Abstract
Significant academic research and moderate commercial process innovation on rare-earth element (REE) processing have been underway for decades. The last several years have seen exponential growth in research due to growth in demand of REE that is threatened by supply risks and environmental obstacles. The REE industry appears to be at the intersection of strong, but conflicting forces, including public and political support for a Green Revolution and sustainability, as well as the need for a clean, reliable, and socially responsible supply chain. REE recoveries from ore are limited by current technology and plant practice to 50–80 percent. Increasing demand can be partially satisfied by recycling, but reliable and continuous increases in supply will also require new mineral resources, improved process efficiency, and lower production costs. This article reviews and summarizes the past and current REE processing technologies, commonly employed REE separation routes and methods, and suggests ways to increase efficiency in future REE processing.
Graphical abstract
Example of a bastnäsite from the Mountain Pass Mine, CA, and (b) monazite concentrate from the Manchester Mine, NJ.

Similar content being viewed by others
Introduction
Rare-earth elements (REEs) are required for use in modern high-tech applications and demand has increased significantly over the last decade.1 However, processing of REE ores poses potential hazards to human health and the environment due to challenges in the management of thorium (Th) and uranium (U) in waste products.2 If well managed, REE can play a pivotal role in the Green Initiative movement to offset the accumulation of greenhouse gasses, including their indispensable role in wind turbine generators and electric vehicle motors.3 In this article, we summarize the technologies currently being researched and implemented for processing of REE ores. A better understanding of ore treatment processes and forthcoming improvements is beneficial to extractive metallurgists and materials scientists as it contributes to more efficient production of REE. We will begin by discussing the classification of REEs, as that sheds light on their chemical behavior.
Classification of REE
The REE comprise the 15 elements in the atomic number range 57 to 71, defined as “lanthanoids” plus scandium and yttrium. Scandium and yttrium are included because they share Group IIIA in the Periodic Table with lanthanum (atomic number 57) and have similar properties. Among the 15 lanthanoids, promethium (Pm) does not occur in nature, so the REE series contains 16 elements. These are scandium (Sc), yttrium (Y), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and lutetium (Lu).
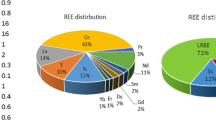
The REE are divided into light REE (LREE) and heavy REE (HREE). The former includes those elements from La through Gd, and the latter includes Tb through Lu. Along with the actinides, the lanthanoids are members of the “f elements” or f block in the Periodic Table. At the electronic structure level, the LREE are those in which the number of unpaired 4f electrons increases as electrons are added to the 4f levels, whereas the HRE are those that experience decreases of unpaired 4f electrons. Yttrium has no 4f electrons, but behaves similar to an HREE. The progressively added electrons are all distant from the valence electrons, resulting in the REE having very similar chemical behavior, especially in aqueous solutions.4
History of REE separation and isolation
Early separation and isolation processes for REE were academic exercises aimed at identification of new elements. The history dates back to 1787 when a Swedish Army Lieutenant, C.A. Arrhenius, collected an unusual black mineral near the Swedish town of Ytterby.5 That specimen was given to Gadolin, a chemist at the University of Åbo in Finland, who investigated a sample of the specimen and isolated two compounds, one that Gadolin named yttria after the specimen’s origin and another that was later named gadolinium.5
In 1803, Berzelius and Hizinger studied a mineral discovered at Båstnäs in Sweden and extracted a metal oxide that they named ceria.5 Hizinger first described another REE mineral, the bastnäsite mineral type, in 1838.5 Concurrently, others studied the crude metal oxides, yttria and ceria, and found that they were mixtures of the oxides of other, yet unknown, elements.5 In 1843, C. Mosander found that crude yttrium oxide also contained the oxides of Tb and Er. The techniques initially used by the early discoverers relied on oxidation/reduction reactions (e.g., with atmospheric oxygen or elemental carbon, and reduction of chlorides with more electropositive metals such as elemental potassium).5 Rather than processes, these were simply qualitative analytical techniques.
The first true separation process was performed in 1907 when G. Urbain isolated Lu from crude Yb by conducting 15,000 sequential nitrate crystallizations.5 Later, scientists discovered that the crude oxides that were initially produced were mixtures of other elemental oxides.
These early separation processes were unsophisticated but sufficient to supply the low demand for REE during the pioneering years in the first half of the twentieth century.5 During the 1950s, many plants produced REE by resin ion exchange, followed by chromatographic elution.5 This technology was replaced during the 1960s by liquid–liquid extraction, as discussed later in the “Hydrometallurgy” section.
A large number of REE refineries continued operations as late as the 1970s in the United States and Europe, but they had been decommissioned by the 1980s for a variety of reasons, including obsolescence, loss of economic viability, and pressure from new health and environmental regulations.5 A popular early product, and one that is still made for applications such as cigarette lighter flints, is misch metal, an REE alloy containing about 50% Ce, 25% La, 15% Nd, and 10% other metals, including iron.5
Economic fundamentals
As is the case with all enterprises that extract natural resources, business success depends on having a predictably positive margin of revenues minus the total production costs. This simple economic model is complicated by China’s quasi-monopoly of the market, as it produces more than 85% of refined REE.5,6 A typical mineral deposit only contains up to five REE at relatively high concentrations (e.g., Ce, La, Nd, Pr, and Sm), and the most abundant elements are usually the least expensive, making it hard to compete with China.4,5,6
Today, price volatility of the REE results from fluctuations in demand, China’s influence on supply, and obsolescence or emergence of end uses.5 The volatility has discouraged many investors from financing new projects. Table I summarizes ranges of average annual prices during the period 2015–2020, and 10-year highs, in US$/kilogram, for various products.8,9 Prices for all REE products and “misch metal” were very high from the early 1990s until they peaked around 2011 and began to decline.8 All 10-year highs were achieved during 2011.8
Geology, deposit types, and ore minerals
There are more than 200 minerals that contain REE.7,8 The most common rare-earth minerals are monazite and bastnäsite (Figure 1a–b and Table II). Monazite exists as a group of arsenates, phosphates, and silicates, but the primary REE-bearing monazite is a complex phosphate.5 Bastnäsite, also known as bastnaesite and bastnasite, is a fluorocarbonate. Table II contains some of the more common REE minerals and their chemical formulas.9,10
REE mineral species are found in carbonatites, pegmatites, and other igneous rocks, and placers (paleoplacers and current marine beaches) derived from weathering of hardrock deposits.5,11,12,13 A carbonatite is a high-carbonate rock derived from hot magmatic fluids, whereas a pegmatite is a coarse-grained rock found near igneous rocks and at the outer margins of intrusive masses. To a lesser extent, REE are also recovered as co-products with other minerals and can be recovered from non-ferrous mineral tailings and industrial wastes.5
The type of deposit has implications on the size of the mineral resource and its REE content. For instance, the carbonatites at Mountain Pass, Calif., Mt. Weld, Australia, and in China (Inner Mongolia) at Bayan Obo are extensive with total REE concentrations in the range 2–10 wt%.14,15,16 Placers are large in size but have low REE concentrations that are typically less than 0.5 wt%. The largest commercial placers are marine beaches found in many parts of the world. The major localities include (1) the states of Orissa and Kerala in India; (2) the beaches at Pulmoddai in northeastern Sri Lanka; (3) the beaches at Eneabba and Jangardup, near Perth in Western Australia; and (4) coastal deposits in the southeastern United States (e.g., Green Cove Springs, Fla).17
Less common occurrences of REE are ionic clays or “ion adsorption clays,” which contain REE ions in structural sites that are readily exchangeable with other cations, especially those in weak electrolytes such as ammonium sulfate.5,18 The mineralogy of these clays has been described by Borst19 as “…leachable 8- to 9-coordinated outer sphere hydrated complexes adsorbed (mainly) onto kaolinite.” Commercial ionic clay deposits are most common in China, Brazil, and Madagascar, but deposits are now being evaluated elsewhere, including Chile.18,20 Ionic clays appear to be especially enriched in Y, La, Nd, Gd, Er, and Yb. However, sampling of in situ clays has not been widely reported. The most reliable data today are for the recovered elements, which means that the apparent relative concentrations may be distorted by the extent to which the REE are displaced from the clays by the leaching solutions.
Many low-grade, but potentially important REE ionic clay resources, are also being studied globally, especially in Canada and the United States. These clay-type deposits merit close attention due to their low radioactivity,19 which can help minimize the environmental issues surrounding management of U- and Th-bearing wastes from processing of monazite and bastnäsite (see later discussion sections).
Beneficiation
In extractive metallurgy, concentration, beneficiation, and physical separation are used interchangeably to describe the treatment of an ore to increase the fraction of the desired mineral(s)’ total solids weight. Beneficiation is done by removing undesired ore constituents (“gangue”) that are associated with ore, but have no commercial interest. Quartz is a typical example, except where quartz itself is desired. Clay usually is another example.
REE minerals can be concentrated in several ways based on physical and/or chemical properties that differ from those of unimportant ore constituents. Compared with the gangue minerals that usually dominate rare-earth ores, the REE minerals generally differ significantly in specific gravity, magnetic susceptibility, and electrical conductivity.5,21 The REE minerals also differ in their response to a common physical/chemical technique, “froth flotation.”15 In almost all cases, the efficiency of the concentrating technique is improved by “comminution,” (i.e., the unit operations of crushing and grinding of ore to a fineness that enables physical “liberation” of the REE minerals from gangue mineral grains).
The following paragraphs briefly summarize the underlying features of the principal beneficiation technologies. These descriptions are offered to provide the reader with an appreciation of the ways REE minerals differ from the gangue and from each other. A typical deposit may contain 1–10 wt% REE and the concentrate usually will contain 50–60 wt% REE, with an overall recovery from ore of 50–80 percent.5
-
Gravity concentration takes advantage of the high specific gravity range, 2.9–7.2, of the REE minerals, compared with 2.5–3.5 for most accessory gangue minerals.5 The technology is exemplified by the classic gold prospector’s pan, wherein worthless sand grains can be washed away from heavier gold particles by applying a gentle shaking motion to a pan loaded with a suspension of “pay dirt” and water. There are many commercial gravity concentration devices, including some that amplify specific gravity differences with centrifugal force.16
-
Magnetic separation depends on applying a magnetic field to a moving stream of gangue and liberated paramagnetic or ferromagnetic minerals, either as a dry powder or as a suspension in water.16 In practice, magnetic particles are attracted to a permanent magnet or electromagnet, usually inside a conveyor head pulley. Non-magnetic particles fly off the end of the head pulley and are discarded, while magnetic particles adhere to the conveyor belt until the space between the pulley and the moving conveyor diminishes the magnetic field’s influence and the particles drop through a chute onto a transfer conveyor.
Many types of devices are available and they are classified as low-intensity and high-intensity magnetic separators, LIMS and HIMS, respectively.16 A choice can also be made between dry separation and wet high-intensity magnetic separation (“WHIMS”). The efficiency and sensitivity of the separation depends on field strength, particle size distribution, and the depth and velocity of the moving stream of ore particles.16,26
Magnetite, Fe3O4, is often present in REE deposits and its high magnetic susceptibility enables removal with LIMS equipment, either wet or dry, depending on processing requirements. Subsequent separations are usually more subtle and require distinguishing between REE minerals that all have only relatively weak responses to magnetic fields. During the last decade, the use of stronger REE-based magnets has reduced both capital costs and energy requirements.16
-
Electrostatic separation takes advantage of differences in electrical conductivity between particles. One type of separator applies a positive static electrical charge to a moving stream of dry particles passing over a rotating horizontal metallic cylinder.16,22,23 Conducting particles quickly lose their induced charge to the grounded cylinder and leave at a high trajectory, whereas the less conductive particles (electrical insulators) temporarily remain statically attracted to the cylinder and leave at a lower trajectory.16,23,24 The two product streams are directed by chutes onto transfer conveyors that deliver them to appropriate points in the process. There are several commercial variations of this basic concept.
-
Froth flotation, a widely used technique for concentrating the ores of most metal sulfide minerals and many non-metallic minerals, is also effective for concentrating some REE minerals.25 Flotation is conducted in an aqueous suspension of ore and gangue particles and the technique relies on several steps. The process begins with addition of reagents that (1) serve as “collectors” that attach selectively to specific mineral grains, either by electrochemical attraction or by chemical adsorption; (2) act as “frothers” by lowering the surface tension of the water; and (3) act as “modifiers” used to depress the flotation response of undesirable minerals such as clays.26
For many years, a popular collector for separation of monazite and bastnäsite from quartz has been a fatty acid derived from tall oil, a residue from the processing of coniferous trees to make wood pulp for paper manufacturing.26 Recently, aryl hydroxamic acids are attracting attention.5
When air bubbles are introduced into the mechanically agitated suspension, each collector molecule acts as a bridge with one end attaching to the surface of the desired mineral grain, while the other end is hydrophobic and attaches to an air bubble. In this manner, liberated grains of REE minerals can be selectively transported to the top surface of the slurry, where the bubbles are stabilized into a persistent froth by frothers. The froth, containing the desired minerals, is removed from the flotation cell as it overflows one edge of the cell into a collection chute or “launder”. Frothers, if needed, include alcohols and plant-based oils. Typical modifiers include several phosphate reagents.5
Beneficiation process flow sheets cannot readily be standardized because the unit operations and equipment selection are dependent on the mineral assemblage being treated, the recommendations of a laboratory-scale process development program, and the preferences of both designer and project owner. REEs are currently recovered commercially from hardrock, usually bastnäsite or monazite, marine placers, and ionic clays.5 Hardrock ores must be comminuted in closed circuit with screens or mechanical classifiers that remove liberated grains before overgrinding occurs. Overgrinding wastes energy, but also may reduce recovery of brittle minerals that are easily ground too finely for efficient beneficiation. Usually, the ore is “deslimed,” a step that typically involves mechanical scrubbing to disaggregate clumps of small particles. Heavy mineral sands from both old and fresh placer deposits may still need feed preparation such as attrition scrubbing and desliming, but comminution is not necessary. Figure 2 is a simplified schematic of REE ore treatment.
Recovery of monazite concentrates from unconsolidated beach sands requires removal of accessory heavy minerals, including ilmenite, leucoxene, magnetite, rutile, and zircon. This typically involves the application of wet gravity concentration, as well as wet and dry magnetic and dry electrostatic separation. Historically, significant REE production has derived from monazite recovered from heavy mineral sands, but current REE production is almost exclusively from hardrock. Due to economics and environmental issues, the monazite currently recovered from beach sand heavy mineral mining operations is being stockpiled or returned to exhausted excavations. See the “Environmental concerns” section.
Hydrometallurgy
The hydrometallurgical treatment technology used by current plants in China and Australia/Malaysia employs variations on baking and/or acid digestion to “crack” the REE minerals, followed by leaching and solvent extraction (liquid ion exchange).5 The term “cracking” is exclusive to REE nomenclature and reflects the extremely refractory nature of the entire REE mineral family. Nearly all of current global REE production is from the following five deposits or districts, and the mining, beneficiation, and concentrate pretreatment techniques can be classified as summarized in Table III, as given by Goode.5
Among the five locations, Mountain Pass in California was in full operation until 2003, when it was placed on care and maintenance pending modernization and resolution of waste treatment issues.5 The mine and processing facility were reactivated by new owners in 2013, but cost overruns and depressed prices drove the resurrected Mountain Pass venture into insolvency and processing operations closed in 2015. In 2017, the project was sold to a new entity called MP Mine Operations, LLC, which later became MP Materials Corp. The company resumed mining and refining operations in 2018, though the concentrate is exported to China for further processing.8
Concentrate leaching
Most REE minerals, especially bastnäsite and monazite, must be decomposed prior to leaching. Decomposition is commonly done by roasting or baking the concentrate in acid-proof kilns at 200–600°C, the optimum temperature being dependent on mineralogy. Strong sulfuric acid (H2SO4) is blended with concentrates in the feed to the kiln. Caustic cracking of concentrates has also been accomplished using strong caustic solutions containing 50–70% sodium hydroxide (NaOH) at 140°C or higher.5,27 Some minerals require a less aggressive treatment, and ion adsorption clays only require a dilute acidic electrolyte. All current operations treating hardrock concentrates require thermal treatment.
Although bastnäsite can be treated with dilute H2SO4, it is a fluorocarbonate and the REE fluorides are insoluble in water or aqueous acid, so they immediately re-precipitate. Therefore, it is necessary to roast bastnasite concentrate at about 650°C to decompose the mineral sufficiently to drive off fluorine, while oxidizing Ce to the tetravalent state. The fluorine gas must then be captured and immobilized.28 One resolution is to pelletize the concentrate with sodium carbonate and roast at 600–700°C, followed by chlorination with ammonium chloride at around 325°C to convert the REE to water-soluble chlorides.5
The current preferred commercial route is baking the REE concentrate at elevated temperatures with excess H2SO4, evolving hydrogen fluoride, which can be absorbed in water to produce hydrofluoric acid. A baking temperature of 600°C or hotter has been reported for Lynas, a REE producer based in Australia,5 but no open-source information is available for the other operations. The resulting REE sulfates are water-soluble and can be separated into individual species. Residual impurities, including the sulfates of Fe, Al, U, and Th can be rejected during solvent extraction, which is further described in the “Separation and refining of REE” section. Specific remedies for the removal of other trace elements may be required.
Precipitation
The aqueous liquid that is produced by leaching the cracked concentrate minerals is called pregnant leach solution (PLS). Residual solids are removed by standard dewatering equipment, usually sedimentation thickeners or centrifuges, followed by vacuum or pressure filtration.5 The filtered residue is washed and filtered again to maximize recovery of soluble REE sulfates. The PLS is clarified by additional settling and/or filtration to remove fine residue particles that otherwise would contaminate the subsequent REE precipitate.
Separation and refining of REE
Until the 1960s, many REE plants constructed in the US, UK, USSR, and France performed separations using column ion exchange, followed by chromatographic elution. These facilities satisfied the low demand for REE and REO (rare-earth oxides) at the time and provided adequate purity for applications such as igniter flints, glass polishing, and catalysts. However, those processes were replaced by liquid–liquid extraction in the 1960s, concurrent with the growing need for Y and Eu in red phosphors for television tubes and Sm, Nd, Dy, Tb, and Pr in various high-performance permanent magnet formulations.29
Liquid–liquid extraction, commonly known as solvent extraction, or SX, is inherently simpler and more efficient, and was rapidly adopted globally. SX is a way of treating a dilute and impure solution, the PLS, to concentrate and purify one or more dissolved constituents. SX is done by contacting the PLS with an organic fluid containing a mixture of a specially developed chemical, called the extractant, and a diluent. The extractant forms complexes with the metal ions while releasing cations into the aqueous solution.30
The resulting organic phase is referred to as “loaded” and is usually “scrubbed” with another aqueous solution to remove impurities that otherwise would accumulate in the organic and reduce its loading capacity. Following scrubbing, the organic fluid is “stripped” by contacting it with an aqueous solution from which the desired REE cations have been partially removed. This stripping solution derives its capability from having been enriched in the cation that originally was exchanged with the PLS during extraction.
REE-bearing PLS is treated in this fashion to yield an enriched extract from which REE can then be precipitated. In practice, though, the partitioning coefficients of individual REEs are nearly identical, so the extraction/stripping cycle must be duplicated many times to achieve acceptable recoveries and purities of the desired REE product. An SX plant for REE production may contain well over 1500 total stages of extraction, scrubbing, and stripping.
There are stringent chemical and physical requirements for the organic phase. The diluent is selected for reasons that include immiscibility with aqueous solutions, low vapor pressure, high flash point (to reduce risk of fire), low toxicity, and minimization of reagent cost. It is the main organic component and is a petroleum distillate, often a kerosene. The extractant usually contributes only 3–10 vol% to the organic phase and is an expensive specialty chemical. The chemical most commonly selected for REE processing is a phosphonic acid, named P 507, that functions as a cationic exchanger.30
Separation circuits based on P 507 as the extractant use hydrochloric acid (HCl) to dissolve the mixed REE feed and also use HCl for both stripping and scrubbing of the loaded organic. Adjustment of acidity is then accomplished with an alkali, usually sodium hydroxide. This results in a sodium chloride (NaCl) brine, which is the primary refinery effluent. This can be a problem because NaCl is almost impossible to remove from groundwater, requiring expensive treatment like reverse osmosis, which still produces a brine. Various extractant and stripping reagent combinations are under constant evaluation for mitigating effluent management challenges. A schematic diagram for leaching, purifying, and precipitating REE is illustrated in Figure 3.
Precipitation and semi-refining of REE salts from the aqueous extract
The semi-purified mixed REE can be precipitated as carbonates or evaporated to yield chloride salts. However, a dominant practice is precipitation with oxalic acid, C2H2O4. The REE oxalates are then calcined at approximately 900°C to drive off water and carbon dioxide, leaving the mixed oxides, REO. Although the aqueous REE solution after SX can be evaporated and the solids sold directly as chlorides, they are often sold as carbonate or oxalate precipitates. Depending on market requirements, the oxalate or carbonate precipitates can be calcined to REO. Some applications may also require specific mixtures or particle size distributions.
REE refining and metal production
While extractive metallurgical processing of ores is influenced and complicated by the similar chemical properties of REE species, refining is made easier by the rather broad variations in physical properties.29
REE that have been reduced to metals by either fused-salt electrowinning or electrothermic methods at temperatures above 1000°C are only 98–99% pure. It seems reasonable to assume that higher purity will lead to better performance in critical applications. Impurities in REE are dependent on the preparation method. Close control of raw materials and process variables can yield 99.99% purity with respect to other metallic elements. However, the REE attract crucible elements as well as oxygen, nitrogen, carbon, hydrogen, and the halides. Producing an REE metal that contains less than 0.1% of these non-metallics is very challenging. Naturally, the best way to minimize the impurity problem is to avoid contamination at the outset.
For industrial-scale production, the refining technique must be compatible with many criteria, including product form, metal yield, batch size, operational simplicity, and economics.29 For research and development purposes, REE must have the highest achievable purity. Impurity removal practices are dominated by vacuum refining, vacuum distillation, and sublimation. Ultra-purification typically involves zone refining and electro-transport.29 These unit operations have been utilized for decades, but the significant demand for high-purity REE for research purposes requires more selective techniques, easier scalability, and improved amenability to automation.
The future of ore processing and REE refining
Current mining and processing operations tend to have low recoveries, on the order of 50–80%, from ore to high-purity REE compounds.5 Inefficient recovery is partly due to the low intrinsic value of the geologic resource compared with the processing costs. The processes for REE ores have been characterized by occasional major developments (e.g., SX, and gradual adoption of new techniques and evolutionary improvements). Today, new research is revealing ways of improving recoveries through development of more powerful and selective reagents,26 more efficient concentrating devices,13,25 and models that fully integrate processing flow sheets.5
While improvements in REE processing have persisted through evaluation and adoption of improved flotation reagents and solvent extractants, the marginal benefits have been small. Further improvements in efficiency can be made by focusing on applying technologies such as ionic or molecular separation with membranes including the microporous hollow fiber contactor,31,32 emulsion liquid membrane extraction,33,34,35 and molecular recognition technology (“MRT”).36,37,38 Examples of these technologies include electrically enhanced extraction,39 phase transition extraction, “tunable” solvents,40 ionic liquids,41,42,43 deep eutectic solvents,44,45 and miniaturized extraction.46
While recycling and re-use of end-of-life REE products are possible, the processes are energy-intensive and complex. Recycling of REE products is complicated by the fact that only a small quantity of REE is incorporated in most recyclable or re-useable materials and that the performance of recycled materials can be impaired.47 However, recycling of manufacturing scrap is much easier than processing end-of-life (EOL) scrap, as the challenges of EOL scrap include collection and dismantling.29,47
Environmental concerns
An over-arching challenge regarding REE mines and processing is that all REE deposits contain Th and U, with lesser concentrations in ionic clays. On-site disposal options are usually limited and subject to strict regulations, whereas transportation to and impoundment in a public landfill are exorbitantly expensive or impossible. Left unmitigated, these radioactive materials can pose human health risks, for example, through breathing of contaminated dusts.
Among the REE minerals, monazite is the primary source of Th.8 A typical composition of monazite from the Kerala deposits in India is 57.5% REO and 7.96% thorium oxide (ThO2).48 Th or U recovered from REE minerals could be used for nuclear power generation. For example, government-supported projects on Th-based nuclear power in India, China, France, Norway, and Brazil could benefit from stockpiling Th extracted from REE processing.49 In the United States, monazite is blended with waste sands and silts and buried in excavations, rather than being stockpiled for potential use in nuclear reactors.50
The separation and removal of U and Th from REE can be done either with SX or by selective precipitation. One approach to removal of U and Th from bastnäsite concentrates is discussed by Zhu.2 In the example cited, the REE concentrate is digested with weak sulfuric acid at pH 1.9 and 95°C and the leach liquor containing most of the uranium and some of the thorium is treated by SX using a tertiary amine extractant. The residue is re-leached with strong (10 wt%) H2SO4 at 95°C, converting most of the REE to their double salts by maintaining a high concentration of sodium in solution. (A double salt is a solid that contains either two different cations or two different anions.) Some of the Th and U dissolve and are recycled to the weak acid leach. The REE double salts are converted to REE hydroxides and then dissolved in HCl, leaving Th in the solid residue for stockpiling or solid waste disposal. Traces of Th and U remain in the REE solution and are removed by resin ion exchange. It is important to note that several techniques are available, but they must be applied after the REE have been extracted into a concentrated solution. There is not yet a way of removing U and Th from monazite-containing mineral concentrates because the monazite must first be chemically “cracked.”
Chu and Serpell51 have observed that the ratio of REE product to waste is about 0.0005, or 2000 tons of solid waste per ton of total recovered REE. They concluded in a July 2021 podcast that “Rare-earth elements are essential to many clean energy policies, yet their production can bring severe environmental impacts.” Chu and Serpell further concluded that “Just as a disruption in supply from China would impact defense preparations, it could also impact domestic clean energy industries. This would be especially troubling if limited supply forced competition between, for example, US defense and domestic energy investments.” This conclusion emphasizes the pressing need for development of Western REE resources and processing technologies.
References
V. Balaram, Geosci. Front. 10(4), 1285 (2019)
Z. Zhu, Y. Pranolo, C.Y. Cheng, Miner. Eng. 77, 185 (2015)
D.D. Imholte, R.T. Nguyen, A. Vedantam, M. Brown, A. Iyer, B.J. Smith, J.W. Collins, C.G. Anderson, B. O’Kelley, Energy Policy 113, 294 (2018)
W. Mercer, R.G. Eggert, Rare-Earth Geology, Mineralogy and Resource Development, (NATO Unclassified Releasable to Aust. PFP Nations, STO-RLS-AVT-285-4, S&T Organ. 4-1 through -19, 2017), pp. 1–22
J.R. Goode, “Rare Earth Elements,” in SME Mineral Processing & Extractive Metallurgy Handbook, eds. R.C. Dunne, S.K. Kawatra, C.A. Young (Society for Mining, Metallurgy & Exploration, Englewood, CO, 2019), vol. 2, pp. 2049–2075
US Geological Survey, Mineral Commodity Summaries 2021. https://www.usgs.gov/centers/nmic/publications
B.S. Van Gosen, P.L. Verplanck, K.R. Long, J. Gambogi, R.R. Seal II, The Rare-Earth Elements—Vital to Modern Technologies and Lifestyles (US Geological Survey Scientific Investigations Report, 2014)
I.M.S.K. Ilankoon, N.P. Dushyantha, N. Mancheri, P.M. Edirisinghe, S.J. Neethling, N.P. Ratnayake, L.P.S. Rohitha, D.M.D.O.K. Dissanayake, H.M.R. Premasiri, A.M.K.B. Abeysinghe et al., “Constraints to rare earth elements supply diversification: Evidence from an industry survey.” in Journal of Clearner Production, 331, 10 (2022)
M. Schlumbohm, L. Muñoz-Giraldo, E. Barth, J. Sieveneck, M. Zeppernick, L. Stefan, Hamburg 2021: Statista Global Business Cities Report 2021 (Statista, Hamburg, Germany)
J.E. Sutton, S. Roy, A.U. Chowdhury, L. Wu, A.K. Wanhala, N. De Silva, S. Jansone-Popova, B.P. Hay, M.C. Cheshire, T.L. Windus, A.G. Stack, A. Navrotsky, B.A. Moyer, B. Doughty, V.S. Bryantsev, ACS Appl. Mater. Interfaces 12(14), 16327 (2020)
S.B. Caster, J.B. Hedrick, Rare Earth Elements, Industrial Minerals Volume, 7th edn. (Society for Mining, Metallurgy & Exploration, Littleton, CO, 2006), pp. 769–792
B. Lehmann, Euro. Geol. 37, 21 (2014)
Y. Kanazawa, M. Kamitani, J. Alloys Compd. 408, 1339 (2006)
K.R. Long, B.S. Van Gosen, N.K. Foley, D. Cordier, The Principal Rare Earth Elements Deposits of the United States: A Summary of Domestic Deposits and a Global Perspective (US Geological Survey Scientific Investigations Report 2010-5220, U.S. Geological Survey, Reston, VA, 2010), p. 96
X. Yang, X. Yang, Y. Zheng, M.J. Le Bas, Mineral. Petrol. 78(1–2), 93 (2003)
B.G. Lottermoser, Lithos 24, 151 (1990)
D. Sengupta, B.S. Van Gosen, “Placer-type rare earth element deposits,” in Reviews in Economic Geology, vol. 18 (Society of Economic Geologists, Littleton, CO, 2016), chap. 4, p. 81
G.A. Moldoveanu, V.G. Papangelakis, Mineral. Mag. 80(1), 63 (2016)
A.M. Borst, M.P. Smith, A.A. Finch, G. Estrade, C. Villanova-de-Benavent, P. Nason, E. Marquis, N.J. Horsburgh, K.M. Goodenough, C. Xu, J. Kynicky, K. Geraki, Nat. Commun. 11, 4386 (2020)
A. Corvalan, P. Javier, Technical-Environmental Feasibility Evaluation for a Rare Earth Extraction Plant in Chile (INS International Nuclear Information System, 2017). http://inis.iaea.org/search/search.aspx?orig_q=RN:50058128
J. Zhang, B. Zhao, B. Schreiner, Separation Hydrometallurgy of Rare Earth Elements, 1st edn. (Springer, Dordrecht, 2016)
A. Jordens, R.S. Sheridan, N.A. Rowson, K.E. Waters, Miner. Eng. 62, 9 (2014)
G.B. Abaka-Wood, J. Addai-Mensah, W. Skinner, “Review of Flotation and Physical Separation of Rare Earth Element Minerals,” presented at the 4th UMaT Biennial International Mining and Mineral Conference, Tarkwa, Ghana, 2016, pp. 55–62
A.F. Taggart, H.A. Behre, Handbook of Mineral Dressing, Ores and Industrial Minerals, 1st edn. (Wiley, 1945)
L.M. Suli, W. Hanisah, W. Ibrahim, B.A. Aziz, M. Rizauddin, Chem. Eng. Res. Bull. 19, 20 (2017)
M.G. Nelson, M.J. Mankosa, D. Lelinski, J.N. Kohmuench, G.H. Luttrell, “Flotation,” in SME Mineral Processing & Extractive Metallurgy Handbook, eds. R.C. Dunne, S.K. Kawatra, C.A. Young (Society for Mining, Metallurgy & Exploration, Englewood, CO, 2019), vol. 2, pp. 891–1065
T.E. Amer, M.A. Gado, B. Attia, E.M. El-Sheikh, Radiochemistry 62(1), 73 (2020)
P. Cen, X. Bian, W. Wu, B. Li, Sep. Purif. Technol. 274, 118380 (2021)
C.K. Gupta, N. Krishnamurthy, Extractive Metallurgy of Rare Earths, 1st edn. (CRC Press, Boca Raton, 2005)
S.C. Bouffard, J.B. Hiskey, J.G. Mansanti, T. McNulty, “Hydrometallurgy,” in SME Mining Processing & Extractive Metallurgy Handbook, eds. R.C. Dunne, S.K. Kawatra, C.A. Young, (Society for Mining, Metallurgy & Exploration, Englewood, CO, 2019), vol. 2, pp. 1179–1367
N. Kunthakudee, N. Sunsandee, U. Pancharoen, P. Ramakul, Desalination Water Treat. 57(9), 3985 (2014)
A.C. Niam, Y.F. Wang, S.W. Chen, G.M. Chang, S.J. You, Chem. Eng. Process. Process Intensif. 148, 107831 (2020)
A.L. Ahmad, A. Kusumastuti, C.J.C. Derek, B.S. Ooi, Chem. Eng. J. 171(3), 870 (2011)
Z. Lichang, C. Qianlin, K. Chao, M.A. Xin, Y. Zunliang, J. Rare Earths 34(7), 717 (2016)
X. Liu, X. Zhang, J. Membr. Sci. 128, 223 (1997)
S.R. Izatt, J.S. McKenzie, N.E. Izatt, R.L. Bruening, K.E. Krakowiak, R.M. Izatt, “Molecular Recognition Technology: A Green Chemistry Process for Separation of Individual Rare Earth Metals,” White Paper on Separation of Rare Earth Elements (2016). http://ucore.com/academic-papers
S. Satoshi, H. Miyake, H. Tsukube, “Molecular Recognition and Sensing via Rare Earth Complexes,” in Handbook on the Physics and Chemistry of Rare Earths, eds. K.A. Gschneider, J.C.G. Bunzli, V.K. Pecharsky (Elsevier, 2005), p. 273
S.R. Izatt, J.S. McKenzie, R.K. Bruening, R.M. Izatt, N.E. Izatt, K.E. Krakowiak, “Selective Recovery of Platinum Group Metals and Rare Earth Metals from Complex Matrices Using a Green Chemistry/Molecular Recognition Technology Approach,” in Metal Sustainability: Global Challenges, Consequences and Prospects, ed. R.M. Izatt, (Wiley, 2016), pp. 317–332
Y. Li, C. Zhang, Y. Jiang, T. Wang, Chemosphere 200, 554 (2018)
L.K. Sinclair, D.L. Baek, J. Thompson, J.W. Tester, R.V. Fox, J. Supercrit. Fluids 124, 20 (2017)
K. Wang, H. Adidharma, M. Radosz, P. Wang, X. Xu, C.K. Russell, H. Tian, M. Fan, J. Yu, Green Chem. 19, 4469 (2017)
F. Yang, F. Kubota, N. Kamiya, M. Goto, Solvent Extr. Res. Dev. Jpn. 20, 225 (2013)
F. Kubota, Y. Shimobori, Y. Baba, Y. Koyanagi, K. Shimojo, N. Kamiya, M. Goto, J. Chem. Eng. Jpn. 44(5), 307 (2011)
W. Chen, J. Jiang, X. Lan, X. Zhao, H. Mou, T. Mu, Green Chem. 21, 4748 (2019)
A. Entezari-zarandi, F. Larachi, J. Rare Earths 37(5), 528 (2019)
K. Wang, G. Luo, Chem. Eng. Sci. 169, 18 (2017)
D. Schueler, M. Buchert, R. Liu, S. Dittrich, C. Merz, Study on Rare Earths and Their Recycling (Final Report for the Green/EFA Group in the European Parliament, Öko-Institut e.V., 2011)
B. Klein, A. Bamber, R.C. Dunne, R.Q. Honaker, A. Gilbert, “Physical Separations,” in SME Mineral Processing & Extractive Metallurgy Handbook, eds. R.C. Dunne, S.K. Kawatra, C.A. Young, (Society for Mining, Metallurgy & Exploration, Englewood, CO, 2019), vol. 2, pp. 761–887
C.J. Rhodes, Sci. Prog. 96(2), 200 (2013)
B.S. Van Gosen, D.I. Bleiwas, G.M. Bedinger, K.J. Ellefsen, A.K. Shah, Min. Eng. 68, 36 (2016)
A. Chu, O. Serpell, “Rare Earth Elements Pose Environmental, Economic Risks for Clean Energy” Kleinman Center for Energy Policy, University of Pennsylvania Podcast on July 27, 2021
Funding
This article is a survey of information available in published technical papers or texts. No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
McNulty, T., Hazen, N. & Park, S. Processing the ores of rare-earth elements. MRS Bulletin 47, 258–266 (2022). https://doi.org/10.1557/s43577-022-00288-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-022-00288-4