Abstract
Rare earth is a crucial mineral resource that has significant applications in the electronic device industry. In recent years, with the continuous improvement and updating of various electronic devices, electric vehicles have been promoted globally. The excellent properties of rare earth fluorescent materials have made rare earth-containing electronic devices widely used in civil transportation, communication and other fields. However, the global consumption of electronic devices is enormous, and their lifespan is limited, which has led to an annual increase in the production of electronic waste worldwide. How to recycle the rare earth elements contained in them is receiving increasing attention. At present, research on the recovery of rare earth elements from electronic waste mainly focuses on the recovery of neodymium iron boron magnets and rare earth fluorescent powders. This article summarizes the main application pathways of rare earth elements in electronic equipment as well as the research status and technological development trend of recovering rare earth elements from electronic waste.
Similar content being viewed by others
Introduction
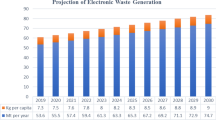
Rare earth is one of the key mineral resources, containing 17 metallic elements including 15 lanthanides (element numbers 57–71), scandium (element number 21) and yttrium (element number 39).1 It is an important raw material for manufacturing electronic equipment and has been widely used in cathode ray tubes, printed circuit boards, permanent magnets and energy storage batteries. Globally, with technological innovation, consumer demand for electronic products has increased, leading to a sharp increase in the generation of electronic waste in recent years. The report of United Nations' 2020 Global Electronic Waste Monitoring is shown in Fig. 1. The total amount of electronic waste (products with batteries or plugs) generated globally has reached 53.6 million tons, representing a 21% increase over 5 years.2 The report predicts that global electronic waste will reach 74 million tons by 2030, making it the fastest growing type of domestic waste in the world.3,4
The composition of electronic waste5 is shown in Fig. 2.
There are many materials in electronic waste that have recycling value, which can be generally divided into five categories: ferrous metal, non-ferrous metal, glass, plastic and other materials.6 Among them, non-ferrous metals mainly refer to copper, aluminum, lead and zinc.7 The content of rare earth elements is not low; however, reports on the recovery process for these elements are rare.8
Overview of Rare Earth Applications
Rare earth elements play a crucial role in many industrial sectors. They can be divided into two categories: light rare earth elements (LREE) and heavy rare earth elements (HREE). Rare earths are naturally found in the form of oxides, carbonates, silicates, phosphates, halides and other minerals. LREEs are generally more abundant than HREEs. The proportion of rare earth element content in the upper crust is shown in Fig. 3.
Globally, REO is widely distributed, but most of its reserves are located in China, Brazil and Vietnam. REO deposits are formed by hydrothermal forces, metamorphism and weathering. Table I shows the distribution of rare earth elements in the world (data updated to 2018) and their production capacity.9
Rare earth elements play an important role in the field of modern industrial manufacturing, with unique characteristics of high magnetism, conductivity, catalysis, electrochemistry and luminescence.10 Therefore, rare earth elements have become an indispensable part of contemporary people's lives as well as the basic raw materials for metal refining, alloy manufacturing, petrochemical processing, locomotive and automobile catalysis, superconductors, colored pigments for glass and ceramic manufacturing, fuel cells, military equipment, permanent magnets, laser manufacturing and other industries. In recent years, due to the widespread promotion of electric vehicles, the demand for electric motors has correspondingly increased, making the importance of rare earths even higher.11 In addition, rare earth elements are widely used in household appliances, such as rechargeable batteries, mobile phone speakers, microphones, fiber optic cables, compact fluorescent lamps, LED lamps, electric motors and hard disk drives.12
Current Situation of Recycling
Recycling can be roughly divided into three steps: (1) disassembly. The selective disassembly of targeted selection of harmful or valuable components for special treatment is an essential process of electronic waste recycling. (2) Enrichment. Mechanical/physical treatment/metallurgical treatment are used to increase the required material content, i.e., raw materials prepared for refining. (3) Refining. The enriched raw material waste is treated and purified by chemical (metallurgical) process, so that it can be reused.13
Magnetic Material
NdFeB is the most common rare earth permanent magnetic material, widely used in small electronic equipment such as mobile phones, headphones, computer hard disks, and electronic equipment, automobile industry and other industries.14,15 Among them, the electronic equipment industry is the most widely used field, such as the hard disk drive in electronic computers. Each hard disk drive has two NdFeB magnets, each of which is in the spindle motor and voice coil motor, with a mass of 10–20 g.16 Each year, about 600 million computer hard disks are produced and manufactured, consuming 6000–12,000 tons of NdFeB magnets.17 In addition, mobile phones, earphones, cameras, speakers and other electronic products contain a large number of NdFeB magnets. These electronic products are rapidly updated, and the corresponding consumption of NdFeB magnets is huge.18 China is one of the major producers of NdFeB magnets in the world, accounting for > 80% of the global output. To obtain the size and shape required by the design, in the polishing and finishing process of NdFeB magnets, 25–30% of the waste will be generated, and the content of rare earth in the waste is about 30%.19,20 In addition, > 600,000–700,000 tons of neodymium iron boron magnets (about 200,000 tons of rare earth) have been used in the world to manufacture wind turbines, motors, hard disk drives, loudspeakers and other products. Most of these products have entered the Chinese market. With the gradual end of the service life of these products, the scrap amount of neodymium, iron and boron will increase correspondingly and will increase rapidly at a rate of > 10% per year in the future.21,22 According to the different sources of magnets and the use after recycling, the recycling methods adopted are also different. For large NdFeB magnets in electric motors and electric vehicle generators, they can be reused directly in their current form or reused after polishing and magnetizing.23 Neodymium iron boron magnets with serious damage at the end of their life cycle need to be recycled by extracting elements. Table II shows the main chemical composition of typical NdFeB magnet waste.24
Due to differences in material composition and chemical properties, the selected recycling process also varies. Currently, the recycling of NdFeB magnets can be divided into the following processes:
Direct Reuse Routes
For magnets similar to those in wind turbines and hybrid electric vehicles, they often have larger volumes, lower pollution levels and lower oxidation levels. After simple pre-treatment, they can be used to manufacture new NdFeB magnets.25 The rare earth elements in NdFeB magnets can react with some solid or gaseous extractants at high temperature to generate corresponding compounds or alloys and then separate them from the ferroboron matrix by simple physical or chemical methods to obtain mixed rare earth products. These direct separation and reuse methods include re-sintering, melt spinning (MS), hydrogen explosion (HD) and hydrogenation disproportionation desorption composite (HDDR) or additive manufacturing (AM). At present, hydrogen explosion method is widely used because of the low energy consumption and simple process. For NdFeB waste materials with little change in composition and content, such as magnets in computer hard disk drives, they can be processed and manufactured into NdFeB magnets after hydrogen pretreatment for reuse to simplify the processing process and reduce the processing cost. The process principle of the hydrogen fragmentation method (HD) is that under certain temperature and hydrogen environments, hydrogen reacts with metals or metal compounds to generate metal hydride, referred to as hydrogen absorption reaction;26 the reaction formula is as follows:
Walton27 used hydrogen to explode sintered NdFeB waste in hard disk drives into demagnetized hydrogenated powder. After the operation of screening and impurity removal, the alloy powder can be directly mixed and then sintered to make a completely dense magnet. By further screening and mechanical separation to reduce the impurity content, the extracted NdFeB powder can be directly used to form new magnetic materials. Pan28 used HD technology to treat commercial magnet waste, spray ground it, sinter it and successfully recover Nd-Fe-B waste magnets through GB modification technology, greatly improving corrosion resistance. The optimization mechanism of corrosion resistance was systematically studied. Xia29 proposed the HD-PLP recovery method, combining the hydrogen decomposition process of sintered NdFeB waste with the pressureless process, sintering the alloy powder in a graphite crucible, which can produce isotropic and anisotropic magnets without adding any new materials.
The HDDR method consists of four stages: hydrogenation, disproportionation, dehydrogenation and recombination. Based on HD treatment, dehydrogenation and recombination processes were carried out.30 The reaction formula is as follows:
During the HDDR process, it is necessary to design process parameters such as the pressure, temperature and time of hydrogen gas injection. Otherwise, it will have a certain impact on chemical reactions, crystal structure and grain size, ultimately causing problems such as abnormal grain size and coercivity.31 Zhao32 prepared anisotropic magnetic powders using the HDDR method. The effects of pressure and disproportionation time on the microstructure of magnetic materials were studied and conditions were optimized. The results indicate that the optimized HDDR powder inherits the grain structure of the initial sintered magnet and has been proven to be a promising recycling method for Ce containing sintered magnets. Christian33 discussed the feasibility of extracting NdFeB waste from automobile engine rotor by hydrogen treatment from three aspects: hydrogen pretreatment, alloy powder purification and the effect of dysprosium content on the hydrogen treatment process. Although the specific detonation kinetics and influencing factors in the hydrogen treatment process have not been explored, it still provides a theoretical basis for the recovery of NdFeB waste from automobile rotor. The above-mentioned short-process regeneration method is an ideal method for recovering NdFeB waste due to its short process and non-pollution. In addition, there are oxidation, chlorination, liquid alloy and other methods. However, the magnetic properties of the regenerated magnets obtained from this often decrease.
Hydrometallurgy
The basic principle of hydrometallurgy is to dissolve rare earths in neodymium iron boron waste into ionic states with acid, then extract or selectively precipitate it, and finally recover it in the form of simple substance, oxide or metal alloy.34 The basic process of hydrochloric acid selective leaching method is to oxidize and roast the NdFeB waste at high temperature, convert all the components into oxides and then control the concentration of hydrochloric acid to selectively dissolve the rare earth oxides to obtain the rare earth chloride solution, and the iron oxide is filtered as the slag. Kumari16 studied the effect of roasting on selective leaching of rare earth in detail. Under the optimum roasting temperature of 1123 K, the leaching behavior of rare earth elements and iron in roasted samples was studied by analyzing the effects of hydrochloric acid concentration, slurry density, stirring speed and temperature. Under the optimized conditions, 98% of rare earth elements are selectively leached. The leaching residue is mainly unreacted iron oxide, which can be used to produce pigments after proper treatment. After oxalic acid precipitation and calcination, the leaching solution generates mixed oxides of neodymium, praseodymium and dysprosium with a purity of 99%. This leaching method has element selectivity, also known as hydrochloric acid selective leaching method. Unlike the hydrochloric acid selective leaching method, which only dissolves the rare earth components in the magnet waste, the hydrochloric acid total dissolution method does not need to oxidize and roast the magnet waste first, but uses hydrochloric acid to dissolve all components in the waste into the solution. Then, hydrogen peroxide solution is added to oxidize ferrous ion into iron ion. The iron ion is extracted into the organic phase with an extractant, thus being separated from the rare earth chloride solution; Then, the extractant is used for multistage extraction to obtain a single rare earth chloride solution. The subsequent steps of oxalic acid precipitation and oxidation roasting are the same as those of hydrochloric acid selective leaching. The hydrochloric acid total dissolution process is characterized by a compact process, high degree of automation, high grade of recovered products and high rare earth recovery rate, but long cycle, large consumption of hydrochloric acid and large consumption of extractant. He35 uses phosphoric acid instead of hydrochloric acid, recovers rare earth elements through one-step selective precipitation and recovers dissolved iron with oxalic acid. The results of phosphoric acid leaching showed that the leaching efficiencies of iron and rare earth elements reached 98.76% and 1.09%, respectively. The remaining rare earth elements remain in the leaching residue in the form of REEPO4 · nH2O precipitation. Subsequently, mixed rare earth oxides with purity of 99.49% and 97.17% were obtained from REEPO4 · nH2O solution and phosphoric acid leaching solution by oxalic acid precipitation. Sahar36 studied using organic acid as leaching agent to leach NdFeB waste from discarded hard disk drives. It was found that acetic acid could leach > 90% of rare earth elements at 60°C for 24 h, but some Fe, Co and B were also leached. Aarti37 proposed a chlorination roasting-water leaching process, which is more energy-saving than the traditional oxidation roasting-acid leaching process. The NdFeB waste was calcined with ammonium chloride at a lower temperature to selectively chlorinate the rare earth elements, leached for 3 h and precipitated with oxalic acid, and the high purity (99.2%) rare earth oxide was recovered after calcination. The Fe2O3 recovered from the leaching residue (purity 96.4%) is a valuable by-product, which can be used to produce pigments, etc. Co in leaching solution also has recovery value. Mehmet38 proposed a method combining pyrometallurgy and hydrometallurgy. After the powder sample is completely converted into metal nitrate mixture at room temperature for 1 hour, low-temperature calcination and water immersion treatment can make the extraction efficiency of Ne, Dy, Pr and Gd reach 95–100%. The main impurity iron is almost completely retained in the obtained residue, forming a mixture of hematite and goethite. Al and Co are also mainly left in the residue. Due to the high purity of rare earth extraction solution, it can be directly treated by the subsequent shortened purification process. Most of the acid used in the process is recyclable, and the acid consumption is not high.
Loy39 proposed a new hydrometallurgical method of recovering rare earth elements and cobalt from permanent magnets by mechanochemical-assisted iron sulfate leaching. Through grinding and activating NdFeB waste powder and iron sulfate powder, the metal is converted into corresponding water-soluble sulfate. The rare earth elements and cobalt are recovered by leaching the activated powder in water, and the solid residues are removed successively by filtration. We precipitate rare earth elements with oxalic acid, filter and remove the mixed rare earth oxalates. Finally, rare earth oxalates are calcined to form corresponding oxides. The hydrometallurgical process does not need to use acid or fire pretreatment steps to avoid the generation of hydrogen or other pollutants, and the whole leaching process can be carried out at room temperature. The final rare earth element leaching efficiency is > 95%, and Co is completely recovered.
In recent years, the ionic liquid extraction method also belongs to the category of wet processes. Ionic liquid is a solvent completely composed of ions, with unique properties such as extremely low vapor pressure and strong conductivity. Because it is not volatile, ionic liquids can replace the organic phase in the liquid-liquid extraction process, making the extraction system safer. Xue40 designed and synthesized two carboxylic acid-based reagents for extracting and separating Ne from NdFeB waste. In the presence of low acidity and salting-out agent, both extractants can effectively extract neodymium, while the extractant with long carbon chains has better extraction effect, and the interface layer of the extraction phase is more obvious, which solves the problem of emulsification of traditional extractant to produce the third phase. Pavón41 uses solvent extraction technology to recover rare earth elements from NdFeB waste by using cationic extractant. The new extractant used can recover rare earth elements by countercurrent process under the condition of pH 1.2. Liu42 uses INET-3 as an extractant to recover and separate Dy, Ne and Co from the NdFeB leaching solution. The purity of neodymium is approximately 99% through three countercurrent extraction stages. About 95% Co with purity of 99.98% remains in the raffinate. Li43 has developed a closed loop process for recovering Ne, Dy and Co from NdFeB waste by using a mixture of trichloride oxide and complex chloride. The process steps include the dissolution of magnets, metal stripping and solvent regeneration. Under proper solid-liquid ratio, NdFeB waste can be completely dissolved, and higher temperature can improve the dissolution rate. Adding complex chloride ions to the leaching system can significantly improve the solubility of trichloride. Liu44 proposed a new carbonization/hydrogenation hydrolysis process, using waste sawdust biochar as extraction agent to recover rare earth elements from waste NdFeB magnets. During carbonization/hydrogenation, NdFeB magnets can react with biochar to form NdFeB-C/H alloy. The final REOH purity and corresponding REE recovery reached 99.43% and 88.4%, respectively.
Pyrometallurgy
Although hydrometallurgy is the main industrial recycling method for NdFeB magnets, pyrometallurgy is also an optional option when facing strict environmental restrictions. Fire metallurgy can avoid the generation of wastewater containing chemicals, but due to its high energy consumption, it is still in the research stage. It can be mainly divided into two processes: (1) using reactive liquid metals (such as Mg and Ag) to rapidly form intermetallic compounds with Nd at high temperatures; (2) selective recovery of Nd from compounds using chlorination or oxidation methods. The principle of chlorination method is basically similar to that of oxidation method. According to the differences in affinity between different elements in magnets and chlorine (oxygen), as well as the different properties of chlorine (oxide) formed by each element, rare earth elements are separated from metallic iron to extract rare earth elements. Stopic et al.45,46 oxidized the material for 2 h in a muffle furnace at 1000°C and then separated the magnet into metal phases and residual phases rich in rare earth oxides through carbothermal reduction. In subsequent conditional experiments, it was also proven that increasing the temperature within a certain range can improve the separation efficiency of rare earth elements. Shirayam47 selectively chlorinates and extracts rare earth elements from the magnet alloy by melting magnesium chloride, which can also successfully extract the rare earth elements from the magnet into the molten salt, while the Fe-B alloy remains solid. Subsequently, excess MgCl2 and Mg are removed through vacuum distillation to effectively recover RECl3. In addition, pyrometallurgy can also combine with other processes to recover rare earth elements. Researchers have proposed combining pyrometallurgy with hydrometallurgy by first alkaline roasting the material, followed by solution leaching and finally precipitation and separation of rare earth elements.48 Some researchers calcine magnetic materials to selectively melt rare earth elements with LiF-CaF2 and directly electrolyze the separated rare earth ions to prepare pure metals.49 However, neither method can avoid the high energy consumption caused by roasting, which limits the cost of the pyrometallurgical process.
Electrochemistry
Electrochemical method has introduced a new idea for the recovery of rare earth from electronic waste. Xiao50 summarized the research progress of electrochemical recovery of NdFeB magnets in recent years. Venkatesan51,52 proposed two recovery routes based on the total dissolution method of hydrochloric acid. First, after the neodymium iron boron magnet is completely dissolved with hydrochloric acid, the ferrous ion in the solution is oxidized to iron ion. The rare earth oxalate was obtained by selective precipitation with oxalic acid, the mixed rare earth oxide with purity of 99.2% was obtained after oxidizing and roasting, and the recovery rate of rare earth reached 97%. The remaining ferric chloride solution is used for ore leaching or water treatment agent. The second is to partially dissolve the NdFeB magnet with hydrochloric acid and then put the dissolved solution and the undissolved magnet into the anode chamber of an electrolytic cell. The anode chamber and the cathode chamber are separated by a layer of anion exchange membrane. Ferrous ions in the anode chamber are oxidized to iron ions after electrolysis and precipitated in the form of iron hydroxide, while rare earth ions remain in the solution. Oxalic acid is added to the solution after the precipitation is filtered to selectively precipitate and recover rare earth oxalate, and hydrochloric acid is returned to dissolve NdFeB magnets for recycling. From waste NdFeB magnets to roasting oxalate to finally obtain rare earth oxides, the whole process only consumes electricity and oxalic acid, which is green and environmentally friendly. The purity of rare earth oxides can reach 99%, and the recovery rate can reach 95%.
Makarova53 found that the effect of element separation can be improved by controlling the composition of electrolyte and operating conditions. The electrostatic attraction between rare earth oxalate particles and cathode is used to selectively recover rare earth elements from waste magnets. Kumari54 used citric acid as electrolyte for electrochemical dissolution research. The dissolution process of waste NdFeB magnets in citric acid was studied. Compared with chemical dissolution, the dissolution of NdFeB magnets in electric field in citric acid is significantly enhanced. D2EHPA was used as extractant to selectively and quantitatively extract rare earth from the electrolyte to obtain mixed oxides of Ne, Pr and Dy with purity of 99.9%.
Fluorescent Materials
Rare earth fluorescent materials are widely used in rare earth fluorescent lamps and other light-emitting electronic devices.55 It is a high-quality light source, commonly used in fluorescent lamps (CFL), liquid crystal displays (LCD), light-emitting diodes (LED) and cathode ray tubes (CRT).56 Although LED is rapidly replacing traditional light sources and requires one order of magnitude less rare earth than traditional fluorescent lamps, there are still many rare earth fluorescent lamps with recycling value, and their service life is generally only 3–5 years.57 Yttrium, europium, terbium and cerium contained in waste rare earth fluorescent powder are the basic raw materials of high-tech rare earth functional materials.58 The recovery methods of rare earth fluorescent materials include the following.
Physical Separation Recovery Method
Direct separation and recovery separate halogenated phosphate phosphors from waste phosphors or directly separate monochromatic phosphors. The commonly used separation methods include flotation, extraction, heavy medium centrifugation, air separation, magnetic separation, etc. Yamashita59 successfully recovered LaPO4: Ce, Tb (LAP) with high terbium content from waste phosphor of waste fluorescent lamp by using high-gradient magnetic separation (HGMS) technology, with purity of 87%. In addition to HGMS, iron oxide and glass powder in waste phosphorus can also be removed through screening and precipitation. Compared with the untreated waste LAP, the luminous intensity of the treated LAP is up to 95%, which improves the quality of the recovered phosphor. Due to the wide variety of recovered monochrome phosphors and the structural damage, it is difficult to meet the purity requirements of commercial phosphors, and the industrial application has not yet been realized. In the future, there is room for improvement in the selectivity of separation, rare earth recovery rate, green process and quality of recycled products.
Direct Leaching Method
Wet acid leaching is to leach the rare earth elements into the solution and recover them by taking advantage of the characteristic that phosphor can be dissolved in acid.60 It mainly aims at Y, Eu in red powder, Tb, Ce, La and Gd in green powder and Eu in blue powder. The leaching agents used are mainly inorganic acid sulfuric acid, hydrochloric acid and nitric acid. To improve the leaching efficiency of rare earth, hydrogen peroxide, ascorbic acid and other leaching aids will also be added. The obtained rare earth leaching solution is usually precipitated with oxalic acid and then calcined at high temperature to obtain rare earth oxide. Wu61 recovered Y and Eu from waste by combining acid leaching and photoreduction. The thermodynamic changes of acid leaching and photoreduction steps were studied, and the process conditions were optimized. Under the optimum conditions, the leaching efficiencies of Y and Eu were 99.9% and 61.2%, respectively.
Pretreatment-Leaching Method
Yurramendi62 found that roasting with sodium carbonate at 900°C can convert rare earth phosphate into oxide and improve the leaching efficiency of rare earth elements. Hydrochloric acid leaching is more effective than sulfuric acid leaching. The recoveries of Ce, La and Tb are 86%, 81% and 85%, respectively. Loy63 compared the mechanical activation mechanism before the hydrometallurgical acid leaching process for recovering rare earth elements from green light phosphors LaPO4: Ce3+and Tb3+and the solvent metallurgical mechanochemical leaching process. After 60-min cyclic mechanical activation, the leaching efficiency of rare earth elements increased by 60%, and the leaching efficiency of rare earth elements increased by 98% after combined mechanical-chemical leaching. A solvent-mechanical chemical method consisting of comprehensive leaching and mechanical activation steps has been developed. Using a small amount of concentrated H2SO4 as the leaching agent at room temperature will not only improve the leaching efficiency but also reduce the processing time to 1/6 of the original. Tan64 innovatively uses mechanical activation method to pretreat waste phosphor and recover rare earth elements by acid leaching. First, a planetary mill is used to strengthen the leaching of rare earth elements from waste phosphors. Although it consumes high power during high-speed milling, it can provide high-density energy input and achieve activation in a short time. The leaching efficiency of Eu and Y increased by 10%, reaching 93.1% and 94.6%, respectively. Song65 uses the disordered crystallinity change caused by mechanical activation to improve the rare earth element leaching effect of waste tricolor phosphor. The maximum leaching efficiencies of Y, Eu and Ce were 96.3%, 91.1% and 77.3%, respectively, by studying the sulfuric acid leaching behavior of activated waste phosphor. After adding alkali to the waste phosphor, He66 carried out mechanical activation, and the leaching efficiencies of Ce and Tb were 85.0% and 89.8%, respectively. The experiment shows that the effect of mechanical activation with alkali is better than that of direct mechanical activation. Liu67 extracted rare earth elements from waste phosphors by microwave alkali leaching method. It was found that the addition of alkali enhanced the microwave heating ability of phosphors and finally the leaching efficiency of Ce and Tb reached 97.86% and 95.75%, respectively, which was higher than that of conventional roasting. Shukla68 studied microwave-assisted acid roasting and then water leaching of waste phosphors recovered from waste fluorescent lamps. The baking parameters such as microwave power, baking time and acid dosage were optimized. Under the optimum conditions, 73 g 98% pure Y-Eu-Tb oxide was produced from 184 g phosphor of 100 tubular lamp units. A typical process in pretreatment is "one-stage leaching-alkali melting pretreatment-water washing-two-stage leaching." That is, red powder Y and Eu are extracted with dilute acid. Then, the residual phosphor is pretreated by alkali melting. Washing with water was carried out to remove impurities such as sodium aluminate and excessive alkali. Finally, two-stage acid leaching was carried out to recover Tb, Ce and Eu in green powder and blue powder. After treatment, the leaching efficiency of Y, Eu and Tb, Ce and La in waste phosphor can reach > 96% and 70–98%, respectively. Alkali melting pretreatment can effectively improve the leaching efficiency of Tb, Ce, Eu and La. Based on the consideration of large-scale production and cost, alkali melting pretreatment is the most promising method for industrial application, but the recovery rate of rare earth is still not high. Reduction-alkali fusion pretreatment can obtain low-cost Tb and Ce oxides that are easier to leach, reduce the dependence on leaching conditions and effectively improve the leaching efficiency, but the selection of reducing agents, control of reaction conditions and process mechanism still need further in-depth study.
Emerging Recycling Methods
In recent years, magnetic suspension treatment, microwave pretreatment,69 ionic liquid leaching,70 chelating agent leaching,71 sub-molten salt leaching,25 biological leaching,72 pressure leaching, supercritical extraction and other methods have emerged. The magnetic suspension method has significant advantages such as environmental friendliness, no strong acid and alkali, no electricity and low cost. It provides a new choice for the separation and recycling of rare earth fluorescent lamps and liquid crystal displays, which have the potential for industrial application. Arunraj73 proposed to use graphene to oxidize a mixture of Aspergillus niger spores (GOeA. niger spores) to adsorb and recover rare earth Eu (III) and optimized the adsorption variables for the developed biosorbent, such as medium pH, adsorbent dose, adsorption kinetics, thermodynamics and isotherm. The biosorbent has the characteristics of high adsorption capacity and fast adsorption, and many of its functional groups have selectivity for the adsorption of other rare earth elements. Shukla74 compared three process routes of hydrochloric acid leaching, mechanical activation leaching and roasting leaching to recover rare earth elements from compact fluorescent lamp (CFL) and fluorescent tube (TL) phosphors. On the scale of laboratory and pilot plant, Liu75 studied the effects of pre-sintering temperature, screening, alkali leaching conditions and alkali melting temperature on glass removal and rare earth element recovery in the process of recovering rare earth elements from waste tricolor phosphors containing glass. Under the optimum technological conditions of decomposition and acid leaching after desilication, the total leaching efficiency of rare earth elements reaches 94%, while the leaching efficiencies of Y, Eu, Ce and Tb are 96%, 99%, 81% and 92%, respectively. The expected economic benefits are good. Ippolito76 evaluated two kinds of rare earth phosphor recovery processes and the technology of extracting rare earth from raw ore in terms of environmental impact and economic benefits. The first includes 950°C thermal pretreatment-sulfuric acid leaching-oxalic acid precipitation and finally recovery of rare earth oxides. The second is to replace thermal pretreatment with mechanical activation. LCA evaluation shows that the secondary production of rare earth oxide (REO) has advantages. The sensitivity analysis shows that the processing scale affects the profit level, and the rare earth market also has an impact.
Conclusion
Rare earth is an important mineral resource, and the recovery of rare earth elements from electronic waste is one of the important ways to source rare earth elements in the future. Although there is currently a large research foundation and certain industrial recycling foundation, large-scale recycling still faces two major problems: first, the reliable source of electronic waste. In the recycling of NdFeB magnets and fluorescent materials, pre-separation is required to recycle electronic waste containing rare earths and peel off most of the components that do not contain rare earths. How to ensure the continuous and stable process and how to handle the stripped low value components restrict the continuous progress of industrial recycling. Second is the control of cost recovery.77,78 At present, several recycling process routes for NdFeB magnets and fluorescent materials have been basically determined, and each has its own advantages and disadvantages. If large-scale recycling is to be carried out, it is still necessary to improve the process methods to reduce recycling costs. The pyrometallurgical process has the advantages of high recovery rate and high purity, but the recovery process may generate harmful emissions and has high energy consumption costs when the rare earth content is low. Hydrometallurgy also has the advantage of high recovery rate, but the generated waste liquid often requires more investment to solve. Compared with wet and fire methods, biological methods have better selectivity at lower rare earth concentrations. However, currently, biological methods have poor kinetic conditions and low recovery rates and are difficult to accurately control. Electrochemical methods can selectively recover specific metals based on their different electrochemical properties and generate less pollution than traditional chemical methods, but there is still a distance from large-scale industrial applications.
In short, although theoretical research has made some progress, there is still a certain distance from large-scale recycling. And currently, there is no single recycling method that can complete the recycling of rare earth elements at a lower cost. How to avoid the shortcomings of various processes through a combination of multiple methods will be the focus of future research. For enterprises engaged in industrialization, recovering rare earths from waste is different from traditional mining. When the production scale expands, it is equally important to ensure the long-term stable supply of raw materials and conduct process research.
References
N. Dushyantha, N. Batapola, I. Ilankoon, S. Rohitha, R. Premasiri, B. Abeysinghe, N. Ratnayake, and K. Dissanayake, Ore Geol. Rev. 122, 17 https://doi.org/10.1016/j.oregeorev.2020.103521 (2020).
Y. Attia, P.K. Soori, and F. Ghaith, Toxics 9, 236 https://doi.org/10.3390/toxics9100236 (2021).
S.M. Al-Salem, G.A. Leeke, M.S. El-Eskandarany, M. Van Haute, A. Constantinou, R. Dewil, and J. Baeyens, J. Environ. Manag. 323, 116181 https://doi.org/10.1016/j.jenvman.2022.116181 (2022).
O.S. Shittu, I.D. Williams, and P.J. Shaw, Waste Manag. 120, 549 https://doi.org/10.1016/j.wasman.2020.10.016 (2021).
F.O. Ongondo, I.D. Williams, and T.J. Cherrett, Waste Manag. 31, 714 https://doi.org/10.1016/j.wasman.2010.10.023 (2011).
R. Widmer, H. Oswald-Krapf, D. Sinha-Khetriwal, M. Schnellmann, and H. Böni, Environ. Impact Assess. Rev. 25, 436 (2005).
K. Brindhadevi, D. Barcelo, N.T. Lan Chi, and E.R. Rene, Environ. Res. 217, 114926 https://doi.org/10.1016/j.envres.2022.114926 (2023).
P. Ramya and V. Ramya, Adv. Eng. Softw. 176, 103353 https://doi.org/10.1016/j.advengsoft.2022.103353 (2023).
C. Ramprasad, W. Gwenzi, N. Chaukura, N. Izyan Wan Azelee, A. Upamali Rajapaksha, M. Naushad, and S. Rangabhashiyam, Chem. Eng. J. https://doi.org/10.1016/j.cej.2022.135992 (2022).
V. Balaram, Spectroscopy 31, 40 (2016).
R. Sasai and N. Shimamura, J. Asian Ceram. Soc. 4, 155 (2016).
V. Balaram, Geosci. Front. 10, 1285 https://doi.org/10.1016/j.gsf.2018.12.005 (2019).
N. Dhawan and H. Tanvar, Sustain. Mater. Technol. 32, e00401 https://doi.org/10.1016/j.susmat.2022.e00401 (2022).
E. Padhan, A.K. Nayak, and K. Sarangi, Hydrometallurgy 174, 210 (2017).
R. Sasai, N. Shimamura, and T. Fujimura, ACS Sustain. Chem. Eng. 8, 1507 (2019).
A. Kumari, M.K. Sinha, S. Pramanik, and S.K. Sahu, Waste Manag. 75, 486 https://doi.org/10.1016/j.wasman.2018.01.033 (2018).
Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P.T. Jones, and K. Binnemans, J. Sustain. Metall. 3, 122 https://doi.org/10.1007/s40831-016-0090-4 (2016).
F. Liu, A. Porvali, J. Wang, H. Wang, C. Peng, B.P. Wilson, and M. Lundström, Miner. Eng. 145, 106097 https://doi.org/10.1016/j.mineng.2019.106097 (2020).
A. Lixandru, P. Venkatesan, C. Jönsson, I. Poenaru, B. Hall, Y. Yang, A. Walton, K. Güth, R. Gauß, and O. Gutfleisch, Waste Manag. 68, 482 (2017).
Y. Yang, C. Lan, Y. Wang, Z. Zhao, and B. Li, Sep. Purif. Technol. 230, 115870 (2020).
J.M.D. Coey, Engineering 6, 119 https://doi.org/10.1016/j.eng.2018.11.034 (2020).
N.A. Mancheri, B. Sprecher, G. Bailey, J.P. Ge, and A. Tukker, Resour. Conserv. Recycl. 142, 101 https://doi.org/10.1016/j.resconrec.2018.11.017 (2019).
P.A. Prokofev, N.B. Kolchugina, K. Skotnicova, G.S. Burkhanov, M. Kursa, M.V. Zheleznyi, N.A. Dormidontov, T. Cegan, A.S. Bakulina, Y.S. Koshkidko, and B. Smetana, Materials 13, 3049 (2020).
Y.B. Zhang, F. Gu, Z.J. Su, S. Liu, C. Anderson, and T. Jiang, Metals 10, 34 https://doi.org/10.3390/met10060841 (2020).
M.M. Yu, G.J. Mei, and X.D. Chen, Miner. Eng. 117, 1 https://doi.org/10.1016/j.mineng.2017.12.001 (2018).
T.H. Kim, M.C. Kang, J.G. Lee, H.W. Kwon, D.S. Kim, and C.W. Yang, J. Alloys Compd. 732, 32 https://doi.org/10.1016/j.jallcom.2017.10.173 (2018).
A. Walton, H. Yi, N.A. Rowson, J.D. Speight, V.S.J. Mann, R.S. Sheridan, A. Bradshaw, I.R. Harris, and A.J. Williams, J. Clean. Prod. 104, 236 https://doi.org/10.1016/j.jclepro.2015.05.033 (2015).
P. Mengjie, L. Xiaolian, J. Weiyang, F. Song, Y. Enxiang, L. Xianguo, Z. Lizhong, Z. Xuefeng, and Y. Mi, J. Mag. Mag. Mater. 550 (2022).
M. Xia, A.B. Abrahamsen, C.R.H. Bahl, B. Veluri, A.I. Søegaard, and P. Bøjsøe, J. Mag. Mag. Mater. 441, 55 https://doi.org/10.1016/j.jmmm.2017.01.049 (2017).
F. Zhang, Y. Liu, J. Li, and R. Wang, J. Alloys Compd. 750, 401 (2018).
Y. Xintong, C. Shuai, L. Ming, J. Zhi, D. Guangfei, C. Renjie, Y. Aru, X. Ailin, and X. Zhiyi, Mater. Lett. 341, 134239 (2023).
Z. Haoyang, Y. Wenzong, D. Guangfei, J. Jinyun, T. Xu, C. Renjie, Y. Jianhui, and Y. Aru, J. Mag. Mag. Mater. 562, 169745 (2022).
C. Jönsson, M. Awais, L. Pickering, M. Degri, W. Zhou, A. Bradshaw, R. Sheridan, V. Mann, and A. Walton, J. Clean. Prod. 277, 124058 https://doi.org/10.1016/j.jclepro.2020.124058 (2020).
K. Rima and S.S. Ranjan, J. Environ. Manag. 320 (2022).
L. He, Q. Xu, W. Li, Q. Dong, and W. Sun, J. Rare Earths 40, 338 https://doi.org/10.1016/j.jre.2021.01.003 (2022).
S. Belfqueh, A. Seron, S. Chapron, G. Arrachart, and N. Menad, J. Rare Earths. https://doi.org/10.1016/j.jre.2022.04.027 (2022).
A. Kumari, R. Raj, N.S. Randhawa, and S.K. Sahu, Hydrometallurgy 201, 105581 https://doi.org/10.1016/j.hydromet.2021.105581 (2021).
M.A.R. Önal, E. Aktan, C.R. Borra, B. Blanpain, T. Van Gerven, and M. Guo, Hydrometallurgy 167, 115 https://doi.org/10.1016/j.hydromet.2016.11.006 (2017).
S. Van Loy, M.A.R. Önal, K. Binnemans, and T. Van Gerven, Hydrometallurgy 191, 105154 https://doi.org/10.1016/j.hydromet.2019.105154 (2020).
W. Xue, R. Liu, X. Liu, Y. Wang, P. Lv, and Y. Yang, J. Mol. Liq. 364, 119919 https://doi.org/10.1016/j.molliq.2022.119919 (2022).
S. Pavon, A. Fortuny, M.T. Coll, and A.M. Sastre, J. Environ. Manag. 222, 359 https://doi.org/10.1016/j.jenvman.2018.05.054 (2018).
Z. Liu, J. Wu, X. Liu, W. Wang, Z. Li, R. Xu, Y. Ding, and J. Wang, J. Rare Earths 38, 1114 https://doi.org/10.1016/j.jre.2020.01.018 (2020).
X. Li, Z. Li, and K. Binnemans, Sep. Purif. Technol. 275, 119158 https://doi.org/10.1016/j.seppur.2021.119158 (2021).
B. Liu, N. Zhu, Y. Li, P. Wu, Z. Dang, and Y. Ke, Process. Saf. Environ. Prot. 124, 317 https://doi.org/10.1016/j.psep.2019.01.026 (2019).
S. Srecko, P. Buse, C. Hanwen, E. Elif, S. Slavko, G. Sebahattin, and F. Bernd, Metals 12 (2022).
C. Hanwen, S. Srecko, E. Elif, G. Sebahattin, and F. Bernd, Metals 12 (2022).
S. Shirayama and T.H. Okabe, Metall. Mater. Trans. B 49, 1067 (2018).
Y.T. Qi, X. Yun, Y.Y. de, M.Z. Chao, M.F. Qiu, Z.M. Lin, W.G. Ling, and Q. Min, Int. J. Miner. Metall. Mater. 28 (2021).
J. Younghwan, H. Jungho, J.G. Yoon, S. Sungjune, and P. Jaeyeong, J. Alloys Compd. 860 (2021).
F.S. Xiao, W.T. Hu, J.Q. Zhao, and H.M. Zhu, Metals 13, 20 https://doi.org/10.3390/met13040779 (2023).
P. Venkatesan, Z.H.I. Sun, J. Sietsma, and Y. Yang, Sep. Purif. Technol. 191, 384 https://doi.org/10.1016/j.seppur.2017.09.053 (2018).
P. Venkatesan, T. Vander Hoogerstraete, T. Hennebel, K. Binnemans, J. Sietsma, and Y. Yang, Green Chem. 20, 1065 https://doi.org/10.1039/c7gc03296j (2018).
I. Makarova, J. Ryl, Z. Sun, I. Kurilo, K. Górnicka, M. Laatikainen, and E. Repo, Sep. Purif. Technol. 251, 117362 https://doi.org/10.1016/j.seppur.2020.117362 (2020).
A. Kumari, Dipali, N.S. Randhawa, and S.K. Sahu, J. Clean. Prod. 309, 127393 https://doi.org/10.1016/j.jclepro.2021.127393 (2021).
N. Swain and S. Mishra, J. Clean. Prod. 220, 884 (2019).
V. Innocenzi, Waste Electrical and Electronic Equipment Recycling (Woodhead Publishing, 2018), p 139.
B. Koen, J.P. Tom, M. Torsten, and Y. Lourdes, J. Sustain. Metall. 4, 126 (2018).
S.-K. Back, B.-M. Joung, E.-S. Lee, J.-H. Sung, A.H.M. Mojammal, Y.-J. Park, and Y.-C. Seo, J. Hazard. Mater. 382, 121094 (2020).
M. Yamashita, T. Akai, M. Murakami, and T. Oki, Waste Manag. 79, 164 https://doi.org/10.1016/j.wasman.2018.07.038 (2018).
M.M. Yu, X.Q. Jiang, G.J. Mei, and X.D. Chen, Physicochem. Probl. Miner. Proccess. 54, 238 https://doi.org/10.5277/ppmp1808 (2018).
Y. Wu, Q. Zhang, and T. Zuo, J. Clean. Prod. 226, 858 (2019).
L. Yurramendi, L. Gijsemans, F. Forte, J.L. Aldana, C. del Río, and K. Binnemans, Hydrometallurgy 187, 38 https://doi.org/10.1016/j.hydromet.2019.04.030 (2019).
S. Van Loy, K. Binnemans, and T. Van Gerven, Engineering 4, 398 https://doi.org/10.1016/j.eng.2018.05.015 (2018).
Q. Tan, C. Deng, and J. Li, J. Clean. Prod. 142, 2187 https://doi.org/10.1016/j.jclepro.2016.11.062 (2017).
G. Song, W. Yuan, X. Zhu, X. Wang, C. Zhang, J. Li, J. Bai, and J. Wang, J. Clean. Prod. 151, 361 https://doi.org/10.1016/j.jclepro.2017.03.086 (2017).
L. He, W. Ji, Y. Yin, and W. Sun, J. Rare Earths 36, 108 (2018).
C. Liu, W. Luo, Z. Liu, J. Long, S. Xu, H. Liu, and X. Wang, Process. Saf. Environ. Prot. 162, 395 https://doi.org/10.1016/j.psep.2022.04.034 (2022).
N. Shukla, S. Agrawal, and N. Dhawan, J. Clean. Prod. 307, 127235 https://doi.org/10.1016/j.jclepro.2021.127235 (2021).
S. Nicolas, F. Xiaofan, G. Sue, and C. Christopher, J. Chem. Technol. Biotechnol. 92 (2017).
S. Pavón, A. Fortuny, M.T. Coll, and A.M. Sastre, Waste Manag. 82, 241 (2018).
H. Hasegawa, Z.A. Begum, R. Murase, K. Ishii, H. Sawai, A.S. Mashio, T. Maki, and I.M.M. Rahman, Waste Manag. 80, 17 (2018).
S. Hopfe, S. Konsulke, R. Barthen, F. Lehmann, S. Kutschke, and K. Pollmann, Waste Manag. 79, 554 (2018).
B. Arunraj, V. Rajesh, and N. Rajesh, J. Rare Earths. https://doi.org/10.1016/j.jre.2021.12.006 (2021).
N. Shukla and N. Dhawan, Miner. Eng. 187, 107759 https://doi.org/10.1016/j.mineng.2022.107759 (2022).
H. Liu, S. Li, B. Wang, K. Wang, R. Wu, C. Ekberg, and A.A. Volinsky, J. Clean. Prod. 238, 117998 https://doi.org/10.1016/j.jclepro.2019.117998 (2019).
N.M. Ippolito, A. Amato, V. Innocenzi, F. Ferella, S. Zueva, F. Beolchini, and F. Vegliò, J. Environ. Chem. Eng. 10, 107064 https://doi.org/10.1016/j.jece.2021.107064 (2022).
L. Alvarado-Hernandez, G.T. Lapidus, and F. Gonzalez, Waste Manag. 95, 53 https://doi.org/10.1016/j.wasman.2019.05.057 (2019).
D. Subhabrata, S. Ankur, D. Fahimeh, G. Tathagata, B. Brandon, and A. Srijan, Chem. Eng. J. 397, 124596 (2020).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2020YFC1807803), the National Natural Science Foundation of China (52004021), the Fundamental Research Funds for the Central Universities (FRF-IP-20-02), and the Open Foundation of State Key Laboratory of Mineral Processing of China (BGRIMM-KJSKL-2020-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Wu, D., Hu, W. et al. Recycling NdFeB Magnets and Rare Earth Fluorescent Materials from Electronic Waste. JOM 76, 1319–1328 (2024). https://doi.org/10.1007/s11837-023-06235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06235-1