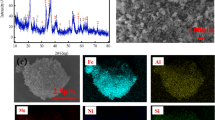
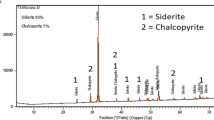
Abstract
The production of metallic copper from low-grade copper ores is generally carried out by hydrometallurgical methods. Leaching is the first prerequisite of any hydrometallurgical process. Solutions containing ammonia may allow for selective leaching of the copper from the ore. In this study, the leaching kinetics of malachite, which is an oxidized copper ore, in ammonium nitrate solutions was examined. The effects of some experimental parameters on the leaching process were investigated, and a kinetic model to represent the effects of these parameters on the leaching rate was developed. It was determined that the leaching rate increased with increasing solution concentration, temperature, and agitation speed, as well as decreasing particle size. It was found that the leaching reaction followed the mixed kinetic controlled model, which includes two different leaching processes including the surface chemical reaction (303 K to 323 K [30 °C to 50 °C]) and diffusion through a porous product layer (323 K to 343 K [50 °C to 70 °C]). The activation energies of these sequential steps were determined to be 95.10 and 29.50 kJ/mol, respectively.












Similar content being viewed by others
References
A. Akçıl: Miner. Eng., 2002, vol. 15, pp. 1193–97.
M.E. Arzutuğ, M.M. Kocakerim, and M. Çopur: Ind. Eng. Chem. Res., 2004, vol. 43, pp. 4118–23.
A. Ekmekyapar, S. Çolak, M. Alkan, and İ. Kayadeniz: J. Chem. Technol. Biotechnol., 1988, vol. 43, pp. 195–204.
S. Venkatachalam: Hydrometallurgy, Narosa Publishing House, Delhi, India, 1998.
N. Habbache, N. Alane, S. Djerad, and L. Tifouti: Chem. Eng. J., 2009, vol. 152, pp. 503–08.
D. Bingöl and M. Canbazoğlu: Hydrometallurgy, 2004, vol. 72, pp. 159–65.
A. Ekmekyapar, R. Oya, and A. Künkül: Chem. Biochem. Eng. Q., 2003, vol. 17, pp. 261–66.
L.J.L. Blanco, V.F.M Zapata, and D.D.J. Garcia, Hydrometallurgy, 1999, vol. 54, pp. 41–48.
J. Moghaddam, R. Sarraf-Mamoory, Y. Yamini, and M. Abdollahy: Ind. Eng. Chem. Res., 2005, vol. 44, pp. 8952–58.
P.D. Oudenne and F.A Olson: Metall. Trans. B, 1983, vol. 14B, pp. 33–40.
A. Künkül, M.M. Kocakerim, S. Yapıcı, and A. Demirbağ: Int. J. Miner. Process., 1994, vol. 41, pp. 167–82.
A. Yartaşı and M. Çopur: Miner. Eng., 1996, vol. 9, pp. 639–98.
D. Bingöl, M. Canbazoğlu, and S. Aydoğan: Hydrometallurgy, 2005, vol. 76, pp. 55–62.
W. Lui, M. Tang, C. Tang, J. He, S. Yong, and J. Yang: T. Nonferr. Metal. Soc. China, 2010, vol. 20, pp. 910–17.
W.J, Albery, P.N. Bartlett, C.P. Wilde, and J.R. Darwent: J. Am. Chem. Soc., 1985, vol. 107, pp. 1854–58.
F.K. Crundwell: Hydrometallurgy, 1995, vol. 39, pp. 321–35.
C.Y. Wen: Ind. Eng. Chem., 1968, vol. 60, pp. 34–54.
O. Levespiel: Chemical Reaction Engineering, Wiley, New York, NY, 1972.
N. Demirkıran: Chem. Eng. J., 2008, vol. 141, pp. 180–86.
N. Mazet: Int. Chem. Eng., 1992, vol. 32, pp. 271–84.
C.F. Dickinson and G.R. Heal: Thermochim. Acta, 1999, vols. 340–341, pp. 89–103.
D. İkiz, M. Gülfen, and A.O. Aydın: Miner. Eng., 2006, vol. 19, pp. 972–74.
F. Habashi: Naturwissenschaften, 1983, vol. 70, pp. 403–11.
M. Ashraf, Z.I. Zafar, and T.M. Ansari: Hydrometallurgy, 2005, vol. 80, pp. 286–92.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted September 22, 2011.
Rights and permissions
About this article
Cite this article
Ekmekyapar, A., Aktaş, E., Künkül, A. et al. Investigation of Leaching Kinetics of Copper from Malachite Ore in Ammonium Nitrate Solutions. Metall Mater Trans B 43, 764–772 (2012). https://doi.org/10.1007/s11663-012-9670-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9670-2