Abstract
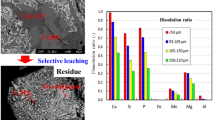
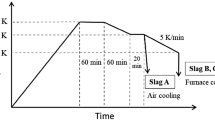
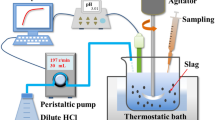
To realize efficient P removal and resource utilization of steelmaking slag, a synthetic slag with appropriate basicity was produced by co-processing the practical dephosphorization slag and basic oxygen furnace slag. The selective leaching behavior and dissolution kinetics of P were investigated. The results indicated that the P dissolution efficiency and reaction rate were significantly influenced by the variety in stirring rate and pH. However, the decreasing solid/liquid ratio and particle size can significantly enhance the reaction rate, while exerting a slight impact on the P dissolution efficiency. The leaching temperature had less influence on both P dissolution efficiency and reaction rate. Under the optimal leaching conditions, the leaching reaction of the P-condensed phase primarily occurred within the initial 3 min, and, finally, the P dissolution efficiency reached 87.5%, with an extremely low dissolved amount of Fe. After leaching, the Fe-rich residue consisted of massive Fe2O3, MgO, and MnO, with only 0.32% P2O5. This residue can be used as a raw material or flux in steel plants. Furthermore, it was found that the selective leaching of P was controlled by diffusion through the product layer, with the apparent activation energy of 5.80 kJ/mol. The empirical equation has been established.










Similar content being viewed by others
References
S. Basu, A.K. Lahiri, and S. Seetharaman, Metall. Mater. Trans. B 38, 357 (2007).
L. Hao, S.X. Zhang, J.H. Dong, and W. Ke, Corros. Sci. 53, 4187 (2011).
M.A. Tayeb, S. Spooner, and S. Sridhar, JOM 66, 1565 (2014).
Y. Ogawa, M. Yano, S.Y. Kitamura, and H. Hirata, Steel Res. Int. 74, 70 (2003).
H. Sun, J. Yang, R.H. Zhang, and W.K. Yang, Metall. Mater. Trans. B 52, 3403 (2021).
H. Ohtake, and S. Tsuneda, Phosphorus Recovery and Recycling (Springer Singaporem, Singapore, 2019), pp3–8.
Y. Sun, M. Chen, X. Ma, Z.X. Zhao, T. Evans, and B.J. Zhao, JOM 73, 1845 (2021).
H.M. Zhou, Y.M. Bao, and L. Lin, Steel Res. Int. 84, 863 (2013).
K. Matsubae-Yokoyama, H. Kubo, and T. Nagasaka, ISIJ Int. 50, 65 (2010).
H.J. Li, H. Suito, and M. Tokuda, ISIJ Int. 35, 1079 (1995).
K. Yokoyama, H. Kubo, K. Mori, H. Okada, S. Takeuchi, and T. Nagasaka, ISIJ Int. 47, 1541 (2007).
C. Li, J.T. Gao, Z. Wang, H.R. Ren, and Z.C. Guo, ISIJ Int. 57, 767 (2017).
J. Xi, G. Ji, Y. Liao, Y. Wu, Q. Liu, and M. Li, J. Sustain. Metall. 8, 51 (2022).
M. Numata, N. Maruoka, S.J. Kim, and S.Y. Kitamura, ISIJ Int. 54, 1983 (2014).
T. Teratoko, N. Maruoka, H. Shibata, and S.Y. Kitamura, High Temp. Mater. Process. 31, 329 (2012).
C.M. Du, X. Gao, S. Ueda, and S.Y. Kitamura, ISIJ Int. 57, 487 (2017).
C.M. Du, X. Gao, S. Ueda, and S.Y. Kitamura, J. Sustain. Metall. 4, 443 (2018).
C.M. Du, Y.H. Yu, L.D. Jiang, and J.K. Yu, J. Clean. Prod. 332, 130087 (2022).
C.M. Du, X. Gao, S. Ueda, and S.Y. Kitamura, Sci. Total. Environ. 819, 153125 (2022).
Q. Zhao, C.J. Liu, X.H. Mei, H. Saxén, and R. Zevenhoven, Fundam. Res. https://doi.org/10.1016/j.fmre.2022.09.023 (2022).
C.M. Du, X. Gao, S. Ueda, and S.Y. Kitamura, ISIJ Int. 58, 1659 (2018).
J. Guo, Y. Bao, and M. Wang, Waste Manag. 78, 318 (2018).
Y.H. Yu, C.M. Du, J.K. Yu, and X. Yang, Metall. Mater. Trans. B 53, 3635 (2022).
T.K. Choo, J. Cashion, C. Selomulya, and L. Zhang, Energy Fuels 30, 1162 (2016).
Y.H. Yu, C.M. Du, S.L. Fan, and W.M. Yu, J. Environ. Chem. Eng. 10, 108394 (2022).
K. Liu, Q. Chen, Z. Yin, H. Hu, and Z. Ding, Hydrometallurgy 125, 125 (2012).
E.A. Abdel-Aal, Hydrometallurgy 55, 247 (2000).
O. Lachkar-Zamouri, K. Brahim, F. Bennour, and I. Khattech, J. Min. Metall. B. 55, 9 (2019).
S. Aydogan, G. Ucar, and M. Canbazoglu, Hydrometallurgy 81, 45 (2006).
P.K. Gbor and C.Q. Jia, Chem. Eng. Sci. 59, 1979 (2004).
Z.M. Jin, G.W. Warren, and H. Henein, Metall. Trans. B 15, 5 (1984).
E.A. Abdel-Aal and M.M. Rashad, Hydrometallurgy 74, 189 (2004).
M. Ashraf, Z.I. Zafar, and T.M. Ansari, Hydrometallurgy 80, 286 (2005).
H. Gao, T. Jiang, Y. Xu, J. Wen, and X. Xue, Min. Proc. Ext. Met. Rev. 41, 22 (2018).
S. Aydogan, A. Aras, and M. Canbazoglu, Chem. Eng. J. 114, 67 (2005).
M. Gharabaghi, M. Noaparast, and M. Irannajad, Hydrometallurgy 95, 341 (2009).
H.H. Wang, G.Q. Li, D. Zhao, J.H. Ma, and J. Yang, Hydrometallurgy 171, 61 (2017).
C.M. Du, X. Gao, S. Ueda, and S.Y. Kitamura, J. Sustain. Metall. 6, 724 (2020).
Y.H. Yu, N.N. Lv, and C.M. Du, Can. Metall. Q. 61, 483 (2022).
A. Ekmekyapar, E. Aktaş, A. Künkül, and N. Demirkiran, Metall. Mater. Trans. B 43, 764 (2012).
M. Gharabaghi, M. Irannajad, and A.R. Azadmehr, Chem. Eng. Res. Des. 91, 325 (2013).
Funding
The funding was provided by National Natural Science Foundation of China (Grant No. 52104326) and Fundamental Research Funds for the Central Universities (Grant No. N2225016).
Author information
Authors and Affiliations
Contributions
YY: Data curation, Formal analysis, Investigation, Validation, Writing-original draft. CD: Conceptualization, Data curation, Project administration, Supervision, Writing-review & editing. YZ: Data curation, Formal analysis, Validation.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Yh., Du, Cm. & Zhang, Yt. Acid Leaching for Phosphorus Separation from the Co-processing of Dephosphorization Slag and Basic Oxygen Furnace Slag: Kinetics Investigation. JOM 76, 2501–2512 (2024). https://doi.org/10.1007/s11837-024-06442-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-024-06442-4