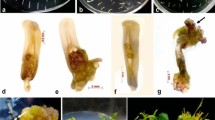
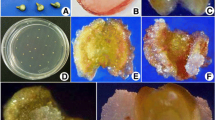
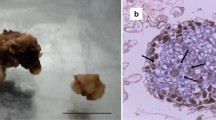
Abstract
Somatic embryos were induced from sliced unpollinated ovaries of cocoa. The influence of genotypes and cold pre-treatment were studied on the induction of callus and haploid embryogenesis. Among the five cocoa genotypes studied, CCRP 5 and CCRP 1 had recorded a maximum callus induction frequency of 66.00% and 62.00%, respectively, from sliced ovaries on WPM medium supplemented with 2-iP (1.0 mg L−1), Zeatin (1.0 mg L−1), and AgNO3 (5.0 mg L−1). Sliced ovaries isolated from cold pre-treated (4 °C for 1 day), 4–6 mm long (containing mature ovule) flower buds recorded the maximum callus induction frequency (67%). The highest percentage of embryogenic calli was noticed from ovaries of pre-treated (4 °C for 1 day) flower buds of CCRP 5 (31.00%). During proliferation and sub-culturing, callus morphogenesis such as white compact, light creamy nodular, proliferative beige shaded embryogenic, and light brown watery spongy non-proliferative calli were observed. Induction of globular and heart stage somatic embryos was noticed in WPM medium supplemented with ascorbic acid (35.2 mg L−1), Zeatin (1.0 mg L−1), Kinetin (3.0 mg L−1), and sucrose (30.0 g L−1). Further, the cotyledonary stage embryos and shoot conversion were observed in WPM medium supplemented with MgSO4 (4.0 g L−1), K2SO4 (12.0 g L−1), glucose (1.0 g L−1), and sucrose (30.0 g L−1). Histological and scanning electron microscopic studies revealed an asynchronous pattern of somatic embryos development (globular, heart, and torpedo stage) from embryogenic calli. The molecular confirmation of embryogenic competence with different types of ovary callus at different stages was confirmed with the detection of the TcSERK gene through semi-quantitative RT-PCR. The TcSERK gene expression was higher in embryogenic friable calli and lower in callus with early embryo induction. Flow cytometry analysis revealed that cells from ovary calli were haploids (1n = 10). This study would be a starting step for the induction of haploid embryogenesis from sliced ovaries of the well-adapted regional genotypes of cocoa, for obtaining rapid homozygosity; as induction of haploids through androgenesis in an earlier study could not yield fruitful results.








Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- WPM:
-
Woody plant medium
- TcSERK:
-
Theobroma cacao L. Somatic embryogenesis receptor like kinase
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- MgSO4 :
-
Magnesium sulfate
- K2SO4 :
-
Potassium sulfate
- SE:
-
Somatic embryogenesis
- AgNO3 :
-
Silver nitrate
- ANOVA:
-
Analysis of variance
- FAA:
-
Formalin–acetic acid–alcohol
- ESEM:
-
Environmental scanning electron microscope
- MgCl2 :
-
Magnesium chloride
- MOPS:
-
3-Morpholinopropane-1-sulfonic acid
- PI:
-
Propidium iodide
- mm:
-
Millimetre
- pg:
-
Picogram
- 2ip:
-
6-(Gamma, gamma-Dimethylallylamino) purine
References
Afoakwa EO (2016) Chocolate science and technology. Wiley, Chichester
Ajijah N, Hartati RS, Rubiyo R, Sukma D, Sudarsono S (2016) Effective cacao somatic embryo regeneration on kinetin supplemented DKW medium and somaclonal variation assessment using SSRS markers. Agrivita J Agric Sci 38(1):80–92
Alemanno L, Berthouly M, Michaux-Ferriere N (1996) Histology of somatic embryogenesis from floral tissues cocoa. Plant Cell Tissue Organ Cult 46(3):187–194
Badu M, Tripathy B, Sahu GS, Jena AK (2017) Role of doubled haploids in vegetable crop improvement. J Pharm Phytochem 6(6):384–389
Baudino S, Hansen S, Brettschneider R, Hecht VF, Dresselhaus T, Loerz H, Rogowsky PM (2001) Molecular characterization of two novel maize LRR receptor- like kinases, which belong to the SERK gene family. Planta 213(1):1–10
Bekele FL, Bekele I, Butler DR, Bidaisee GG (2006) Patterns of morphological variation in a sample of cacao (Theobroma cacao L.) germplasm from the International Cocoa Genebank, Trinidad. Genet Resour Crop Evol 53(5):933–948
Benelli C, Germana MA, Ganino T, Beghe D, Fabbri A (2010) Morphological and anatomical observations of abnormal somatic embryos from anther cultures of Citrus reticulata. Biol Plant 54(2):224–230
Bhat JG, Murthy HN (2007) Factors affecting in-vitro gynogenic haploid production in niger (Guizotia abyssinica (L. f.) Cass.). Plant Growth Regul 52(3):241–248
Bhojwani SS, Dantu PK (2013) Plant tissue culture: an introductory text. Springer, India
Bohanec B (2009) Doubled haploids via gynogenesis. In: Advances in haploid production in higher plants. Springer, Dordrecht, pp 35–46
Cardoso JC, Abdelgalel AM, Chiancone B, Latado RR, Lain O, Testolin R, Germana MA (2016) Gametic and somatic embryogenesis through in vitro anther culture of different Citrus genotypes. Plant Biosyst Int J Deal All Asp Plant Biol 150(2):304–312
Chen JF, Cui L, Malik AA, Mbira KG (2011) In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tissue Organ Cult 104(3):311–319
Couto EGDO, Pinho EVDRV, Pinho RGV, Veiga AD, Bustamante FDO, Dias KODG (2015) In vivo haploid induction and efficiency of two chromosome duplication protocols in tropical maize. Ciência e Agrotecnologia 39:435–442
Dan Y (2008) Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Biol-Plant 44(3):149–161
Diao WP, Jia YY, Song H, Zhang XQ, Lou QF, Chen JF (2009) Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenerants using SSR markers. Sci Hortic 119(3):246–251
Doi H, Hoshi N, Yamada E, Yokoi S, Nishihara M, Hikage T, Takahata Y (2013) Efficient haploid and doubled haploid production from unfertilized ovule culture of gentians (Gentiana spp.). Breed Sci 63(4):400–406
Dpoolezel J, Binarová P, Lcretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31(2):113–120
El-Mahrouk ME, Maamoun MK, El-Banna AN, Omran SA, Dewir YH, El-Hendawy S (2018) In vitro gynogenesis and flow cytometry analysis of the regenerated haploids of black cumin (Nigella sativa). HortScience 53(5):681–686
Eshaghi ZC, Abdollahi MR, Moosavi SS, Deljou A, Segui-Simarro JM (2015) Induction of androgenesis and production of haploid embryos in anther cultures of borage (Borago officinalis L.). Plant Cell Tissue Organ Culture (PCTOC) 122(2):321–329
Falque M, Kodia A, Sounigo O, Eskes A, Charrier A (1992) Gamma-irradiation of cacao (Theobroma cacao L.) pollen: effect on pollen grain viability, germination and mitosis and on fruit set. Euphytica 64(3):167–172
Fayos O, Valles MP, Garces-Claver A, Mallor C, Castillo AM (2015) Doubled haploid production from Spanish onion (Allium cepa L.) germplasm: embryogenesis induction, plant regeneration and chromosome doubling. Front Plant Sci 6(384):1–11
Figueira A, Janick J (1995) Somatic embryogenesis in cacao (Theobroma cacao L.). In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 2. Klumer Academic Publishers, Netherlands, pp 291–310
Figueira A, Janick J, Goldsbrough P (1992) Genome size and DNA polymorphism in Theobroma cacao. J Am Soc Horticult Sci 117(4):673–677
Gallego Rua AM, Henao Ramirez AM, Urrea Trujillo AI, Atehortua Garces L (2016) Polyphenols distribution and reserve substances analysis in cacao somatic embryogenesis. Acta Biologica Colombiana 21(2):335–345
Germana MA (2011) Anther culture for haploid and doubled haploid production. Plant Cell Tissue Organ Cult 104(3):283–300
Grewal S, Ahuja A, Atal CK (1980) Clonal multiplication of medicinal plants by tissue culture. In vitro proliferation of shoot apices of Eucalyptus citriodora Hook. Indian J Exp Biol 18:775–776
Gurel S, Gurel E, Kaya Z (2000) Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep 19(12):1155–1159
Hadziabdic D, Wadl PA, Reed SM (2011). Haploid cultures. Plant Tissue Cult Dev Biotech pp 385–396
Han Y, Wei A, Zhang L, Liu N, Zhang G, Zhao G (2010) A new cucumber cultivar’Jinmei 3’bred by unfertilized ovary culture. Acta Horticulturae Sinica 37(3):509–510
Han Y, Wei A, Liu N, Chen Z, Zhang Y, Li P (2019) A new cucumber cultivar for protected cultivation “Kerun 99.” Acta Horticulturae Sinica 46(3):609–610
Hazarika RR, Mishra VK, Chaturvedi R (2013) In vitro haploid production—a fast and reliable approach for crop improvement. Crop improvement under adverse conditions. Springer, New York, pp 171–212
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, De Vries SC (2001) The Arabidopsis SERK 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127(3):803–816
Huang X, Lu XY, Zhao JT, Chen JK (2010) MaSERK1 gene expression associated with somatic embryogenic competence and disease resistance response in banana (Musa spp.). Plant Mol Biol Report 28(2):309–316
Koli SP, Murthy HN (2013) Haploid plant regeneration from unpollinated ovules of Cucumis melo L. var. conomon cv. Mudicode. Biotech J Int 3(4):605–613
Kurtar ES, Balkaya A, Ozer MO (2018) Production of callus mediated gynogenic haploids in winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.). Czech J Genetics Plant Breed 54(1):9–16
Lentini Z, Gonzalez A, Tabares E, Buitrago ME, Wedzony M (2020) Studies on gynogenesis induction in cassava (Manihot esculenta Crantz) unpollinated ovule culture. Front Plant Sci 11(365):1–14
Li Z, Traore A, Maximova S, Guiltinan MJ (1998) Somatic embryogenesis and plant regeneration from floral explants of cacao (Theobroma cacao L.) using thidiazuron. In Vitro Cell Dev Biol-Plant 34(4):293–299
Li JW, Si SW, Cheng JY, Li JX, Liu JQ (2013) Thidiazuron and silver nitrate enhanced gynogenesis of unfertilized ovule cultures of Cucumis sativus. Biol Plant 57(1):164–168
Li F, Cheng Y, Zhao X, Yu R, Li H, Wang L, Li S, Shan Q (2020) Haploid induction via unpollinated ovule culture in Gerbera hybrida. Sci Rep 10(1):1–9
Liu L, Wang J, Zhao H, Yang H, Zhang F (2015) Studies on embryo induction from un-pollinated ovary culture and regenerated plant in cucumber. China Veg 6:48–53
Lloyd GB, McCown BH (1980) Commercially feasible micropropagation of mountain laurel (Kalmia latifolia) by use of shoot tip culture. Proc-Int Plant Propagators’ Soc 30:421–437
Lopez-Puc G, Herrera-Cool GJ, Alberto UV, Ramos-Diaz A, Gongora-Canul CC, Aguilera-Cauich EA, Martínez-Sebastián G (2021) In vitro gynogenesis of Jatropha curcas L. var ALJC01. Trop Subtrop Agroecosyst 24(1):1–10
Ma J, He Y, Hu Z, Xu W, Xia J, Guo C, Lin S, Cao L, Chen C, Wu C, Zhang J (2012a) Characterization and expression analysis of AcSERK2, a somatic embryogenesis and stress resistance related gene in pineapple. Gene 500(1):115–123
Ma J, He YH, Wu CH, Liu HP (2012b) Cloning and molecular characterization of a SERK gene transcriptionally induced during somatic embryogenesis in Ananas comosus cv. Shenwan. Plant Mol Biol Report 30(1):195–203
Maximova SN, Alemanno L, Young A, Ferriere N, Traore A, Guiltinan MJ (2002) Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma cacao L. In Vitro Cell Dev Biol-Plant 38(3):252–259
Minyaka E, Niemenak N, Sangare A, Omokolo DN (2008) Effect of MgSO4 and K2SO4 on somatic embryo differentiation in Theobroma cacao L. Plant Cell Tissue Organ Cult 94(2):149–160
Mishra VK, Chaturvedi R (2012) In vitro haploid production–fast forward technique for improved crop production. Botanica 59(61):35–42
Mishra VK, Gowswami R (2014) Haploid production in higher plant. Int J Chem Biol Sci 1:26–45
Modeste KK, Eliane MT, Daouda K, Brahima SA, Tchoa K, Kouablan KE, Mongomaké K (2017) Effect of antioxidants on the callus induction and the development of somatic embryogenesis of cocoa [Theobroma cacao (L.)]. Aust J Crop Sci 11(1):25–31
Montalban IA, Garcia-Mendiguren O, Goicoa T, Ugarte MD, Moncalean P (2015) Cold storage of initial plant material affects positively somatic embryogenesis in Pinus radiata. New for 46(2):309–317
Nath S, Mallick SK, Jha S (2014) An improved method of genome size estimation by flow cytometry in five mucilaginous species of Hyacinthaceae. Cytometry A 85(10):833–840
Nolan KE, Irwanto RR, Rose RJ (2003) Auxin upregulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133(1):218–230
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the Somatic Embryogenesis Receptor-Like Kinase1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60(6):1759–1771
Pazuki A, Aflaki F, Gurel E, Ergul A, Gurel S (2018) Gynogenesis induction in sugar beet (Beta vulgaris) improved by 6-benzylaminopurine (BAP) and synergized with cold pretreatment. Sugar Tech 20(1):69–77
Perez-Nunez MT, Souza R, Sáenz L, Chan JL, Zuniga-Aguilar JJ, Oropeza C (2009) Detection of a SERK-like gene in coconut and analysis of its expression during the formation of embryogenic callus and somatic embryos. Plant Cell Rep 28(1):11–19
Popova T, Grozeva S, Todorova V, Stankova G, Anachkov N, Rodeva V (2016) Effects of low temperature, genotype and culture media on in vitro androgenic answer of pepper (Capsicum annuum L.). Acta Physiol Plant 38(11):1–11
Porras-Murillo R, Andrade-Torres A, Solis-Ramos LY (2018) Expression analysis of two somatic embryogenesis receptor kinase (SERK) genes during in vitro morphogenesis in Spanish cedar (Cedrela odorata L.). 3 Biotech 8(11):1–9
Puddephat IJ, Robinson HT, Smith BM, Lynn J (1999) Influence of stock plant pretreatment on gynogenic embryo induction from flower buds of onion. Plant Cell Tissue Organ Cult 57:145–148
Ramirez AMH, De la Hoz VT, Osorio TMO, Garcés LA, Trujillo AIU (2018) Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa (Theobroma cacao L.) via somatic embryogenesis. Sci Hortic 229:148–156
Rekha HR, Rakhi C (2013) Establishment of dedifferentiated callus of haploid origin from unfertilized ovaries of tea (Camellia sinensis (L.) O. Kuntze) as a potential source of total phenolics and antioxidant activity. In Vitro Cell Dev Biol-Plant 49(1):60–69
Santa-Catarina C, Hanai LR, Dornelas MC, Viana AM, Floh EI (2004) SERK gene homolog expression, polyamines and amino acids associated with somatic embryogenic competence of Ocotea catharinensis Mez.(Lauraceae). Plant Cell Tissue Organ Cult 79(1):53–61
Santana M, Velasquez S, Mata J (2010) Carbon source effect on cacao organogenesis and somatic embryogenesis. Cytogenetic analysis. Agronomía Tropical 60(2):193–202
Santos M, Romano E, Yotoko KSC, Tinoco MLP, Dias BBA, Aragao FJL (2005) Characterisation of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Sci 168(3):723–729
Santos MO, Romano E, Vieira LS, Baldoni AB, Aragao FJL (2009) Suppression of SERK gene expression affects fungus tolerance and somatic embryogenesis in transgenic lettuce. Plant Biol 11(1):83–89
Schellenbaum P, Jacques A, Maillot P, Bertsch C, Mazet F, Farine S, Walter B (2008) Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27(12):1799–1809
Schmidt ED, Guzzo F, Toonen MA, De Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124(10):2049–2062
Shalaby TA (2007) Factors affecting haploid induction through in vitro gynogenesis in summer squash (Cucurbita pepo L.). Sci Hortic 115(1):1–6
Sharma SK, Millam S, Hein I, Bryan GJ (2008) Cloning and molecular characterization of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 228(2):319
Shimada T, Hirabayashi T, Endo T, Fujii H (2005) Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CitSERK1) from Citrus unshiu Marc. Scientia Horticulturae-Amsterdam 103(2):233–238
Singla B, Khurana JP, Khurana P (2008) Characterization of three somatic embryogenesis receptor kinase genes from wheat Triticum aestivum. Plant Cell Rep 27(5):833–843
Sivachandran R, Gnanam R, Sudhakar D, Suresh J, Ganesh Ram S (2017a) Influence of genotypes, stages of microspore, pre-treatments and media factors on induction of callus from anthers of cocoa (Theobroma cacao L.). J Plant Crop 45(3):162–172
Sivachandran R, Gnanam R, Sudhakar D, Suresh J, Ganesh Ram S, Gangai Selvi R (2017b) Development of in vitro gynogenesis system in cocoa (Theobroma cacao L.). Chem Sci Rev Lett 6(24):2169–2177
Somleva MN, Schmidt EDL, De Vries SC (2000) Embryogenic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep 19(7):718–726
Sorntip A, Poolsawat O, Kativat C, Tantasawat PA (2017) Gynogenesis and doubled haploid production from unpollinated ovary culture of cucumber (Cucumis sativus L.). Can J Plant Sci 98(2):353–361
Srivastava P, Chaturvedi R (2008) In vitro androgenesis in tree species: an update and prospect for further research. Biotechnol Adv 26(5):482–491
Svirshchevskaya AM, DolezeF J (2000) Production and performance of gynogenetic sugarbeet lines. J Sugar Beet Res 37(4):117–133
Tan C, Furtek D (2003) Development of an in vitro regeneration system for Theobroma cacao from mature tissues. Plant Sci 164(3):407–412
Tantasawat PA, Sorntip A, Poolsawat O, Chaowiset W, Pornbungkerd P (2015a) Evaluation of factors affecting embryo-like structure and callus formation in unpollinated ovary culture of cucumber (Cucumis sativus). Int J Agric Biol 17(3):613–618
Tantasawat PA, Sorntip A, Pornbungkerd P (2015b) Effects of exogenous application of plant growth regulators on growth, yield, and in vitro gynogenesis in cucumber. HortScience 50(3):374–382
Thomas TD, Bhatnagar AK, Razdan MK, Bhojwani SS (1999) A reproducible protocol for the production of gynogenic haploids of mulberry, Morus alba L. Euphytica 110(3):169–173
Tomaszewska-Sowa M, Keutgen AJ (2021) Plant regeneration from unpollinated ovules of sugar beet (Beta vulgaris L.) on growing media with different carbohydrates. Sugar Tech 1–9
Traore A, Guiltinan MJ (2006) Effects of carbon source and explant type on somatic embryogenesis of four cacao genotypes. Hortic Sci 41(3):753–758
Ulrich I, Ulrich W (1991) High-resolution flow cytometry of nuclear DNA in higher plants. Protoplasma 165(1–3):212–215
Voora V, Bermúdez S, Larrea C (2019) Global Market Report: Cocoa. International Institute for Sustainable Development
Yang C, Zhao TJ, Yu DY, Gai JY (2011) Isolation and functional characterization of a SERK gene from soybean [Glycine max (L.) Merr.]. Plant Mol Biol Report 29(2):334–344
Zheng L, Ma J, Mao J, Fan S, Zhang D, Zhao C, An N, Han M (2018) Genome-wide identification of SERK genes in apple and analyses of their role in stress responses and growth. BMC Genomics 19(1):1–18
Zou T, Su HN, Wu Q, Sun XW (2018) Haploid induction via unfertilized ovary culture in watermelon. Plant Cell Tissue and Organ Culture (PCTOC) 135(2):179–187
Zou T, Song H, Chu X, Tong L, Liang S, Gong S, Yanga H, Sun X (2020) Efficient induction of gynogenesis through unfertilized ovary culture with winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.). Sci Hortic 264:109152
Acknowledgements
The authors thank the Department of Spices and Plantation crops, Tamil Nadu Agricultural University for explants source; Sanjaya K Mallick, Cytometry Solution Pvt. Ltd. for flow cytometry experiment and T. G. Menon, Microtomy Lab, Chennai for Histology experiment.
Funding
The authors thank Mondelez International Private Ltd., U.S for providing financial support to carry out the research work.
Author information
Authors and Affiliations
Contributions
RG, RS, DS, and MR conceived and designed the experiments. VJ maintained cocoa accessions in the field for the collection of flower buds. RG, RS, RNS, and KR performed the experiments and analysed the results. RG, RS, RNS, SR, and RR drafted the manuscript. RG, SR, RR, DS, and MR were responsible for the manuscript editing, and also supervision of the entire work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not Applicable.
Consent to participate
Not applicable.
Consent for publication
The work is presented in the manuscript with the consent of all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramasamy, G., Ramasamy, S., Ravi, N.S. et al. Haploid embryogenesis and molecular detection of somatic embryogenesis receptor-like kinase (TcSERK) genes in sliced ovary cultures of cocoa (Theobroma cacao L.). Plant Biotechnol Rep 16, 283–297 (2022). https://doi.org/10.1007/s11816-022-00756-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-022-00756-y