184.
Balsamo, M., Semprucci, F., Frontalini, F., & Coccioni, R. (2012). Meiofauna as a tool for marine ecosystem biomonitoring. Marine Ecosystems, 4, 77–104.
Ben-David, A., & Davidson, C. E. (2014). Estimation method for serial dilution experiments. Journal of Microbiological Methods, 107, 214–221.
Brown, A., & McLachlan, A. (2010). The ecology of sandy shores. Elsevier.
Costa, A., Valenca, A., & Santos, P. (2016). Is meiofauna community structure in Artificial Substrate Units a good tool to assess anthropogenic impact in estuaries? Marine Pollution Bulletin, 110, 354–361.
Coull, B. (1999). Role of meiofauna in estuarine soft-bottom habitats. Australian Journal of Ecology, 24, 327–343.
Coull, B., & Fleeger, J. W. (1977). Long-term temporal variation and community dynamics of meiobenthic copepods. Ecology, 58, 1136–1143.
Cunningham, R. B., & Lindenmayer, D. B. (2005). Modeling count data of rare species: Some statistical issues. Ecology, 86, 1135–1142.
Danovaro, R. (1996). Detritus-bacteria-Meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Marine Biology, 127(121), 113.
Danovaro, R., Dinet, A., Duineveld, G., & Tselepides, A. (1999). Benthic response to particulate fluxes in different trophic environments: A comparison between the Gulf of Lions-Catalan Sea (western-Mediterranean) and the Cretan Sea (eastern-Mediterranean). Progress in Oceanography, 44, 287–312.
Defeo, O., McLachlan, A., Schoeman, D. S., Schlacher, T. A., Dugan, J., Jones, A., Lastra, M., & Scapini, F. (2009). Threats to sandy beach ecosystems: A review. Estuarine, Coastal and Shelf Science, 81, 1–12.
Dorgham, M. M., Hamdy, R., El Rashidy, H. H., Atta, M. M., & Musco, L. (2014). Distribution patterns of shallow water polychaetes (Annelida) along the Alexandria coast, Egypt (Eastern Mediterranean). Mediterranean Marine Science, 15, 635.
Dowidar, N. M. (1984). Phytoplankton biomass and primary productivity of the South-Eastern Mediterranean (pp. 983–1000). Faculty of Science, Alexandria University.
EEAA. (2015). Alexandria Coastal Zone Management Project (ACZMP). Ministry of Environmental Affairs & Egyptian Environmental Affairs Agency (pp. 1–15). SFG1484V2.
El-Raey, M. F., Nasr, S., & Hendy, D. (2015). Integrating knowledge to assess coastal vulnerability to sea-level rise. In El- Mex Bay (Ed.), Egypt: The Application of the DIVA Tool. International Journal of Marine Science.
El-Shenawy, M. A., & El-Shenawy, M. (2009). Enterohaemorrhagic Escherichia coli O157 in coastal environment of Alexandria. Egypt. Microbial Ecology in Health and Disease, 17, 103–106.
El Nemr, A., El-Sadaawy, M. M., Khaled, A., & Draz, S. O. (2013). Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments of the Mediterranean: Assessment and source recognition of petroleum hydrocarbons. Environmental Monitoring and Assessment, 185, 4571–4589.
El Wakeel, S., & Riley, J. (1957). The Determination of organic carbon in marine muds. ICES Journal of Marine Science, 22, 180–183.
Evrard, V., Huettel, M., Cook, P. L. M., Soetaert, K., Heip, C. H. R., & Middelburg, J. J. (2012). Importance of phytodetritus and microphytobenthos for heterotrophs in a shallow subtidal sandy sediment. Marine Ecology Progress Series, 455, 13–31.
First, M. R., & Hollibaugh, J. T. (2010). Environmental factors shaping microbial community structure in salt marsh sediments. Marine Ecology Progress Series, 399, 15–26.
Folk, R. L., & Ward, W. C. (1957). Brazos River bar [Texas]; A study in the significance of grain size parameters. Journal of Sedimentary Research, 27, 3–26.
Frihy, O. E. (2009). Morphodynamic implications for shoreline management of the western-Mediterranean sector of Egypt. Environmental Geology, 58, 1177–1189.
Frihy, O. E., & Deabes, E. (2012). Erosion chain reaction at El Alamein Resorts on the western Mediterranean coast of Egypt. Coastal Engineering, 69, 12–18.
Frihy, O. E., Dewidar, K. M., & El Raey, M. M. (1996). Evaluation of coastal problems at Alexandria, Egypt. Ocean & Coastal Management, 30, 281-295.
Gambi, M. C., & Dappiano, M. (2004). Mediterranean marine benthos: A manual of methods for its sampling and sudy. Società Italiana di Biologia Marina.
Gansfort, B., Fontaneto, D., & Zhai, M. (2020). Meiofauna as a model to test paradigms of ecological metacommunity theory. Hydrobiologia, 847, 2645–2663.
George, E. M. (2009). Egypt State of Environment Report. Egyptian Environmental Affaris Agency www.eeaa.gov.egeport. pp. 1–356.
Gheskiere, T., Hoste, E., Kotwicki, L., Degraer, S., Vanaverbeke, J., & Vincx, M. (2002). The sandy beach meiofauna and free-living nematodes from De Panne (Belgium). Bulletin de l’institut Royal des sciences Naturelles De Belgique, 72, 43–49.
Gheskiere, T., Vincx, M., Weslawski, J. M., Scapini, F., & Degraer, S. (2005). Meiofauna as descriptor of tourism-induced changes at sandy beaches. Marine Environment Research, 60, 245–265.
Giere, O. (2009). Meiobenthology: The microscopic motile fauna of aquatic sediments (2nd ed., pp. 1–537). Springer-Verlag Berlin Heidelberg.
Grémare, A., Amouroux, J.-M., Cauwet, G., Charles, F., Courties, C., De Bovée, F., Dinet, A., Devenon, J. L., De Madron, X. D., Ferre, B., Fraunie, P., Joux, F., Lantoine, F., Lebaron, P., Naudin, J.-J., Palanques, A., Pujo-Pay, M., & Zudaire, L. (2003). The effects of a strong winter storm on physical and biological variables at a shelf site in the Mediterranean. Oceanologica Acta, 26, 407–419.
Ha, S. Y., Min, W.-K., Kim, D.-S., & Shin, K.-H. (2014). Trophic importance of meiofauna to polychaetes in a seagrass (Zostera marina) bed as traced by stable isotopes. Journal of the Marine Biological Association of the United Kingdom, 94, 121–127.
Hamdan, A. M., El-Sayed, A. F., & Mahmoud, M. M. (2016). Effects of a novel marine probiotic, Lactobacillus plantarum AH 78, on growth performance and immune response of Nile tilapia (Oreochromis niloticus). Journal of Applied Microbiology, 120, 1061–1073.
Hamdy, R., & Ibrahim, H. (2019). Recent changes in polychaete community along the Alexandria coast, Egy. Egyptian Journal of Aquatic Biology & Fisheries, 23, 1–12.
Higgins, R. P., & Thiel, H. (1988). Introduction to the study of meiofauna. Smithsonian Institution Press.
Huys, R., Gee, J. M., Moore, C., & Hamond, R. (1996). Marine and brackish water harpacticoid copepods Part I. Synopses of the British Fauna (New Series) Book. In Kermack, D. M., Barnes, R. S .K. & Crothers (Eds.) (pp.1–352). London.
Iskander, M. M., Frihy, O. E., El Ansary, A. E., El Mooty, M. M., & Nagy, H. M. (2007). Beach impacts of shore-parallel breakwaters backing offshore submerged ridges, Western Mediterranean Coast of Egypt. Journal of Environmental Management, 85, 1109–1119.
Ismail, M. M., El Zokm, G. M., & El-Sayed, A. A. M. (2017). Variation in biochemical constituents and master elements in common seaweeds from Alexandria Coast, Egypt, with special reference to their antioxidant activity and potential food uses: prospective equations. Environmental Monitoring and Assessment, 189, 648.
Itoh, M., Kawamura, K., Kitahashi, T., Kojima, S., Katagiri, H., & Shimanaga, M. (2011). Bathymetric patterns of meiofaunal abundance and biomass associated with the Kuril and Ryukyu trenches, western North Pacific Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 58, 86–97.
Jammo, K. M. T. (2004). Biodegredation of organic matter in the marine environment of Alexandria (Eastern Harbor) (pp. 1–303). Ph D Alexandria University.
Khairy, M. A., Kolb, M., Mostafa, A. R., El-Fiky, A., & Bahadir, M. (2012). Risk posed by chlorinated organic compounds in Abu Qir Bay, East Alexandria. Egypt. Environ Sci Pollut Res Int, 19, 794–811.
Korajkic, A., McMinn, B. R., & Harwood, V. J. (2018). Relationships between microbial indicators and pathogens in recreational water settings. International Journal of Environmental Research and Public Health 15.
Kotwicki, L., Szymelfenig, M., De Troch, M., Urban-Malinga, B., & Weslawski, J. M. (2005). Latitudinal biodiversity patterns of meiofauna from sandy littoral beaches. Biodiversity and Conservation, 14, 461–471.
Landres, P. B., Morgan, P., & Swanson, F. J. (1999). Overview of the use of natural variability concepts in managing ecological systems. Ecological Applications, 9, 1179–1188.
Lee, K., & Levy, E. M. (1991). Bioremediation: Waxy crude oils stranded on low-energy shorelines. International Oil spill conference Proceeding, 1991, 541–547.
Legendre, P., & Anderson, M. J. (1999). Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs, 69, 1–24.
Lo, H. S., Xu, X., Wong, C. Y., & Cheung, S. G. (2018). Comparisons of microplastic pollution between mudflats and sandy beaches in Hong Kong. Environmental Pollution, 236, 208–217.
Maria, T. F., Paiva, P., Vanreusel, A., & Esteves, A. M. (2013). The relationship between sandy beach nematodes and environmental characteristics in two Brazilian sandy beaches (Guanabara Bay, Rio de Janeiro). Annals of the Brazilian Academy of Sciences, 85, 257–270.
Maria, T. F., Vanaverbeke, J., Vanreusel, A., & Esteves, A. M. (2016). Sandy beaches: State of the art of nematode ecology. Anais da Academia Brasileira de Ciências, 88, 1635–1653.
McLachlan, A., & Defeo, O. (2017). The ecology of sandy shores. Academic Press.
McLachlan, A., Defeo, O., Jaramillo, E., & Short, A. D. (2013). Sandy beach conservation and recreation: Guidelines for optimising management strategies for multi-purpose use. Ocean & Coastal Management, 71, 256–268.
Milner, A. M., Collier, R. E. L., Roucoux, K. H., Müller, U. C., Pross, J., Kalaitzidis, S., Christanis, K., & Tzedakis, P. C. (2012). Enhanced seasonality of precipitation in the Mediterranean during the early part of the Last Interglacial. Geology, 40, 919–922.
Mitwally, H. (1999). Ecological and systematic studies of the interstitial fauna and benthic diatoms in the sandy beaches of Alexandria. Ph. D thesis, Faculty of Science, Alexandria University, 324.
Mitwally, H., & Abada, A. E. (2008). Spatial variability of meiofauna and macrofauna in a Mediterranean protected area, Burullus Lake. Egypt. Meiofauna Marina, 16, 185–200.
Mitwally, H., Montagna, P., Halim, Y., Khalil, A., Dorgham, M., & Atta, M. (2004). Egyptian sandy beach meiofauna and benthic diatoms. Rapp Comm Int Mer Medit, 37, 537.
Moens, T., Braeckman, U., Derycke, S., Fonseca, G., Gallucci, F., Gingold, R., Guilini, K., Ingels, J., Leduc, D., Vanaverbeke, J., Van Colen, C., Vanreusel, A., & Vincx, M. (2013). Ecology of free-living marine nematodes. Handbook of Zoology.
Montagna, P. A. (1984). In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Marine Ecology - Progress Series, 18, 119–130.
Montagna, P. A., Bauer, J. E., Hardin, D., & B, R., . (1989). Vertical distribution of microbial and meiofaunal populations in sediments of a natural coastal hydrocarbon seep. Journal of Marine Research, 47, 657–680.
Montagna, P. A., Bauer, J. E., Toal, J., Hardin, D., & Spies, R. B. (1987). Temporal variability and the relationship between benthic meiofaunal and microbial populations of a natural coastal petroleum seep. Journal of Marine Research, 45, 761–789.
Moreno, M., Ferrero, T. J., Granelli, V., Marin, V., Albertelli, G., & Fabiano, M. (2006). Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuarine, Coastal and Shelf Science, 66, 357–367.
Nafaa, M. G., & Frihy, O. E. (1993). Beach and nearshore features along the dissipative coastline of the nile delta. Egypt Journal of Coastal Research, 9, 423.
Nordstrom, K. F. (2004). Beaches and dunes of developed coasts. Cambridge University Press.
Olausson, E. (1975). Methods for the chemical analysis of sediments. FAO Fisheries Technical Papers (FAO).
Ortega Cisneros, K., Smit, A. J., Laudien, J., & Schoeman, D. S. (2011). Complex, dynamic combination of physical, chemical and nutritional variables controls spatio-temporal variation of sandy beach community structure. PLoS One, 6, e23724.
Papageorgiou, N., Moreno, M., Marin, V., Baiardo, S., Arvanitidis, C., Fabiano, M., & Eleftheriou, A. (2007). Interrelationships of bacteria, meiofauna and macrofauna in a Mediterranean sedimentary beach (Maremma Park, NW Italy). Helgoland Marine Research, 61, 31–42.
Pascal, P.-Y., Dupuy, C., Mallet, C., Richard, P., & Niquil, N. (2008). Bacterivory by benthic organisms in sediment: Quantification using15N-enriched bacteria. Journal of Experimental Marine Biology and Ecology, 355, 18–26.
Pascal, P.-Y., Dupuy, C., Richard, P., Mallet, C., du Chatelet, E. A., & Niquilb, N. (2009). Seasonal variation in consumption of benthic bacteria by meio- and macrofauna in an intertidal mudflat. Limnology and Oceanography, 54, 1048–1059.
Pereira, T. J., Gingold, R., Villegas, A. D. M., & Rocha-Olivares, A. (2017). Patterns of spatial variation of meiofauna in sandy beaches of northwestern Mexico with contrasting levels of disturbance. Thalassas: An International Journal of Marine Sciences, 34, 53–63.
Power, D. M. (1999). Recovery in aquatic ecosystems: An overview of knowledge and needs. Journal of Aquatic Ecosystem Stress and Recovery, 6, 253–257.
Pusceddu, A., Gambi, C., Manini, E., & Danovaro, R. (2007). Trophic state, ecosystem efficiency and biodiversity of transitional aquatic ecosystems: Analysis of environmental quality based on different benthic indicators. Chemistry and Ecology, 23, 505–515.
Ranta, E., Kaitala, V., & Lundberg, P. (1998). Population variability in space and time: the dynamics of synchronous population fluctuations. Oikos, 83, 376–382.
Reiss, H., & Kroncke, I. (2005). Seasonal variability of benthic indices: An approach to test the applicability of different indices for ecosystem quality assessment. Marine Pollution Bulletin, 50, 1490–1499.
Riera, R., Núñez, J., Carmen Brito, M. D., & Tuya, F. (2011). Temporal variability of a s ubtropical intertidal meiofaunal ass emblage: Contrasting effects at the species and assemblage-level. Vie et milieu - Life and Environment, 61, 129–137.
Rodrı́guez, J.G., Lastra, M., López, J., . (2003). Meiofauna distribution along a gradient of sandy beaches in northern Spain. Estuarine, Coastal and Shelf Science, 58, 63–69.
Santos, G. H. C., Cardoso, R. S., & Maria, T. F. (2019). Bioindicators or sediment relationships: Evaluating ecological responses from sandy beach nematodes. Estuarine, Coastal and Shelf Science, 224, 217–227.
Schlacher, T. A., Jones, A. R., Dugan, J. E., Weston, M. A., Harris, L., Schoeman, D. S., Hubbard, D. M., Scapini, F., Nel, R., & Lastra, M. (2014). Open-coast sandy beaches and coastal dunes. Coastal Conservation, 19, 37–92.
Schlacher, T. A., Schoeman, D. S., Dugan, J., Lastra, M., Jones, A., Scapini, F., & McLachlan, A. (2008). Sandy beach ecosystems: Key features, sampling issues, management challenges and climate change impact. Marine Ecology. ISSN, 0173–9565(29), 70–90.
Schratzberger, M., Lampadariou, N., Somerfield, P. J., Vandepitte, L., & Vanden Berghe, E. (2009). The impact of seabed disturbance on nematode communities: Linking field and laboratory observations. Marine Biology, 156, 709–724.
Semprucci, F., Colantoni, P., Sbrocca, C., Baldelli, G., Rocchi, M., & Balsamo, M. (2011). Meiofauna in sandy back-reef platforms differently exposed to the monsoons in the Maldives (Indian Ocean). Journal of Marine Systems, 87, 208–215.
Semprucci, F., Frontalini, F., Sbrocca, C., du Chatelet, E. A., Bout-Roumazeilles, V., Coccioni, R., & Balsamo, M. (2015). Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environment Monitoring and Assessment, 187, 187–251.
Sevastou, K., Lampadariou, N., & Eleftheriou, A. (2011). Meiobenthic diversity in space and time: The case of harpacticoid copepods in two Mediterranean microtidal sandy beaches. Journal of Sea Research, 66, 205–214.
Shreadah, A. M., Abdel-Mohsen M, El-Sayed, A., Mohamed A, A., & Hamam A. R. H. (2019). Evaluation of Different Anthropogenic Effluents Impacts on the Water Quality Using Principal Component Analysis: A Case Study of Abu-Qir Bay-Alexandria-Egypt. International Journal of Environmental Monitoring and Analysis, 7, 56.
Shreadah, M., Masoud, M., Khattab, A., & El Zokm, G. (2014). Impacts of different drains on the seawater quality of El-Mex bay (Alexandria, Egypt). Journal of Ecology and The Natural Environment, 6, 287–303.
Smith, E. P. (2019). Ending reliance on statistical significance will improve environmental inference and communication. Estuaries and Coasts, 43, 1–6.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis. The Journal of the Fisheries Research Board of Canada Ottawa, Ont'ario (pp. 310) Canada.
Sun, X., Zhou, H., Hua, E., Xu, S., Cong, B., & Zhang, Z. (2014). Meiofauna and its sedimentary environment as an integrated indication of anthropogenic disturbance to sandy beach ecosystems. Marine Pollution Bulletin, 88, 260–267.
Swanson, F. J., Jones, J. A., Wallin, D. O., & Cissel, J. H. (1994). Natural variability-implications for ecosystem management. ecosystem management: Principles and applications. Jensen and Bourgeron (editors), II, 80–90.
Szymelfenig, M., Kotwicki, L., & Graca, B. (2006). Benthic re-colonization in post-dredging pits in the Puck Bay (Southern Baltic Sea). Estuarine, Coastal and Shelf Science, 68, 489–498.
Tolhurst, T. J., Defew, E. C., & Dye, A. (2010). Lack of correlation between surface macrofauna, meiofauna, erosion threshold and biogeochemical properties of sediments within an intertidal mudflat and mangrove forest. Hydrobiologia, 652, 1–13.
Torres-Bejarano, F., González-Márquez, L. C., Díaz-Solano, B., Torregroza-Espinosa, A. C., & Cantero-Rodelo, R. (2016). Effects of beach tourists on bathing water and sand quality at Puerto Velero, Colombia. Environment, Development and Sustainability, 20, 255–269.
Traunspurger, W., & Majdi, N. (2017). Meiofauna. Methods in stream. Ecology, 3, 273–295.
Urban-Malinga, B., Kotwicki, L., Gheskiere, T. A., Jankowska, K., Opalinski, K., & Malinga, M. (2004). Composition and distribution of meiofauna, including nematode genera, in two contrasting Arctic beaches. Polar Biology 27.
Zaki, H., Goma, R., Tadros, A., & Mahmoud, M. (2009). Environmental parameters of Alexnadria Inshore North western Coastall Area, Alexandria. World Applied science, 7, 715–725.
Zeppilli, D., Sarrazin, J., Leduc, D., Arbizu, P. M., Fontaneto, D., Fontanier, C., Gooday, A. J., Kristensen, R. M., Ivanenko, V. N., Sørensen, M. V., Vanreusel, A., Thébault, J., Mea, M., Allio, N., Andro, T., Arvigo, A., Castrec, J., Danielo, M., Foulon, V., … Fernandes, D. (2015). Is the meiofauna a good indicator for climate change and anthropogenic impacts? Marine Biodiversity, 45, 505–535.
Acknowledgements
The authors deeply thank Mr. A.S. Qamara for collecting and sorting samples. The first author is grateful to Prof Dr. John W Fleeger, Louisiana State University, USA, for reading the manuscript and for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights for review
Meiofaunal Natural variability is a good indicator of beach ruin.
Stochastic distribution, winter storms, and touristic activities are the fundamental causes of the large fraction of the unexplained variation in meiofaunal communities.
The beach that experiences the synergetic effect of natural forces and human-made activities has a worse ecological state.
Human-made disturbances; mechanical engineering, pollution, tourism activities, and the natural forces; winter storms, high energy, and rip currents are essential contributors for management decision-makers.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitwally, H.M., Hamdan, A.M. Environmental drivers of meiofaunal natural variability, Egypt, Southeastern Mediterranean. Environ Monit Assess 193, 185 (2021). https://doi.org/10.1007/s10661-021-08927-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08927-0