Abstract
Honey bees play a crucial role in agricultural production. Farmers rely on commercial bee pollination to obtain optimal yields, and beekeepers on the income from pollination fees. However, commercial pollination confronts honey bees with pests and pathogens, pesticides, and low-quality food, which in many cases do not fulfill the minimal nutritional requirements of honey bees. In this work, we evaluated honey bee nutrition in avocado and blueberry plantations under organic and conventional management, by assessing the nutritional quality of pollen and bee bread based on their protein, fatty acid, and microbial content, and by analyzing honey bee health through the examination of abdominal fatty acid profiles and head protein content. Low protein content in honey bee hemolymph was evident under conventional management. Regardless of management, avocado pollen showed higher protein levels, which were translated into higher honey bee head protein levels being 60% higher in avocado under conventional management when compared to blueberry under the same management. However, higher protein levels in avocado pollen were also translated into lower amounts of fatty acids in bee bread and in honey bee fat. In particular, the total amount of fatty acids in bee bread from conventional management was 50% lower than that from organic management. Additionally, the saturated fatty acids in conventional blueberries were lower compared to that of organic blueberries. Crop system and bee bread microbial composition altered the plant-honey bee nutrition cascades transforming pollen to bee bread with increased amount of saturated, unsaturated, and total fatty acids. In conclusion, both crop species and crop systems determine honey bee nutrition through alterations in the pollen transformation, affecting protein and fatty acid assimilation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
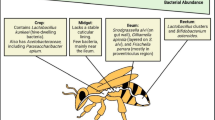
Honey bees are among the most important pollinators, playing a crucial role in agroecosystems (Reilly et al. 2020). Semi-domestication of bees allows beekeepers to provide pollination services with hives to farmers at a commercial scale assuring world food supply (Aizen and Harder 2009). However, agricultural crops do not always fulfill the minimal honey bee nutritional demands in terms of protein and carbohydrates (Colwell et al. 2017). Pollen and nectar are the only sources of proteins and lipids for honey bees (Corby-Harris et al. 2018). After pollen collection by forager honey bees, the pollen is mixed with honey, inoculated with microorganisms, and stored. After hatching, honey bees start to consume bee bread, which is known to be important in relation to larval growth, longevity, body size and ovary development (Vanderplanck et al. 2014). Biochemical transformation of pollen into bee bread is mainly made by yeasts (Haydak 1958) and Lactobacillus spp. (Chevtchik 1950), transforming pollen into a rich and complex food, increasing nutrient bioavailability of carbohydrates, enzymes, vitamins, and lactic acids (Khalifa et al. 2020). Besides pollen pre-digestion, pollen-borne microorganisms play an important role for bee nutrition and deficiencies of microorganisms in their diet have been shown to cause poor development and survival (Dharampal et al. 2019). In hives, microorganisms are transmitted between honey bees via social contact between nestmates and by adult bees feeding recently emerged honey bees. Hence, the bee microbiome is well conserved, but susceptible to environmental changes, diseases, feeding habits, and pesticide exposition (Kwong and Moran 2016; Diaz et al. 2019).
Protein is one of the key elements of honey bee nutrition and is naturally obtained by plant pollen. The nutritional value of pollen is defined by its protein percentage and amino acid composition (Crailsheim 1990), which has been shown to fluctuate from 9 to 37% depending on the origin (Liolios et al. 2015). Lipids are also an important component for honey bee nutrition, which are crucial in the production of fat, glycogen, and cell membranes (Graham 1992). Oleic and palmitic fatty acids are two of the principal fatty acids found in honey bees (Manning 2001) and imbalance of these fatty acids may cause honey bee learning deficiencies (Arien et al. 2018; Bennett et al. 2022) and alterations in immune reactions (Bedick et al. 2001). Fatty acids not only can give us insight of the honey bee nutritional status, but can also provide qualitative and quantitative information of the microbial community through specific microbial biomarkers (Grubbs et al. 2015).
As before mentioned, bee bread fermentation is made principally by microorganism like yeast and bacteria. Pesticides like fungicides, herbicides, and insecticides are known to have strong non-target effects on bee bread microorganisms and in this way indirectly affect honey bee health and nutrition (El Agrebi et al. 2020).
Protein and lipid ratios define the pollen preferences of bees (Vaudo et al. 2024) according to the nutritional requirements of the colony and the amount of essential fatty acids in pollen (Zarchin et al. 2017; Hendriksma et al. 2019). The fatty acid composition of pollen plays a significant role in shaping the normal development of the colony (Arien et al. 2020). Therefore, the availability of adequate resources in the environment is crucial. Diversified floral resources in agroecosystems provide spatial and temporal nutrient supply (Decourtye et al. 2010). In contrast, conventional crop systems with monoculture represent a challenge for honey bee nutrition (Colwell et al. 2017) since pollen and nectar nutritional values differ between crops of which some have low levels of essential fatty acids, amino acids, and minimal protein content to fulfill the honey bee nutritional needs to support colony development. In conventional crop systems, pesticides adversely affect honey bee nutrition through intensive herbicide applications which reduce bee food sources (Bretagnolle and Gaba 2015). In addition, pesticides can lead to alterations in bee bread microbial composition (El Agrebi et al. 2020). Added to this, in general, pesticides negatively affect fatty acid metabolism by suppressing fatty acid synthesis (Erban et al. 2019) and by reducing food consumption and sugar levels in the hemolymph (Tosi et al. 2017).
Avocado (Persea americana Mill 1768) and blueberry (Vaccinium corymbosum L. 1753) are two high value crops that require optimal fertilization and pest management practices to achieve maximum yield. These practices can involve the use of either biological methods (organic) or agrochemicals (conventional), such as pesticides and mineral fertilizers (Aktar et al. 2009). In Mexico, avocado is one of the leading crops produced in large areas as monocultures, while blueberry is commonly produced under closed plastic tunnels limiting natural pollination (Ramírez-Mejía et al. 2022; Wu and Guan 2021; Kendall et al. 2021). The direct effects of agricultural management have been widely studied in terms of honey bee pesticide exposure, but knowledge about the indirect effects of agricultural management on honey bee nutrition is limited (Sánchez-Bayo et al. 2016). Pesticides can reach honey through different routes, including oral intake, inhalation, or contact (Crenna et al. 2020).
Here, we studied the effects of crop system (organic and conventional) and crop type (avocado and blueberry) on honey bee nutritional cascades focusing on fatty acids and proteins in pollen, bee bread, and in honey bees. We hypothesized that conventional management would have negative effects on honey bee nutrition cascades reducing potential honey bee food sources. Our prediction is that honey bee nutrition depends on both crop species and management with conventional blueberry systems representing the lowest nutritional value for honey bees.
Materials and methods
Field site location
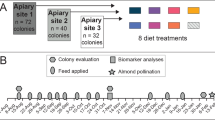
In central Mexico, newly created nucleus hives were established in avocado and blueberry agroecosystems both with organic and conventional production systems. Organic systems were certified by third-party organizations according to the federal regulatory program for organic produced agricultural products. Conventional sites followed the guidance of the secretary of agriculture and rural development (Secretariat of Agriculture 2006). Sites with similar size plots and characteristics (climate, age of the crop, weed management, pesticide, and fertilization schemes) were selected in avocado and blueberry (blueberry: organic 19°22′13.3"N 101°28′54.4"W, and conventional 19°22′37.7"N 101°29′21.6"W and avocado: Organic 19°28′16.9"N 102°01′32.3"W, and conventional 19°28′49.1"N 102°01′24.8"W).
45 days before peak blooming, 10 nucleus hives with a newly mated hybrid Italian/Africanized queen obtained from a local supplier and three frames of different age brood and one as reserve (honey and bee bread) were placed in each site. Hives were supplied with empty frames with organic certified bee wax foundation. At peak blooming, and after one month of hive placement, three randomly selected hives were sampled per crop, management, and site (n = 3). Only colonies showing normal development, with a solid brood pattern and a healthy amount of brood, eggs, larvae, pupae, and young adults covering all the frames, were selected for the study. These colonies had varroa levels below 1%, measured by alcohol wash (Gregorc and Sampson 2019) and showed no visible signs of other diseases. In each hive, a capped frame with pupae ready to emerge was caged with a metallic mesh frame with a 3 mm opening to allow trophallaxis, while preventing the bees from escaping. Emerged bees were marked daily on the thorax with a color code, and after 7 days, ten adult honey bees per hive were sampled for subsequent analysis. Also, five grams of randomly collected bee bread was collected from newly introduced foundation frames, using an alcohol disinfected spatula.
Pollen
Pollen from the different sites and crops above mentioned was collected from flower anthers of three randomly selected plants per site, a compound sample was made for each plant. Pollen was cold macerated using 1 ml of saline phosphate buffer, vortexed 10 s, and the supernatant was separated by centrifugation 1 min at 3000 g. Hereafter, pollen protein from the supernatant was measured using a BCA Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions at 595 nm. Final protein concentration was calculated according to the corresponding standard curve (Degrandi-Hoffman et al. 2015). Fatty acid methyl esters (FAMEs) were extracted from 200 mg of hand collected pollen in a three-step analysis: saponification with sodium hydroxide, methanol, and water for 30 min at 100 °C; methylation with chlorohydric acid and methanol for 10 min at 80 °C and extraction with hexane and methyl tert-butyl methyl ether. Fatty acids were detected on a gas chromatograph (Agilent 7890B) at 300 °C equipped with a fused silica capillary column and analyzed by Sherlock software version 3.1 (MIDI Inc. Delaware, USA). Fatty acid identification was made by comparison with an external calibration standard and quantification with the addition of an internal standard 19:0 (nonadecanoic methyl ester, Sigma) of known concentration and comparing peak areas. To determine pollen quality, saturated, unsaturated, and total fatty acid biomarkers were summed and compared in the different treatments.
Bee bread
Floral origin of bee bread was determined by microscopy at 400 ×magnification in an Olympus BX41 microscope, according to the standard procedures adapted for bee bread (Bakour et al. 2019). Identification was made by morphological comparison with pollen collected from the main crop and with the pollen atlas database (pollenatlas.net, accessed on November 22, 2022) of 500 grains per sample. Two hundred milligrams of bee bread per hive was homogenized with a mortar and pestle at 4 °C until no visible intact grains were observed at 400 ×magnification. Hereafter, bee bread pollen diversity and microbial composition were evaluated by FAME analysis as described above. For each treatment, fatty acid biomarkers were grouped according to taxonomy (eukaryotic and prokaryotic) and type (saturated, unsaturated, and total). To compare the relative contribution of the prokaryotic and eukaryotic fatty acids in the bee bread, we summed iso and ante-iso and monounsaturated fatty acids as biomarkers of prokaryotes, while eukaryotic fatty acids were calculated by summing polyunsaturated fatty acids (Table 1). Protein percentage was measured as before mentioned.
Bee head
After thawing, heads of the bees collected earlier were dissected and crushed in a mortar containing a phosphate buffer solution. The protein was separated from debris by centrifugation at 3000 rpm for 10 min and the protein percentage was assessed as before mentioned.
Bee fat body
Fatty acids were extracted from bee recently crushed abdomens according to the analysis as described above. Fat body quality was assessed by comparing the amount of saturated, unsaturated, and total fatty acids.
Honey bee gut yeasts and Lactobacillus
In order to identify the beneficial microorganisms associated with honey bee guts, nine dissected honey bee midguts per site, three for each hive, were added to two different broths. MRS (Man, Rogosa and Sharpe) to promote Lactobacillus growth and YEPD for yeasts (yeast extract, peptone, and dextrose) acidified to a pH of 4.5 adding HCl 1N as needed. Broth enrichment cultures were incubated for 72 h at 37 °C for MRS and 30 °C for YEPD. Hereafter, 40 mg of cells from the exponential growth phase was harvested and characterized by biomarker fatty acid analysis as described above.
Statistics
Fatty acids in pollen, bee bread and abdomens, pollen protein, and bee head protein were analyzed with generalized linear mixed model (GLMM) with gamma distribution, using crop and management as fixed effects and random effect for hive ID was included (Bates 2010). For visualization of the dataset, a principal component analysis (PCA) was performed in R studio (Team, R. C. 2013 version 1.2.5019) with Factoextra package (Kassambara 2017). Structural equation models were used to test the direct and indirect effects of pollen protein and fatty acids on honey bee nutrient assimilation, all the plausible variables and their interactions are tested by d-separation test for the evaluation of possible missing paths among unconnected variables (Shipley and Douma 2021). Overall fit of the network of causality was made by piecewiseSEM and model acceptance based on Fisher´s C statistics (α > 0.05) and selecting the model with the best global goodness of fit with the piecewiseSEM package (Lefcheck 2016) in R studio (Team, R. C. 2013 version 1.2.5019).
Results
Protein in pollen was significantly higher in avocado than in blueberry, regardless management type (Fig. 1A). In the same manner, bee bread protein content showed higher levels in avocado when compared with blueberry (Fig. 1B). Neither in pollen protein nor in bee bread, significant interactions were observed between management and crop.
Saturated fatty acids of pollen from both avocado and blueberry plantations were significantly higher with organic management compared to conventional management. In conventional management, pollen from blueberry had even lower levels of saturated fatty acids when compared with avocado pollen (Fig. 2A). In terms of unsaturated fatty acids, pollen from avocado with conventional management showed the highest levels, whereas conventional blueberry showed the lowest. The opposite was observed with organic management, where the amount of unsaturated fatty acids was higher in blueberry than in avocado (Fig. 2B). In terms of total fatty acids, the only difference observed was that blueberry pollen from conventional management had the lowest amount of total fatty acids (Fig. 2C).
Microscopy analysis of bee bread showed that in organic avocado, 78% of identifiable pollen belongs to the main crop, 83% in conventional avocado, 92% in organic blueberry, and 92% in conventional blueberry.
Total fatty acids in bee bread showed significant differences for crop management, with organic management resulting in higher levels of total fatty acids in bee bread than conventional (Fig. 3A). Saturated fatty acids of bee bread showed higher levels in organic blueberry than in organic avocado, and for conventional management, lower levels of saturated fatty acids in bee bread were observed in avocado and blueberry crops (Fig. 3B). In terms of unsaturated fatty acids in bee bread, no significant differences were found, neither for crop management nor for crop (Table 1).
The ratio between eukaryotic and prokaryotic fatty acid biomarkers in bee bread was higher in organic than in conventional management (Fig. 4A) and higher in blueberry than in avocado (Fig. 4B), while no interaction was observed for these two factors. Yeast biomarkers in bee bread showed no significant differences neither for crop management nor for crop (Table 2). Lactobacillus biomarkers in bee bread were higher in organic management when compared with conventional, whereas no significant differences were found for the factor crop (Fig. 5). Protein percentage of the bee head was significantly higher in avocado in both organic and conventional management, whereas bees from conventional blueberry showed the lowest level of head protein percentage (Fig. 6).
The amount of saturated fatty acids in bee abdomen was highest in organic avocado and lowest in conventional avocado (Fig. 7). No differences were observed between the treatments for unsaturated fatty acids in honey bee abdomen.
The first two components of the principal component analysis contributed to the 61.6% of the experiment variance. Eigenvalues and factor loadings of the principal component analysis are presented in Table 3. In the first component (Dim1), a clear separation was observed between the conventional avocado and blueberry groups. In the first component (Dim 1), conventional blueberry group is in the bottom followed by conventional avocado, organic blueberry, and in the top, organic avocado. This separation is caused principally by an increase in saturated fatty acids in bee bread, total fatty acids in bee bread, saturated fatty acids in pollen, and unsaturated fatty acids in bee bread (Fig. 8).
The structural equation model of honey bee nutrition cascades best fitted model p = 0.27, explained 68% of the variation in bee bread protein, 67% in bee bread microorganisms, 76% of bee protein, 80% in linoleic fatty acid, and 92% on oleic fatty acid. Oleic and linoleic acids in fat were significantly positively correlated with total fatty acid in pollen. Besides, bee bread protein, bee protein, linoleic acid in fat, and bee microorganism abundance in pollen were positively correlated with pollen protein. In addition, bee bread microorganisms were positively correlated with total fatty acids in pollen and linoleic fatty acid with unsaturated fatty acids of bee bread. Finally, oleic acid in fat was negatively correlated with bee bread protein and multigroup analysis showed that all paths are constrained to the global models showing no differences depending on the management or crop (Fig. 9).
Structural equation best fitted model (p = 0.27) of nutrition cascades in honey bees. Green and red arrows are significant positive and negative coefficients. The number in the circles represent regression coefficients. Only significant regressions are presented, and d-separation test showed no significant missing paths
Discussion
Here, we show that both crop species and crop system determine honey bee nutrition, though crop species to a higher extent than crop system. Overall, our results clearly show that avocado pollen provides higher-quality honey bee nutrition than blueberry pollen. As predicted, crop species and system significantly affected honey bee nutrition with the strongest contrast found in conventional blueberry providing lowest values for honey bee nutrition. Crops, define the availability of alternative pollen sources to the main crop, being blueberry the one with the poorest pollen diversity in bee bread, most likely because bees are kept inside plastic macro tunnels. Also, avocado crops in the area are widely distributed in large extensions in the avocado strip, preventing bees to obtain alternative sources of pollen to the main crop (Saenz-Ceja et al. 2022). In addition, regulatory programs prohibit weeds in crops destined to exportation reducing alternative sources of pollen (De la Federación 2002). Our results showing low pollen protein in blueberry is in accordance with Colwell et al. (2017), which has been further shown to lead to poor colony development (Dufour et al. 2020), since such low protein levels do not fulfill the minimal honey bee nutritional requirements (Haydak 1970). Though a slight increase in protein content in bee bread compared to pollen was seen for blueberry systems, this was still lower than what was found for avocado systems. Assimilation of protein in honey bee head seems to be linked with both crop species and management, where bees from plantations with low pollen protein percentage showed low levels of protein in the head, which was further underlined in conventional crops. Protein is important for nursing, hypopharyngeal, and mandibular glands, which is provided to larvae and young bees by nurse bees storing and disseminating protein (Crailsheim 1990). After emerging, honey bee head protein has been shown to increase by 92% (Haydak 1934), which is crucial for carbohydrate metabolism, antioxidant activities, detoxification, and learning (Zaluski et al. 2020).
The observed increase in saturated fatty acids in pollen from organic agroecosystems could be attributed to several factors associated with organic farming practices. Organic management often involves the use of natural fertilizers and reduced pesticide application, which may contribute to alterations in the fatty acid profile of pollen. Overall, the amount of fatty acids, saturated as well as unsaturated, was higher in bee bread than in pollen, most likely mediated by microbial fermentation and the addition of other pollen sources. However, though avocado pollen from conventional agroecosystems had the highest amount of unsaturated fatty acids, this was not transformed into higher levels of unsaturated fatty acids in bee fat. Unsaturated fatty acids play an important role in honey bee nutrition (Manning 2001) and disease prevention (Feldlaufer et al. 1993).
The ratio between eukaryotic (plants and fungi) and prokaryotic (bacteria) fatty acids can provide information about the general microbial composition in the bee bread during pollen fermentation revealing useful information about the relative abundance of microorganisms and their fatty acid contribution to honey bee nutrition. Interestingly, in the present study, this ratio differed both between crop species and management system. Organic management increased the eukaryotic/prokaryotic fatty acid ratio and in terms of crop species. Bee bread from avocado agroecosystems showed a better balance between eukaryotic and prokaryotic biomarkers compared to blueberry. Bee bread microbial diversity and abundances are related to pollen origin and nutrients (Vásquez and Olofsson 2009) and can be altered by pesticide exposition (Bartlewicz et al. 2016). Myristic, palmitic, and linoleic fatty acids play an important role in honey bee attraction to pollen (Manning 2001), which, in the present study, were more abundant in bee bread from organic agroecosystems than in the conventional ones.
Contrary to other bees, honey bees seem to lack the ability to select pollen with desirable nutritional characteristics (Corby-Harris et al. 2018), which, however, in this study, was quantified from corbicular pollen without considering possible effects of pollen-borne microorganisms. Indeed, it has been shown that pollen-borne microorganisms like yeasts and Lactobacillus represent a decision choice for the bees controlled by volatile emissions (Rering et al. 2018). Pollen microorganisms not only release basic nutrients to bees, but also play a direct role in bee nutrition (Dharampal et al. 2019). Principal component analysis showed clear differences in the crop species and management studied, because of the changes in the fatty acid profiles, protein content of bee bread and pollen, and in bee nutrient acquisition. Although the specific mechanisms underlying these differences require further investigation, it is possible that variations in management practices and pesticide usage, or nutrient availability might be responsible for the observed differences. Several studies have tested the effects of organic farming on the nutritional profiles of the crops and the consequent effects on human nutrition (Capouchová et al. 2021; Maggio et al. 2013; Montgomery and Biklé 2021). However, to our knowledge, this is one of the first studies to evaluate this on bees. As an overall synthesis of our work, the structural equation model showed how fatty acid and protein assimilation is mediated by different drivers in the nutrition cascades, which should be taken into consideration, when studying honey bee nutrition. Despite the negative effects of monocultures in the honey bee nutrition, pollen diversity, protein, and bee bread microorganisms honey bee nutrition cascades seem to be very well conserved, in concordance with the nutrition pathways in bees (Kunieda et al. 2006).
This study presents basic findings regarding fatty acid and protein cascades in honey bees. The main results show that both crop species and management systems alter fatty acid and protein assimilation but not the honey bee nutrition cascades. Site replication is labor intensive due to the difficulty of finding sites with similar characteristics, but in future experiments, this should be considered thoroughly to improve the robustness of the results. Our findings suggest that pollen quality should not only be addressed with protein content, but that also fatty acids should be taken into consideration, which is important in honey bee health and nutrition.
References
Aizen MA, Harder LD (2009) The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr Biol 19:915–918
Arien Y, Dag A, Yona S, Tietel Z, Cohen TL, Shafir S (2020) Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J Insect Physiol 124:104074
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1
Arien Y, Dag A, Shafir S (2018) Omega-6:3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Front Psychol 9:1001
Bartlewicz J, Pozo MI, Honnay O, Lievens B, Jacquemyn H (2016) Effects of agricultural fungicides on microorganisms associated with floral nectar: susceptibility assays and field experiments. ESPR 23:19776–19786
Bates D (2010) lme4: linear mixed-effects models using S4 classes. http://CRAN.R-project.org/package=lme4
Bedick JC, Tunaz H, Aliza AN, Putnam SM, Ellis MD, Stanley DW (2001) Eicosanoids act in nodulation reactions to bacterial infections in newly emerged adult honey bees, Apis mellifera, but not in older foragers. Comp Biochem Physiol Part C 130:107–117
Bennett MM, Welchert AC, Carroll M, Shafir S, Smith BH, Corby-Harris V (2022) Unbalanced fatty acid diets impair discrimination ability of honey bee workers to damaged and healthy brood odors. J Exp Biol 225:jeb244103
Bretagnolle V, Gaba S (2015) Weeds for bees? A review. ASD 35:891–909
Capouchová I, Kouřimská L, Pazderů K, Škvorová P, Božik M, Konvalina P, Dvořáček V (2021) Fatty acid profile of new oat cultivars grown via organic and conventional farming. J Cereal Sci 98:103180
Colwell MJ, Williams GR, Evans RC, Shutler D (2017) Honey bee-collected pollen in agro-ecosystems reveals diet diversity, diet quality, and pesticide exposure. Ecol Evol 7:7243–7253
Corby-Harris V, Snyder L, Meador C, Ayotte T (2018). Honey bee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS one, 13(1): e0191050
Crailsheim K (1990) The protein balance of the honey bee worker. Apidologie 21:417–429
Crenna E, Jolliet O, Collina E, Sala S, Fantke P (2020) Characterizing honey bee exposure and effects from pesticides for chemical prioritization and life cycle assessment. Environ Int 138:105642
Chevtchik V (1950) Mikrobiologie pyloveho kvaseni. Publ Fac Sci Univ Masaryk 323:103–130
Dharampal PS, Carlson C, Currie CR, Steffan SA (2019) Pollen-borne microbes shape bee fitness. Proc R Soc Lond Ser B 286:20182894
Decourtye A, Mader E, Desneux N (2010) Landscape enhancement of floral resources for honey bees in agro-ecosystems. Apidologie 41:264–277
Degrandi-Hoffman G, Eckholm B, Huang M (2015) Methods for comparing nutrients in beebread made by Africanized and European honey bees and the effects on hemolymph protein titers. JoVE 97:e52448
De la Federación DO (2002) NORMA Oficial Mexicana NOM-066-FITO-2002, Especificaciones para el manejo fitosanitario y movilización del aguacate. Primera sección (49)
Diaz T, del-Val E, Ayala R, Larsen J, (2019) Alterations in honey bee gut microorganisms caused by Nosema spp. and pest control methods. Pest Manag Sci 75:835–843
Dufour C, Fournier V, Giovenazzo P (2020) Diversity and nutritional value of pollen harvestedby honey bee (Hymenoptera: Apidae) colonies during lowbush blueberry and cranberry (Ericaceae) pollination. Can Entomol 152:622–645
El Agrebi N, Tosi S, Wilmart O, Scippo ML, de Graaf DC, Saegerman C (2020) Honeybee and consumer’s exposure and risk characterisation to glyphosate-based herbicide (GBH) and its degradation product (AMPA): residues in beebread, wax, and honey. Sci Total Environ 704:135312
Erban T, Sopko B, Talacko P, Harant K, Kadlikova K, Halesova T, Pekas A (2019) Chronic exposure of bumblebees to neonicotinoid imidacloprid suppresses the entire mevalonate pathway and fatty acid synthesis. J Proteom 196:69–80
Feldlaufer MF, Knox DA, Lusby WR, Shimanuki H (1993) Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 24:95–99
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617
Gilliam M (1979) Microbiology of pollen and bee bread: the yeasts. Apidologie 10:43–53
Graham JM (ed) (1992) The hive and the honey bee. Dadant & Sons, Hamilton, p 1324
Gregorc A, Sampson B (2019) Diagnosis of Varroa Mite (Varroa destructor) and sustainable control in honey bee (Apis mellifera) colonies—a review. Diversity 11:243
Grubbs KJ, Scott JJ, Budsberg KJ, Read H, Balser TC, Currie CR (2015). Unique honey bee (Apis mellifera) hive component-based communities as detected by a hybrid of phospholipid fatty-acid and fatty-acid methyl ester analyses. PLoS One, 10(4): e0121697
Haydak MH (1934) Changes in total nitrogen content during the life of the imago of the worker honeybee. US Government Printing Office
Haydak MH (1958) Pollen-pollen substitutes-beebread. Amer Bee J 98:145–146
Haydak MH (1970) Honey bee nutrition. Annu Rev Entomol 15:143–156
Hendriksma HP, Toth AL, Shafir S (2019) Individual and colony level foraging decisions of bumble bees and honey bees in relation to balancing of nutrient needs. Front Ecol Evol 7:177
Kassambara A (2017) Practical guide to principal component methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra (Vol. 2). Sthda
Kendall LK, Evans LJ, Gee M, Smith TJ, Gagic V, Lobaton JD, Rader R (2021) The effect of protective covers on pollinator health and pollination service delivery. Agric Ecosyst Environ 319:107556
Khalifa SA, Elashal M, Kieliszek M, Ghazala NE, Farag MA, Saeed A, El-Seedi HR (2020) Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci Technol 97:300–316
Kroppenstedt RM (1992) The genus Nocardiopsis. In: Balows A, Trüpper HG, Harder DMW, Schleifer KH (eds) The Prokaryotes, vol 2. Springer, Berlin, pp 1139–1156
Kunieda T, Fujiyuki T, Kucharski R, Foret S, Ament SA, Toth AL, Maleszka R (2006) Carbohydrate metabolism genes and pathways in insects: insights from the honey bee genome. Insect Mol Biol 15:563–576
Kwong WK, Moran NA (2016) Gut microbial communities of social bees. Nat Rev Microbiol 14:374
Lefcheck JS (2016) “piecewiseSEM: piecewise structural equation modeling in R for ecology, evolution, and systematics. MEE 7:573–579
Liolios V, Tananaki C, Dimou M, Kanelis D, Goras G, Karazafiris E, Thrasyvoulou A (2015) Ranking pollen from bee plants according to their protein contribution to honey bees. J Apicult Res 54:582–592
Maggio A, De Pascale S, Paradiso R, Barbieri G (2013) Quality and nutritional value of vegetables from organic and conventional farming. Sci Hortic 164:532–539
Manning R (2001) Fatty acids in pollen: a review of their importance for honey bees. Bee World 82:60–75
Montgomery DR, Biklé A (2021) Soil health and nutrient density: beyond organic vs. conventional farming. Front Sustain Food Syst 5:417
O’leary WM, (1988) Gram positive bacteria. Microbial Lipids 1:117–202
Ramírez-Mejía D, Levers C, Mas JF (2022) Spatial patterns and determinants of avocado frontier dynamics in Mexico. Reg Environ Change 22:28
Ratledge C, Wilkinson SG (1998) Microbial lipids. Ratledge C, Wilkinson SG (eds). London: AP
Reilly JR, Artz DR, Biddinger D, Bobiwash K, Boyle NK, Brittain C, Winfree R (2020) Crop production in the USA is frequently limited by a lack of pollinators. Proc R Soc Lond B Biol Sci 287:20200922
Rering CC, Beck JJ, Hall GW, McCartney MM, Vannette RL (2018) Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol 220:750–759
Sáenz-Ceja JE, Sáenz-Reyes J, Castillo-Quiroz D (2022) Pollinator species at risk from the expansion of avocado monoculture in Central Mexico. Conservation 2:457–472
Sánchez-Bayo F, Goulson D, Pennacchio F, Nazzi F, Goka K, Desneux N (2016) Are bee diseases linked to pesticides?—a brief review. Environ Int 89:7–11
Secretariat of Agriculture (2006, 07 de febrero) DOF. https://www.gob.mx/cms/uploads/attachment/file/718371/Organic_products.pdf
Shipley B, Douma JC (2021) Testing piecewise structural equations models in the presence of latent variables and including correlated errors. Struct Equ Model 28:582–589
Tosi S, Nieh JC, Sgolastra F, Cabbri R, Medrzycki P (2017) Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc R Soc Lond Ser B 284:20171711
Team R C (2013) R: a language and environment for statistical computing
Van Aarle IM, Olsson PA (2003) Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl Environ Microbiol 69:6762–6767
Vanderplanck M, Leroy B, Wathelet B, Wattiez R, Michez D (2014) Standardized protocol to evaluate pollen polypeptides as bee food source. Apidologie 45:192–204
Vásquez A, Olofsson TC (2009) The lactic acid bacteria involved in the production of bee pollen and bee bread. J Apicult Res 48:189–195
Wu F, Guan Z (2021) An overview of the Mexican blueberry industry: FE1106, 12/2021. EDIS. https://doi.org/10.32473/edis-fe1106-2021
Zaluski R, Bittarello AC, Vieira JCS, Braga CP, de Magalhaes PP, da Silva FM, de Oliveira OR (2020) Modification of the head proteome of nurse honeybees (Apis mellifera) exposed to field-relevant doses of pesticides. Sci Rep 10:1–11
Zarchin S, Dag A, Salomon M, Hendriksma HP, Shafir S (2017) Honey bees dance faster for pollen that complements colony essential fatty acid deficiency. Behav Ecol Sociobiol 71:1–11
Vaudo AD, Dyer LA, Leonard AS (2024). Pollen nutrition structures bee and plant community interactions. Proceedings of the National Academy of Sciences, 121(3): e2317228120
Funding
We thank the National Council of Science and Technology (CONACyT) for the PhD stipend 568609 and for the founding with the National Strategic Program (PRONACE) 316049.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial and non-financial interest related to the work to declare.
Additional information
Handling Editors: Michael Lattorff and Ingeborg Menzler-Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diaz, T., del-Val, E., Vega, E. et al. Honey bee protein and lipid nutrition in avocado and blueberry agroecosystems with conventional and organic management. Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10078-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10078-1