Abstract
Spherical titanium alloy powder is an important raw material for near-net-shape fabrication via a powder metallurgy (PM) manufacturing route, as well as feedstock for powder injection molding, and additive manufacturing (AM). Nevertheless, the cost of Ti powder including spherical Ti alloy has been a major hurdle that prevented PM Ti from being adopted for a wide range of applications. Especially with the increasing importance of powder-bed based AM technologies, the demand for spherical Ti powder has brought renewed attention on properties and cost, as well as on powder-producing processes. The performance of Ti components manufactured from powder has a strong dependence on the quality of powder, and it is therefore crucial to understand the properties and production methods of powder. This article aims to provide a cursory review of the basic techniques of commercial and emerging methods for making spherical Ti powder. The advantages as well as limitations of different methods are discussed.
Similar content being viewed by others
Introduction
In the most recent decade, with the advent of additive manufacturing (AM) technologies, the manufacturing of Ti components using selective laser melting (SLM), electron beam melting (EBM), and directed energy deposition (DED) techniques emerged as one of the most important areas of Ti manufacturing.1,2,3,4 One challenge for the development of these manufacturing technologies is to have high-quality and low-cost spherical Ti alloy powder. Other advanced near–net-shape (NNS) manufacturing methods including metal injection molding (MIM) and hot isostatic pressing (HIP) also use spherical Ti or Ti alloy powders to make bulk materials and components. The critical characteristics of spherical Ti powder include particle size and size distributions, flowability, and chemical compositions, especially oxygen content. The requirements of particle size distribution (PSD) vary with applications: −45 μm for MIM, 20–45 μm for SLM, 10–45 μm for cold spraying, and 45–106 μm for EBM, as shown in Fig. 1. Oxygen is a strong solution strengthener for titanium material, but an excess will compromise ductility and fracture toughness.5,6,7,8 To meet the oxygen requirement of industrial standards for final manufactured components, which is less than 0.2 wt.%,9,10,11 most applications require the oxygen content in Ti powder to be less than 0.15 wt.%.
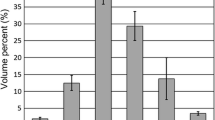
Commercial spherical Ti powder production methods include gas atomization (GA), plasma atomization (PA), and plasma rotating electrode process (PREP). The PREP powder is widely recognized to have very high purity and near-perfect spherical shape. Nevertheless, the particle size of PREP powder is typically coarser (e.g. 50–350 μm),12 as shown in Fig. 2, which is coarser than desired for SLM, EBM, or MIM applications. The finer spherical powder can, however, be produced via GA and PA methods. Typical particle sizes of GA and PA Ti alloy powders range from 10 μm to 300 μm.13 , 14 Although atomized powder can be classified to produce desired size cuts, classification reduces the yield of usable size cuts, further increasing the cost of the material. The low yield of fine powder produced by the current commercial methods is the main technical reason for the high cost of the powder used for the advanced NNS processes, especially for AM. Therefore, the recent R&D efforts have mostly been focused on improving the yield of fine powder (<45 µm) with an acceptable increase in operating and feedstock cost, which is mainly driven by the boost in the market of the powder-bed-based additive manufacturing. The “fine” powder used in this article refers to −325 mesh powder (<45 µm) unless otherwise noted. The improvements in this regard will be discussed in this article.
Curves of typifcal particle size distribution of Ti alloy powder produced by free-fall gas atomization (FFGA),13 electrode induction gas atomization (EIGA),31 plasma atomization (PA),14 and plasma rotating electrode process (PREP).12 Note: The PSD curves of EIGA, PA, and PREP are for Ti-6Al-4V. Ref 13 did not specify which Ti alloy it was. Reported PSD is quoted from the corresponding references. They are not meant to be the limit of the methods
Needless to say, the quality and performance of Ti alloy and components depend strongly on the quality and cost of the Ti alloy powders used. The AM application set the stringent requirements of chemical compositions and physical properties: high purity, high sphericity and flowability, and no trapped gas-bubble porosity. One challenge for the production of titanium powder is the control of oxygen content in the powder, especially fine powder. In general, the oxygen content of Ti powder is inversely proportional to the particle sizes.15 In other words, the smaller the particle size, the higher the oxygen content as shown in Fig. 3.14 Additionally, most NNS methods mentioned earlier require powder to have excellent flowability. The powder flowability can be affected by a few factors including powder shape and size, interparticle friction, type of material, and environmental factors.16,17,18 In general, powder with good flowability should have a spherical shape and the particle sizes should be reasonably large. The flowability of the powder decreases with decreasing particle size. Furthermore, the powders must have good apparent density and tap density, which also affect the density and uniformity of manufactured parts. In short, spherical Ti alloy powder with low oxygen and good flowability is in high demand. Unfortunately, high-quality spherical Ti alloy powders that meet these requirements, especially powders for AM Ti, are all very costly and in short supply, which hinders the development of Ti for broad applications using AM and other advanced manufacturing techniques. Therefore, a strong need exists in the industry to develop new methods for the production of low-cost Ti alloy powders that meet all requirements for chemical composition and physical properties. Therefore, in addition to reviewing the basic techniques, this article also examine the factors that have significant effects on the cost of spherical Ti alloy powder.
Strong dependence of oxygen content in (plasma-atomized) spherical Ti-6Al-4V powder on the particle size.14 (Four curves represent four runs of plasma atomization with different operating conditions.)
Atomization Techniques
Commercial spherical titanium powder in the current market is almost all produced by atomization methods or plasma spheroidization. There are a large variety of atomization techniques. Among them, GA, PA, and PREP are used commercially for the production of spherical Ti alloy powders. All atomization processes consist of three main integrated steps: melting, atomization, and solidification. Melting can be accomplished by techniques such as vacuum induction melting, plasma arc melting, induction drip melting, or direct plasma heating. Atomization is the process during which liquid metal is broken into droplets, which solidify during flight in a cooling chamber under inert gas protection. Atomization is normally accomplished using a high pressure gas to break up liquid stream through a nozzle. Droplets can also be formed by the spinning of a liquid stream off of a disc, causing molten droplets to form and undergo centrifugal acceleration away from the center of spin. The droplet will subsequently solidify during flight. The commercial atomizing processes are usually conducted in ultra-high-purity inert gas (Ar or He) to minimize oxygen pick up, which can usually be controlled within the range of 100–500 ppm depending on the specific process and size of the powder. Each specific atomization technique varies in details from others, but they all share the same three main steps as described earlier. These different techniques are presented and compared with each other below.
Gas Atomization
Gas atomization of titanium was originally developed by Crucible Materials Corporation in the 1980s.19 , 20 In this process, elemental raw materials or pre-alloyed titanium alloy ingots or bars are induction skull-melted in a water-cooled copper crucible under vacuum. Once the composition of alloy becomes homogenous after being held in the molten state for a certain period of time, the melt is poured into a refractory metal nozzle in a tundish and then atomized by high-pressure streams of inlet gas (Ar is usually chosen over helium for economic reasons). As the stream of molten metal falls freely as a result of gravitational forces for a certain distance before atomization, this technique is called free-fall gas atomization (FFGA). The basic configuration of the FFGA process is shown in Fig. 4a.
The gas-to-metal ratio (G/M) is one of the important parameters that determines the PSD of the gas-atomized powder.21 Lubanska developed an empirical equation for the relation between the d 50 (median particle size-the droplet size that corresponds to the 50% cumulative frequency) and the G/M:22
where K is a constant, D is the diameter of the melt stream, η m and η g are the kinematic viscosity of the metal and gas, respectively. M and G are the metal and gas flow rate, respectively. W is the Weber number (\( W = \frac{{V^{2} \rho_{\text{m}} D}}{{\gamma_{\text{m}} }} \), where ν is the gas velocity at the impact of the gas jets with the metal stream; ρ m, γ m are the density and surface tension of the melt, respectively). From the equation, it can be clearly seen that with a fixed gas nozzle design and melt composition, the particle size is dominated by the gas-to-metal ratio (G/M). In the other words, a higher G/M can result in a higher yield of fine powder. The G/M is thus indirectly related to the economics of atomization; it has to be optimized to minimize the production cost of fine powder. FFGA usually produces Ti powder in a wide size range (up to 500 μm).13 As shown in Fig. 2, the yield of fine powder is only around 15% with a reasonable G/M.13
The technique of close-coupled gas atomization (CCGA) was developed to increase the yield of fine powder.23 As shown in Fig. 4b, the melt is disintegrated by the direct impact of high-pressure gas right below the tip of an extended melt guide tube. Compared with FFGA, CCGA is a more efficient method of producing fine spherical powder by maximizing gas velocity and density in contact with metal. The investigation on CCGA dates back to the 1940s;24 nevertheless, it was only successfully used for making spherical titanium powder in the past decade25 , 26 because the choice of material for the guide tube is very limited. The inner surface of the guide tube has to be inert to the extremely reactive titanium melt. Researchers at Ames Laboratory fabricated composite pour tubes (Y2O3-W-YSZ, from interior to exterior),27 which enabled the atomization of titanium with the CCGA technique. In general, compared with FFGA, CCGA can produce powders with the same PSD with a relatively lower G/M as a result of its better atomization efficiency. The yield of fine powder by CCGA method can be much higher than that of the FFGA method. Nevertheless, there is no data of fine titanium powder yield of GGCA method in the archived literature to date.
As mentioned, molten titanium is very reactive to most common metals and ceramics; electrode induction gas atomization (EIGA) was developed by ALD Vacuum Technologies to produce “ceramic-free” powder, in which the melt is not in contact with any refractory metals or other ceramic components that might introduce contamination.28 , 29 As shown in Fig. 4c, a prealloyed rod (25–70 mm) is rotated at a very slow speed and is melted in a conical induction coil;30 then the melt falls into a gas nozzle to be broken up into small droplets. The diameter of the electrode rod can be increased to up to 90 mm31 or 120 mm28 to increase productivity. To minimize the possible pickup of contaminants during atomization, a gas-atomization apparatus with a Ti coating on the inner wall of the atomization chamber and other components in the flow path was designed and developed.32
Although gas atomization is a mature technology, there are a few issues worth noting. Fine particles are flown back to collide with partially molten particles as a result of the circulation of gas in the atomizing chamber, causing the formation of satellite particles (see Fig. 5a). The satellite particles have a negative influence on the free-flowing of the particles, which are thus not desired for some applications. Another issue with the gas atomization is that the high-pressure gas used for atomization may be trapped in the liquid metal, which would remain to become gas pores or gas bubbles in the powder. These gas pores cannot be entirely eliminated even by HIP,33 which is thus detrimental to the mechanical properties, especially fatigue properties.
SEM picture of Ti-6Al-4V powder produced by (a) gas atomization, (b) plasma atomization, (c) plasma rotating electrode process, (d) plasma spheroidization from −140 + 200 mesh HDH powder (reprinted with permission from Ref. 44), and (e) granulation-sintering-deoxygenation
Plasma Atomization
Plasma atomization (PA) was developed to produce fine and spherical powder in 1996.34,35 In the plasma atomization process, as shown in Fig. 4d, a pre-alloyed wire (e.g., 1/16” or 1/8”) is fed into a hot zone (around 10,000 K) heated by plasma torches. The wire is melted and broken into droplets that would cool rapidly. A typical cooling rate is in the range of 10–1000°C/s. Besides the feed material, another major difference between plasma atomization and gas atomization is that in PA, wire is melted and atomized by extremely high-temperature plasma simultaneously, whereas in GA, metal is melted by an induction coil or other source and then atomized by cold high-pressure gas. The plasma-atomized Ti powder has high purity because the liquid metal does not contact any refractory metals or other solid materials that may contaminate the powder before solidification.
In general, the yield of fine powder using the plasma wire atomization technique is significantly higher than that of conventional gas atomization processes. As shown in Fig. 2, the yield of fine powder from plasma atomization is greater than 40% for Ti-6Al-4V.14 The yield of fine powder and the capacity of plasma atomization can be adjusted by varying the diameter and the feed rate of the wire, the inlet gas pressure (or gas-to-metal ratio), the angle of attack between the wire and plasma jets, and the distance between the wire and the plasma outlet.36 It was reported that the yield of fine Ti64 powder can be improved from 39.9% to 59.6% by increasing the G/M from 8.7 to 12.9 and shortening the distance from the wire and the plasma outlet from 25 mm to 19 mm.37 Furthermore, the production rate can be significantly improved by adding an induction coil to preheat the wire before being fed into the plasma.36
As shown in Fig. 5b, the plasma-atomized powder has very good sphericity, and fewer satellites particles than the gas-atomized powder. Nevertheless, the issues of inner porosity resulting from trapped gas during atomization and satellite particles are still concerns for plasma-atomized Ti powder. The main drawback of this process is that the feedstock has to be in the form of wires. In addition to the high cost of Ti wires, this technique cannot be used to produce an alloy powder if the wire form of the alloy is not available (e.g., Ti3Al).
Plasma Rotating Electrode Process
The plasma rotating electrode process (PREP) is a refinement of a powder production method called the rotating electrode process (REP) that was developed by Nuclear Metals, Inc. in the 1960s.38 In the rotating electrode process, the metal electrode rod is melted by the arc from a tungsten-tipped cathode. The rod (usually with diameters of 89 mm or 63.5 mm) spins at a speed 3000–15,000 rpm,39 so the liquid melt is spun off from the electrode surface to form droplets because of centrifugal force. After that, the droplets solidify to form solid spherical particles during flight. S. Abkowitz reported the production of spherical Ti alloy (Ti-7Al-2Nb-1Ta) powder using REP in 1966, and the particle size was approximately 150 µm.40 Nevertheless, discrete tungsten particles were found in hot-isostatic-pressed Ti64 from REP powder, which is detrimental to the fatigue properties.41 Later, the heat source was replaced with a transferred arc plasma torch to avoid tungsten inclusion,12 as shown in Fig. 4e. Helium is preferred because of its improved heat transfer properties and electric arc characteristics.12
The plasma rotating electrode process is one of the most recognized techniques for making spherical Ti alloy powders, as a result of its advantage over other production methods. First, PREP Ti powder has high purity. As descried earlier, the liquid metal has no contact with other metals or ceramics before solidification. Also, the pickup of interstitial impurities (i.e., O, N) during the process is minimal as a result of its relatively large particle size or low specific surface area. Second, PREP Ti powder has no or minimal gas pores because the metal droplets are produced by centrifugal forces rather than by high-pressure gas. Third, PREP powder has fewer satellite particles compared with the other productions methods using high-pressure gas. As discussed earlier, the satellite particles formed during gas atomization are likely a result of the back flow of very fine particles to the spray plume. In PREP, the droplets fly radially away from the metal surface in a centrifugal force; in other words, it moves in order, so the chance of collisions of droplets and particles to form satellites is very low.39
Nevertheless, PREP also has its challenges. PREP typically produces spherical Ti64 powder in sizes ranging from 50 μm to 350 μm,12 as shown in Fig. 2. This particle size range is suitable for HIP powder metallurgy applications, whereas it is too coarse for powder-bed-based additive manufacturing or powder injection molding applications. The dependence of the mean particle size (d 50) on the rotation speed (S) and the electrode diameter (D e) is expressed in the following equation:12
where K is the materials constant determined by surface tension and density of the material. According to the equation, the yield of fine powder can be improved by increasing rotation speed and the diameter of the electrode, which has also been shown in experiments.42 It was reported that the yield of fine Ti alloy powder can be increased to ~16% using an electrode rod with a diameter of 100 mm and a rotating speed of 30,000 rpm.42 It should be noted that, with a bigger diameter for the electrode and higher rotating speed, the requirement of the precision of electrode dimensions is more stringent to minimize out-of-balance forces.39 It is also worth mentioning that the PSD can be adjusted by the electric current applied to the plasma arc and the distance between the tip of the plasma gun and the end of the rod.42
Other Methods
As discussed previously, the R&D efforts on conventional methods for producing low-cost spherical Ti powder were focused on increasing the yield of fine powder by modifying the design and optimizing the processing parameters. Recently, a few emerging technologies have been developed, aiming to produce more affordable spherical titanium powder.
Plasma Spheroidization
The plasma spheroidization (PS) of powders is a relatively new but popular technique. Plasma spheroidization of powders has been applied to a variety of different powders, including refractory metals such as tungsten.43
During plasma spheroidization, the metal powder is melted by a plasma torch and forms molten droplets, which solidify to form spherical solid powder before reaching the bottom of the reactor chamber.43 A unique characteristic of plasma spheroidization is that the particle sizes do not change during plasma processing. Plasma-spheroidized particles typically have the same nearly perfect round shape as the other atomized powders (Fig. 5d).44 Feedstock materials can be hydride-dehydride (HDH) powder,44 or any irregular shaped Ti powder made by a range of processes such as Armstrong process,45 and the FFC Cambridge process.46 Irregular-shape Ti powder by the HAMR process47 , 48 is expected to be able to be plasma-spheroidized as well. Another example is a continuous method during which low-cost Ti sponge fines, HDH powder, or electrolytically produced Ti and alloy powders are fed through a plasma transferred arc torch to make spherical alloy powder.49
The challenge for PS is to produce fine spherical Ti alloy powder with low oxygen at low cost. It was reported that using Ti hydride powder as feedstock helps to improve the yield of fine powder of plasma spheroidization.50 The impurity level of the plasma-spheroidized Ti powder is largely determined by the feed powder; however, the availability of low-oxygen and low-cost fine Ti powder is very limited and costly currently. Another potential issue of the PS process for making Ti alloy powder is at risk of losing the low-melting-point element (e.g., Al) as a result of evaporation at the plasma temperatures.
Granulation-Sintering-Deoxygenation
Recently, a new approach, called granulation-sintering-deoxygenation (GSD), for making spherical Ti powder was developed by the present authors.51,52 Figure 6 illustrates the key steps of this process. There are three main steps as indicated by its name: (I) Granulation-Ti alloy hydride or Ti hydride with master alloy (hydrogenated from Ti sponge or Ti alloy scrap) was milled to fine particles, and then granulated to spherical granules in the desired size range using spray-drying. (II) Sintering—The spherical granules are sintered to obtain dense Ti particles. (III) Deoxygenation—The densified spherical Ti powder with high oxygen content is deoxygenated with Mg to meet industry standards.
Flow chart of granulation-sintering-deoxygenation (GSD) method. (Reprinted with permission from Ref. 52)
The spherical fine Ti alloy powder by GSD method is low cost as it has much lower waste and is able to use low-cost starting powder. Most importantly, the yield from the GSD process is near 100%. The powder product from GSD process can be controlled in a very narrow PSD without much loss of yield as all the powder that is either under- or oversized particles can be recirculated through the process. It should be mentioned here that the de-oxygenation technology used in this process can also be used as a standalone process to reduce the oxygen content of recycled powder that may have oxygen content higher than 0.2 wt.% after repeated use through 3D-printing processes.53
There are a number of key issues when using this process to make spherical Ti powder. First, to produce fine spherical powders, the particle sizes of the initial powder must be less than a few microns. The finer the initial particle size, the better the granules will be with respect to sinter-ability. Nevertheless, the limiting factor is that the oxygen contents can increase with decreasing initial particle size, which needs to be managed. Second, particles may bond to each other during sintering. Therefore, measures must be taken to prevent the sintering of particles to each other. Figure 5e shows that the particles are discrete.
Spheroidization by Mechanical Means
In addition to spheroidizing or producing particles in a molten state, there are reports of modifying the particle shape in the solid state by mechanical means.54,55 The flowability of irregularly shaped powders was reportedly improved by removing sharp angles on the particles through high-speed blending or high sheer milling. Nonetheless, the particles produced by this method are only quasi-spherical shaped, which may limit its applications.
Conclusion
The features of the commercial and emerging spherical Ti powder making methods are summarized in Table I.
Recently, many large companies including GE, GKN, Praxair, and Carpenter Technology entered the market of spherical Ti powder, motivated primarily by the rapid expansion of additive manufacturing. The dominant commercial processes today are gas atomization, plasma atomization, and plasma rotating electrode process. Spherical Ti powder produced by any one of these three methods has its advantages as well as its disadvantages. Great progress has been made toward improving the yield of fine powder by these three relatively mature processes. Nevertheless, there is a strong market demand for further improvements to reduce the cost to make the fine powder affordable for broader range of end-use applications. Newer production techniques such as plasma spheroidization and the GSD process are yet to realize their market potential.
References
I. Gibson, D.W. Rosen, and B. Stucker, Additive Manufacturing Technologies: Rapid Prototyping to Direct Digital Manufacturing (New York: Springer, 2010).
D.D. Gu, W. Meiners, K. Wissenbach, and R. Poprawe, Int. Mater. Rev. 57, 133 (2012).
L.E. Murr, S.M. Gaytan, D.A. Ramirez, E. Martinez, J. Hernandez, K.N. Amato, P.W. Shindo, F.R. Medina, and R.B. Wicker, J. Mater. Sci. Technol. 28, 1 (2012).
B.E. Carroll, T.A. Palmer, and A.M. Beese, Acta Mater. 87, 309 (2015).
R.I. Jaffee and I.E. Campbell, Met. Trans. 185, 646 (1949).
Z. Liu and G. Welsch, Metall. Trans. A 19, 527 (1988).
R. Boyer, G. Welsch, and E.W. Collings, Materials Properties Handbook—Titanium Alloys (Materials Park: ASM International, 1994), p. 239.
T. Horiya and T. Kishi, Nippon Steel Tech. Rep. 62, 85 (1994).
ASTM F2885, Standard Specification for Metal Injection Molded Titanium-6 Aluminum-4 Vanadium Components for Surgical Implant Applications (West Conshohocken: ASTM International, 2011).
ASTM F1580, Standard Specification for Titanium and Titanium-6 Aluminum-4 Vanadium Alloy Powders for Coatings of Surgical Implants (West Conshohocken: ASTM International, 2012).
ASTM F2924, Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion (West Conshohocken: ASTM International, 2014).
P.R. Roberts, Proceedings 1989 PM Conference: Advances in Powder Metallurgy (San Diego: Metal Powder Industries Federation, 1989), pp. 427–438.
J.H. Moll, JOM 52, 32 (2000).
M.E. Smagorinski and G. Tsantrizos, Proceedings of the 2002 World Congress on Powder Metallurgy & Particulate Materials (Orlando: Metal Powder Industries Federation, 1989).
C.G. McCracken, C. Motchenbacher, and D.P. Barbis, Int. J. Powder Metall. 46, 19 (2010).
L.C.Y. Chan and N.W. Page, Powder Technol. 90, 259 (1997).
J.W. Carson and B.H. Pittenger, ASM Handbook, Volume 7: Powder Metal Technologies and Applications, ed. P.W. Lee, Y. Trudel, R. Iacocca, R.M. German, B.L. Ferguson, W.B. Eisen, K. Moyer, D. Madan, and H. Sanderow (Materials Park: ASM International, 1998), pp. 287–301.
A.B. Spierings, M. Voegtlin, T. Bauer, and K. Wegener, Prog. Addit. Manuf. 1, 9 (2016).
C.F. Yolton, Proceedings of PM in Aerospace and Defense Technologies, ed. F.H. Froes (Seattle: Metal Powder Industries Federation, 1989), pp. 123–131.
C.F. Yolton, U.S. Patent 5,084,091, 1992
B. Zheng and F.J. Lavernia, Handbook of Atomization and Sprays: Therory and Applications, ed. N. Ashgriz (New York: Springer, 2011), pp. 837–848
H. Lubanska, JOM 22, 45 (1970).
S. Motaman, High-Speed Imaging and Computational Modelling of Close-Coupled Gas Atomization (Leeds: University of Leeds, 2013).
J.S. Thompson, J. Inst. Metall. 74, 101 (1948).
A.J. Heidloff, J.R. Rieken, I.E. Anderson, and D. Byrd, Proceedings of 2011 International Conference on Powder Metallurgy And Particulate Materials (San Francisco: Metal Powder Industries Federation, 2011).
A.J. Heidloff, J.R. Rieken, I.E. Anderson, D. Byrd, J. Sears, M. Glynn, and R.M. Ward, JOM 62, 35 (2010).
I.E. Anderson, A.J. Heidloff, J.R. Rieken, and D.J. Byrd, PowderMet 2010: Advances in Powder Metallurgy & Particulate Processing (Hollywood: Metal Powder Industries Federation, 2010), pp. 33–44.
H. Franz, L. Plochl, and F.P. Schimansky, Proceedings of 24th Annual ITA Conference Titanium 2008 (Las Vegas: International Titanium Association, 2008).
M. Hohman, N. Ludwig, U.S. Patent 5,284,329 A, 1994
S. Pleier, W. Goy, B. Schaub, M. Hohmann, M. Mede, and R. Schumann, 2004 International Conference on Powder Metallurgy &Particulate Materials (Princeton: Metal Powder Industries Federation, 2004), pp. 2–49.
W. Garcia, Titanium Europe Conference 2015 (Birmingham, UK: International Titanium Association, 2015).
W.M. Hanusiak, D.R. McBride, Patent Application 13/414,769, 2013
R. Cunningham, A. Nicolas, J. Madsen, E. Fodran, E. Anagnostou, M.D. Sangid, and A.D. Rollett, Mater. Res. Lett. (2017). http://dx.doi.org/10.1080/21663831.2017.1340911
M. Entezarian, F. Allaire, P. Tsantrizos, and R.A.L. Drew, JOM 48, 53 (1996).
P.G. Tsantrizos, F. Allaire, M. Entezarian, U.S. Patent 5,707,419, 1998.
C.A.D. Dion, W. Kreklewetz, P. Carabin, Plasma apparatus for the production of high quality spherical powders at high capacity. Publication number WO2016191854 A1, 2016
F. Larouche, M. Balmayer, F. Trudeau-lalonde, Plasma atomization metal powder manufacturing processes and systems therefore. Publication number WO2017011900 A1, 2017
A.R. Kaufmann, U.S. Patent 3,099,041, 1963.
S.A. Miller and P.R. Roberts, ASM Handbook Volume 7, Powder Metal Technologies and Applications (Materials Park: ASM International, 1990), pp. 97–101.
S. Abkowitz, JOM 18, 458 (1966).
R.F. Vaughan, P.A. Blenkinsop, and P.H. Morton, Titanium and Titanium Alloys: Scientific and Technological Aspects, Vol. 3, ed. J.C. Williams and A.F. BelovSpringer, (MA: Boston, 1982), pp. 2377–2388.
Y. Dai and L. Li, Adv. Mater. Ind. (in Chinese) 2016, 57 (2016).
M. Boulos, Metall. Powder Rep. 59, 16 (2004).
D.P. Barbis, R.M. Gasior, G.P. Walker, J.A. Capone, and T.S. Schaeffer, Titanium Powder Metallurgy: Science, Technology and Applications, ed. M. Qian and F.H. Froes (Oxford: Butterworth-Heinemann, 2015), pp. 101–105.
K. Araci, D. Mangabhai, and K. Akhtar, Titanium Powder Metallurgy: Science, Technology and Applications, ed. M. Qian and F.H. Froes (Oxford: Butterworth-Heinemann, 2015), pp. 149–162.
I. Mellor, L. Grainger, K. Rao, J. Deane, M. Conti, G. Doughty, and D. Vaughan, Titanium Powder Metallurgy: Science, Technology and Applications, ed. M. Qian and F.H. Froes (Waltham: Elsevier, 2015), pp. 51–67.
Y. Zhang, Z.Z. Fang, Y. Xia, Z. Huang, H. Lefler, T. Zhang, P. Sun, M.L. Free, and J. Guo, Chem. Eng. J. 286, 517 (2016).
Y. Zhang, Z.Z. Fang, Y. Xia, P. Sun, B. Van Devener, M. Free, H. Lefler, and S. Zheng, Chem. Eng. J. 308, 299 (2017).
J.C. Withers, R.O. Loutfy, U.S. Patent 7,985,326 B2, 2011
C.C. Liu, X. Lu, L. Zhang, W.L. Song, J.B. Tong, S.D. Yang, and X.H. Qu, Powder Metall. 59, 229 (2016).
Z.Z. Fang, Y. Xia, P. Sun, Y. Zhang, U.S. Patent 9,421,612, 2016
P. Sun, Z.Z. Fang, Y. Xia, Y. Zhang, and C. Zhou, Powder Technol. 301, 331 (2016).
Y. Zhang, Z.Z. Fang, P. Sun, T. Zhang, Y. Xia, C. Zhou, and Z. Huang, J. Am. Chem. Soc. 138, 6916 (2016).
G. Gai, Y. Yang, L. Jin, X. Zou, and Y. Wu, Powder Technol. 183, 115 (2008).
Y.Y. Sun, S. Gulizia, C.H. Oh, C. Doblin, Y.F. Yang, and M. Qian, JOM 67, 564 (2015).
Acknowledgements
The authors acknowledge the funding support by the Advanced Research Project Agency for Energy (ARPA-E) of the U.S. DOE (DE-AR0000420) through the Modern Electro/Thermochemical Advances in Light-Metal Systems (METALS) program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, P., Fang, Z.Z., Zhang, Y. et al. Review of the Methods for Production of Spherical Ti and Ti Alloy Powder. JOM 69, 1853–1860 (2017). https://doi.org/10.1007/s11837-017-2513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2513-5