Abstract
Background
Cancer-related fatigue (CRF) is well documented in cancer survivors, but little is known about the personal and societal impact of CRF. This study aimed to examine the impact of CRF in relation to social and vocational functioning and health care utilization in a large sample of post-treatment cancer survivors.
Methods
We conducted a cross-sectional descriptive study of early stage breast and colorectal cancer survivors (n = 454) who were within 5 years from treatment completion. Social difficulties (SDI-21), work status, absenteeism and presenteeism (WHO-HPQ) and healthcare utilization (HSUQ) were compared in those with (CFR +) and without (CRF −) clinically significant fatigue (FACT-F ≤ 34).
Results
A total of 32% met the cut-off criteria for CRF (≤ 34). Participants with CRF + had significantly higher scores on the SDI-21 across all domains and 55% of CRF + vs. 11% in CRF − was above the SDI cut-off (> 10) for significant social difficulties. Participants with CRF + were 2.74 times more likely to be unemployed or on leave (95% CI 1.62, 4.61, p < 0.001). In the subgroup of participants who were currently working (n = 249), those with CRF + reported working on average 27.4 fewer hours in the previous 4 weeks compared to CRF − (p = 0.05), and absolute presenteeism was on average 13% lower in the CRF + group (95% CI 8.0, 18.2, p < 0.001). Finally, individuals with CRF + reported significantly more physician (p < 0.001), other health care professional (p = 0.03) and psychosocial visits (p = 0.002) in the past month.
Conclusions and implications for cancer survivors
CRF is associated with substantial disruption in social and work role functioning in the early transitional phase of cancer survivorship. Better management of persistent CRF and funding for the implementation of existing guidelines and recommended evidence-based interventions are urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In North America alone, there are over 19 million people living with a personal history of cancer [1,2,3,4], and this number is expected to grow by 24% over the next decade [3]. With increasing proportions of people now surviving cancer and transitioning into the extended survival phases of cancer care, the long-term effects of cancer and its treatments and previously unrecognised chronic morbidity and related disability are of increasing importance.
Cancer-related fatigue (CRF) [5] has been reported as a prevalent and disabling side effect of cancer treatment [6,7,8,9,10,11,12] and has been defined as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [13]. During cancer treatment, CRF is an almost universal symptom [14,15,16,17] and 25–40% of post-treatment survivors will experience persistent CRF (> 6 months) up to 10 years post-treatment completion [5, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
While the primary goal of cancer treatments is to eradicate the underlying disease, the long-term goal is for individuals to regain normalcy and return to pre-morbid social and vocational roles after completing treatment [32, 33]. However, CRF can result in significant disability and may disrupt this reintegration process [26, 28, 30, 34]. Despite this, and the evidence of effective interventions and guidelines to manage CRF [13, 17], it remains a poorly managed problem for both cancer patients and survivors [12, 19, 35,36,37]. This may be due to several factors. Oncology health care providers (HCP) may not routinely ask about fatigue[38] nor its impact on social functioning or vocational roles, and cancer survivors may not report it because they view it as unavoidable [19, 39]. HCPs may also underestimate the importance of CRF [11, 12, 36, 40] and are not always aware of effective interventions and access to supportive care services to manage CRF can be inconsistent and inaccessible [41,42,43,44,45,46,47,48].
To date, while the prevalence and predictors of CRF have been well documented in the literature, little research has focused on the impact of CRF on outcomes such as social functioning, work outcomes and health care utilization [49,50,51,52,53]. Understanding the personal and societal impact of CRF and its impact on cancer survivors’ reintegration to social and vocational roles can help provide a broader understanding and appreciation of its significance, identify targets and outcomes for interventional research and provide useful data to drive change to health care policy and remuneration systems. This study aimed to examine the impact of CRF in relation to social difficulties, work status, work absence (absenteeism), ability to perform work (presenteeism) and health care utilization in a large sample of post-treatment breast and colorectal cancer survivors. Breast and colorectal cancers were selected as they are highly prevalent cancers that are known to be associated with persistent CRF [26]. We hypothesised that cancer survivors suffering from CRF (CRF +) would have increased difficulty with social and vocational functioning and have higher health care and social service utilization compared to those who do not suffer from CRF (CRF −).
Methods
Study design and patient selection
This was a cross-sectional descriptive study conducted at Princess Margaret Cancer Centre and Mount Sinai Hospital in Toronto, Canada. Individuals were eligible if they (1) were within 1–5 years of completing primary treatment for early stage (0–III) breast or colorectal cancer, (2) were not receiving current cancer therapies other than adjuvant endocrine therapy, (3) did not have evidence of recurrent or metastatic disease, (4) spoke and read English and (5) were at least 18 years old. After screening for eligibility, individuals attending follow-up clinic visits were approached to participate. Patients who provided consent to participate were given the questionnaire package to complete and return in-clinic or via pre-paid postage envelope.
This study was reviewed and approved by the University Health Network Research Ethics Board and the Mount Sinai Hospital Research Ethics Board.
Study assessments
Demographic and clinical information
A Patient Information Questionnaire was used to assess demographic variables (age, sex, education, income, employment, marital status, ethnicity). Clinical data was extracted from the electronic patient record.
Cancer-related fatigue
CRF was assessed using the 13-item Functional Assessment of Cancer Therapy-Fatigue (FACT-F) subscale [54]. The FACT-F has been extensively used in a range of cancer populations [55, 56] and correlates well with the International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria for cancer-related fatigue [18]. Each item is answered on a five-point scale and scores range from 0 (maximum fatigue) to 52 (minimum fatigue) with lower scores indicating higher fatigue. The recommended cut-off score of ≤ 34 on the FACT-F has a sensitivity of 0.91 and a specificity of 0.75 and can accurately predict ICD status for CRF in 93% of patients [57].
Social functioning
The Social Difficulties Inventory (SDI) is a valid and reliable [58,59,60,61] 21-item questionnaire designed to assess social difficulties experienced by cancer patients over the preceding month [60, 62]. The SDI-21 is scored by calculating a 16-item summary score (SD-16), ranging from 0 to 44, which further comprises three factor analysis-derived subscales: Everyday Living, Money Matters and Self-and-Others [59]. A cut-off of ≥ 10 has been recommended to identify patients experiencing social distress and has 80% sensitivity and 75% specificity compared to clinical assessment, and a difference of 2 points on the sub-scales and 3 on the SD-16 are considered to represent a meaningful clinically important difference [59, 63].
Work status, absenteeism and presenteeism
Participants were asked about their work status and type of work. Participants who indicated that they were currently employed (full or part-time) or self-employed were then asked to complete the absenteeism and presenteeism questions of the World Health Organization’s Health and Work Performance Questionnaire short form (WHO-HPQ) [64, 65]. The WHO HPQ has good validity and reliability [66,67,68,69] and provides a measure of absenteeism and presenteeism. Absolute absenteeism focuses on the total number of hours an employee is absent, while relative absenteeism provides a more contextualised view by comparing the absenteeism rate to the expected number of hours by the employer. Absolute absenteeism (AA) is measured in terms of work hours lost in the past 4 weeks and reported in raw hours. A higher score on AA indicates more absenteeism (hours lost). Relative absenteeism (RA) is reported as the percentage of the hours one is expected to work and can range from a negative value (when a person works more than expected) to a maximum of 1.0 (when the person is always absent). Absolute presenteeism (AP) measures self-rated overall job performance on the days worked over the past 4 weeks and has a lower bound of 0 (worst possible performance) and an upper bound of 100 (best possible performance). Presenteeism is a measure of actual performance in relation to possible performance as rated by the individual. In this case, a higher score indicates a lower amount of lost performance. Relative presenteeism (RP) is the ratio of one’s self-rated performance compared to their rating of the performance of most workers at the same job. The distribution of RP is restricted to the range of 0.25 to 2.0, with the lowest score indicating the worst relative performance (25% or less of other workers’ performance), and the highest score the best performance (200% or more of other workers’ performance) [70]. Respondents were also asked to compare their overall job performance over the past 4 weeks with the performance of most other workers in the same job on a 7-point scale from “You were a lot better than others” to “You were a lot worse than others”.
Health service utilization
Health Service Utilization was assessed using the Health Services Utilization Questionnaire [71, 72] modified for use with a cancer population [73]. Service types are grouped into five categories: Physician visits, Other Health Professionals (nursing, allied health, pharmacist), Hospital visits and services, Psychosocial Services (professional counselling, support group, information supports, financial counselling or assistance programmes, spiritual support) and Home Support (community care access centre services, housekeeping, transportation services, home delivered meals). Participants were asked which of these services they have used in the past month including the frequency of use.
Statistical analysis
Participant characteristics were described for each cancer site (breast or colorectal). Participants were classified as having CRF if their FACT-F score was \(\le 34\) (CRF +). Categorical demographic correlates of CRF were compared with \({\chi }^{2}\) tests and Wilcoxon rank sum test for continuous variables. To assess the association between CRF and social functioning, independent sample t tests were conducted to compare mean SDI domain scores (everyday living, money matters and self) and the SDI-16 score between those CRF + and CRF − and presented on box plots to illustrate the distribution of SDI scores. Cohen’s d effect sizes were also calculated. Working status was classified into four categories: working (full or part-time), retired, on leave/disability or not in paid employment and compared between those CRF + and CRF − using a \({\chi }^{2}\) test. The odds ratio of working (full or part-time) vs. not working (not working/on leave), excluding retired persons, was also calculated. Comparisons of workplace performance, including AA, RA, AP and RP between the groups, were conducted using independent t tests for the sub-sample of patients who reported working full, part-time, or in self-employment (n = 228), and Cohen’s d effect sizes were calculated. Relative workplace performance scores were collapsed to three levels (better than others, about the same, worse than others), and responses between those CRF + and CRF − were compared using Fisher’s exact test. The use of health care resources, measured as number of visits in the previous month, was compared using independent sample t tests, and Cohen’s d effect sizes were calculated. For each research question, a Holm’s correction [74] was applied to control for multiple testing, and results were considered statistically significant if the adjusted two-sided p-value was < 0.05. Sensitivity analyses were conducted using multivariate regression to determine if age or cancer type influenced the findings as follows: logistic modelling of the risk of CRF + as a function of SDI score, age and cancer type for each SDI domain, the odds of working as a function of CRF + , age and cancer type, and linear modelling of the number of health care visits as a function of CRF + , age and cancer type. Analyses were conducted using R version 4.2.2 [75] and all tests were two-sided.
Results
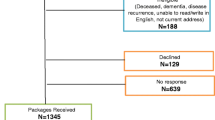
A total of 625 eligible patients were approached and 454 consented and returned the completed questionnaire (overall participation rate 73%; breast (n = 302) 75% and colorectal (n = 152) 68%). Demographic and clinical data are presented in Table 1. Participants were on average 2.4 years from end of treatment (median, 2.2 years; range, 0.2–5.0 years). Just over half the sample (54%) was born in Canada and most were living with a partner (67%).
Of the 454 participants, the mean (sd) score on the FACT-F was 38.0 ± 11.5, and 147 (32%) met the cut-off criteria for CRF (≤ 34). No demographic or clinical variables were significantly associated with the presence of CRF.
Social functioning
CRF + was associated significantly with higher scores on the SDI across all domains (Fig. 1): everyday living (5.0 ± 3.9 vs. 1.1 ± 1.7, p < 0.001, Cohen’s d 1.13), money matters (3.6 ± 3.9 vs. 1.3 ± 1.9, p < 0.001, Cohen’s d 0.68), self (4.3 ± 3.3 vs. 1.6 ± 1.9, p < 0.001, Cohen’s d 0.87) and on overall social distress (SD-16) (12.8 ± 9.5 vs. 4.0 ± 4.2, p < 0.001, Cohen’s d 1.04). Fifty-five percent of individuals’ CRF + and 11% of those CRF − were above the SDI cut-off (> 10) for significant social difficulties.
Work status and performance
Participants with CRF + had significantly different work profiles than those CRF − (Fig. 2) and more likely to be not working or on leave/disability (OR = 2.72 95% CI 1.62, 4.61, p < 0.001).
In the subgroup of participants who were currently working (n = 249), those who were CRF + reported working on average 27.4 fewer hours in the previous 4 weeks vs. those who were CRF − (95% CI 5.3, 49.4, p = 0.05) (Table 2). There was no significant difference in absolute or relative absenteeism scores. However, absolute presenteeism for those CRF + was on average 13% lower (95% CI 8.0, 18.2, p < 0.001). Furthermore, those with CRF + reported a greater discrepancy between themselves and other workers in their jobs (relative presenteeism), than those CRF − (95% CI 0.1, 0.2, p < 0.001).
Reported relative performance of workers with and without fatigue was also compared, and there was a significant difference in the proportion of participants with fatigue who rated their work performance as better vs. worse than their co-workers (Fig. 3) (p < 0.001).
Health care use
The proportion of participants with and without fatigue who reported accessing services is shown in Fig. 4.
Those with fatigue reported significantly more physician, other health care professional and psychosocial visits (Table 3).
Effect of age and cancer type
The results of the sensitivity analyses are reported in the supplemental data. Controlling for age and cancer type did not modify the relationship between CRF and social function (Table S1), work status (Table S2), workplace performance (Table S3), nor health care use (Table S4). Neither age nor cancer type was significant predictors in any of the sensitivity analyses with the exception that, on average, an extra year of age was associated with 1.7 fewer hours worked in the previous 4 weeks (Table S4).
Discussion
This study provides a detailed examination of the association between CRF and social and vocational functioning, and health care utilization in a large sample of post-treatment cancer survivors using a validated fatigue scale. The findings demonstrate that cancer survivors with CRF experience challenges to reintegration including social difficulties and problems with work attendance and work performance. Furthermore, cancer survivors with CRF report more health care utilization over the past month with higher rates of health care provider visits and hospital visits, and more psychosocial support visits. This is important information that suggests a significant personal and societal impact of CRF and can be helpful evidence to advocate for the funding of the implementation of existing evidence-based guidelines and the services and interventions recommended. Furthermore, given the association with CRF, social and vocational functioning and health care utilization can be important outcomes to measure in future interventional studies addressing CRF.
The post-treatment transitional phase of cancer survivorship has been described as one of the most stressful times for survivors, and they describe needing support and guidance as to how to recover their health and to re-engage in their social and work-life roles [76] . Social functioning is an important reintegration target, and social connections have been shown to be beneficial to overall physical health, well-being and longevity [77,78,79,80], as well as better cancer survival and a lower risk of cancer mortality [81,82,83,84]. To date, deficits in social roles and activities have been reported in cancer survivors [85, 86], but little research has examined the related factors. One study examining social functioning in cancer survivors reported a negative association between social activities and CRF, though this was not the primary focus of the study [50]. In our current study, we found a difference of almost 9 points between those with and without CRF on the SDI-16, which has a minimal clinically important difference of 3, and 55% of individuals with CRF met cut-off for clinically significant social difficulties.
In terms of work and work function, we found CRF was associated with a 2.72 times higher odds of being unemployed or on leave. Previous studies have shown that cancer survivors experience higher rates of unemployment compared to non-cancer peers [87, 88], and fatigue and exhaustion are barriers to return to work [89, 90]. Unemployment can result in significant wage loss and financial burden and can impact quality of life [91, 92]. In some situations, survivors must return to work despite physical limitations due to lack of support and financial and medical insecurity [93, 94]. In those who were currently working, individuals with CRF + worked fewer hours over the prior 4 weeks (− 27.4) compared to those who were CRF − , and there was a difference in work performance (presenteeism). Presenteeism, along with absenteeism, can impact earnings, and individuals experiencing dysfunctional presenteeism have lower earnings on average [95] and an increased risk for financial toxicity [96]. Along with the personal impact, there is a significant societal and economic cost of not returning to work and not performing at work [97, 98]. The National Institute of Health in the USA estimated that in 2010, the cost of lost productivity accounted for 61% of the total cost of cancer, compared to 39% for the direct costs related to treatment [99]. Furthermore, estimates of the cost of illness to businesses have reported that impaired presenteeism results in significantly more costs compared to absenteeism [100, 101]. Taken together, these findings highlight the need to improve employment rates and job performance among cancer survivors with fatigue. Interestingly, the effects of interventions to facilitate return to work and work performance, which have primarily targeted work-related factors, have not been effective [102, 103]. This is likely because health-related factors such as persistent fatigue, which are associated with work status and productivity factors, have not been targeted. Moving forward, multifaceted interventions targeting return to work and work function must also consider CRF as a potential mediator and target for intervention [104, 105]. For example, exercise interventions, which are known to effectively reduce cancer-related fatigue [106, 107], have also been shown to promote return to work and reduce missed work hours [108, 109] and are more effective than occupational support or counselling interventions alone [110, 111].
Finally, we found that cancer survivors with CRF had more visits to physicians and other health care professionals (i.e. in-home nursing care, physiotherapists, pharmacist) and accessed more psychosocial services (i.e. social worker, psychologist, support group, financial counselling). Increased health care utilization has been reported in post-treatment cancer survivors compared to age-matched controls [112,113,114,115], and fatigue has been reported as one of the most common presenting complaints. While future research using proper health costing estimates are required, it is clear that CRF results in a significant cost to our health care system.
Based on a growing body of intervention research, guidelines for the management of CRF have been developed and adopted by cancer organisations [13, 17, 116, 117]. Exercise has the strongest evidence of reductions in CRF [118,119,120]. However, simply recommending that people with CRF exercise is likely not effective, and CRF can be a significant barrier to participation in physical activity [121, 122]. In the presence of clinically significant fatigue, current guidelines suggest referral to rehabilitation or exercise specialist for a supervised cancer exercise programme [123]. This has led to recommendations to include exercise science professionals and implement exercise-based rehabilitation as an integral part of cancer care [124, 125]. These programmes can help to address fatigue and optimise physical functioning so that survivors can engage in activities of daily living and participate in the broader community [44]. Despite this, oncology rehabilitation and cancer-specific exercise services have been omitted from large-scale cancer initiatives, and government health care funding dedicated to cancer rehabilitation remains limited in many countries including Canada [41, 45, 46, 124].
The results of the present study should be interpreted within the context of its limitations. This study was conducted in a large urban centre in a high-income country, and the labour market conditions and cultural values are not representative of all settings. This was cross-sectional and therefore the nature of these relationships cannot be determined. While we obtained a good response rate of 73%, there is a possibility of non-response bias. Due to ethical restrictions, data on the non-responders was not available, though previous work by our group with this population found no differences between responders and non-responders based on age, stage of disease, treatments received, or current hormone therapy [26], and work from other groups has shown non-responders may not systematically differ from responders on important baseline variables [126]. Additionally, it is important to note that CRF often co-occurs or clusters with other symptoms such as pain, insomnia and mood disturbances [127], which were not measured in the current study and may mediate or moderate the relationship between CRF and our measured outcomes. Future studies are needed to longitudinally examine the differential role of these symptoms overtime. Finally, the study sample was restricted to breast and colorectal cancer survivors within 5 years of treatment completion. Future research should examine these outcomes in long-term survivor populations to examine if the impact of CRF diminishes as individuals adapt. Future studies should also assess the economic burden of CRF including the burden on the healthcare system, society and those individuals with CRF and their caregivers. Furthermore, studies are needed to demonstrate the benefits and costs of different interventions and clinical services, as this has greater value to policymakers than efficacy of a programme without consideration of its feasibility or the costs of delivery [128].
Despite the limitations, this is the first study to examine the impact of CRF on social and vocational functioning and health care utilization in a large sample of post-treatment cancer survivors using validated tools. Although CRF is a prevalent and consequential symptom and there are guidelines on its detection and management, CRF remains poorly managed. The results from this study provide evidence on the substantial disruptive impact of CRF on the lives of cancer survivors and suggest broader social and financial repercussions. Funding of the implementation of existing guidelines and the recommended evidence-based interventions is urgently needed.
Data availability
Due to REB restrictions, we are not permitted to upload data to a public data repository. However, we can make data available upon request with a data transfer agreement.
References
Ferlay J, Colombet M, Soerjomataram I, Parkin D, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–89.
CancerProgressReport.org [Internet]. Philadelphia: American Association for Cancer Research; ©2022 [2023 July] Available from http://www.CancerProgressReport.org/.
American Cancer Society. Cancer treatment & survivorship facts & figures 2022-2024. Atlanta: American Cancer Society; 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-andsurvivorship-facts-and-figures/2022-cancer-treatment-and-survivorship-fandf-acs.pdf.
Canada. PHAo. Fact sheet: cancer in Canada. 2018 [1–7]. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/fact-sheet-cancer-canada/fact-sheet-cancer-canada.pdf.
Abrahams H, Gielissen M, Schmits I, Verhagen C, Rovers M, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–74.
Piper B, Cella D. Cancer-related fatigue: definitions and clinical subtypes. J Natl Compr Canc Network. 2010;8:958–66.
Bower J. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609.
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–53.
Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? Breast Cancer Res Treat. 2008;112(1):5–13.
Curt G, Breitbart W, Cella D, Groopman J, Horning S, Itri L, et al. Impact of cancer-related fatigue on the lives of patients. Oncologist. 2000;5(5):353–60.
Stone P, Richardson A, Ream E, Smith AG, Kerr DJ, Kearney N. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum Annal Oncol. 2000;11(8):971–5.
Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey The Fatigue Coalition. Semin Hematol. 1997;34(3:Suppl 2):4–12.
Berger A, Mooney K, Alvarez-Perez A, Breitbart W, Carpenter K, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw J Natl Compr Canc Netw. 2015;13(8):1012–39.
Wang XS, Zhao F, Fisch MJ, O’Mara AM, Cella D, Mendoza TR, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–32.
Hickok J, Roscoe J, Morrow G, Mustian K, Okunieff P, Bole C. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104:1772–8.
Roila F, Fumi G, Ruggeri B, Antonuzzo A, Ripamonti C, Fatigoni S, et al. Prevalence, characteristics, and treatment of fatigue in oncological cancer patients in Italy: a cross-sectional study of the Italian network for supportive care in cancer (NICSO). Support Care Cancer. 2019;27:1041–7.
Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2014;32(17):1840–50.
Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. JClinOncol. 2001;19(14):3385–91.
Fitch M, Zomer S, Lockwood G, Louzado C, Shaw Moxam R, Rahal R, et al. Experiences of adult cancer survivors in transitions. Support Care Cancer. 2019;27(8):2977–86.
Husson O, Mols F, van de Poll-Franse L, de Vries J, Schep G, Thong M. Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Support Care Cancer. 2015;23:2165–74.
Reinertsen K, Cvancarova M, Loge J, Edvardsen H, Wist E, Fosså S. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–14.
Fabi A, Falcicchio C, Giannarelli D, Maggi G, Cognetti F, Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast. 2017;34:44–52.
Steen R, Dahl A, Hess S, Kiserud C. A study of chronic fatigue in Norwegian cervical cancer survivors. Gynecol Oncol. 2017;146:630–5.
Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–8.
Meeske K, Smith A, Alfano C, McGregor B, McTiernan A, Baumgartner K, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL study report. Qual Life Res. 2007;16:947–60.
Jones J, Olson K, Catton P, Catton C, Fleshner N, Krzyzanowska M, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10(1):51–61.
Kreissl S, Mueller H, Goergen H, Mayer A, Brillant C, Behringer K, et al. Cancer-related fatigue in patients with and survivors of Hodgkin’’s lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol. 2016;10:1453–62.
Ruiz-Casado A, Álvarez-Bustos A, de Pedro C, Méndez-Otero M, Romero-Elías M. Cancer-related fatigue in breast cancer survivors: a review. Clin Breast Cancer. 2021;1:10–25.
Thong MS, Mols F, Wang XS, Lemmens VE, Smilde TJ, van de Poll-Franse LV. Quantifying fatigue in (long-term) colorectal cancer survivors: a study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur J Cancer. 2013;49(8):1957–66.
Deimling G, Bowman K, Wagner L. The effects of cancer-related pain and fatigue on functioning of older adult, long-term cancer survivors. Cancer Nurs. 2007;30(6):421–33.
Vaz-Luis I, Di Meglio A, El-Mouhebb M, Dumas A, Charles C, Menvielle G, et al. Breast cancer (BC) related fatigue: a longitudinal investigation of its prevalence, domains and correlates. Annal Oncol. 2018;29:viii603–40.
Ore M, Foli K. Reintegration for post-treatment cancer survivors: a concept analysis. J Holist Nurs. 2020;38(3):300–17.
Urquhart R, Murnaghan S, Kendell C, Sussman J, Porter G, Howell D, et al. What matters in cancer survivorship research? A suite of stakeholder-relevant outcomes. Curr Oncol. 2021;28(4):3188–200.
Cheng K, Lee D. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2011;78:127–37.
National Coalition for Cancer Survivorship. Cancer survivorship survey: findings from an online survey of adult cancer patients. 2019 [Available from: https://www.canceradvocacy.org/wp-content/uploads/2019/09/NCCS-Survivorship-Survey-Final-Report.pdf.
Koornstra R, Peters M, Donofrio S, van den Borne B, de Jong F. Management of fatigue in patients with cancer - a practical overview. Cancer Treat Rev. 2014;40:791–9.
Mitchell S. Cancer-related fatigue: state of the science. . PM R. 2010;2(364–383).
Schmidt M, Bergbold S, Hermann S, Steindorf K. Knowledge, perceptions, and management of cancer-related fatigue: the patients’perspective. . Support Care Cancer 2021;29(2063–2071).
Luthy C, Cedraschi C, Pugliesi A, Di Silvestro K, Mugnier-Konrad B, Rapiti E, et al. Patients’ views about causes and preferences for the management of cancer-related fatigue—a case for non-congruence with the physicians? Support Care Cancer. 2011;19:363–70.
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris M, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–9.
Smith S, Zheng J, Silver J, Haig A, Cheville A. Cancer rehabilitation as an essential component of quality care and survivorship from an international perspective. Disabil Rehabil. 2020;42(1):8–13.
Brennan L, Sheill G, O’Neill L, O’Connor L, Smyth E, Guinan E. Physical therapists in oncology settings: experiences in delivering cancer rehabilitation services, barriers to care, and service development needs. Phys Ther 2022;102(3).
Silver J, Baima J, Mayer S. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. Cancer J Clin. 2013;63(5):295–317.
Alfano C, Cheville A, Mustian K. Developing high-quality cancer rehabilitation programs: a timely need. Am Soc Clin Oncol Educ. 2016;36:241–9.
Canestraro ANA, Stack M, Strong K, Wright A, Beauchamp M, Berg K, Brooks D. Oncology rehabilitation provision and practice patterns across Canada. Physiother Can. 2013;65(1):94–102 Winter.
Dennett A, Peiris C, Shields N, Morgan D, Taylor N. Exercise therapy in oncology rehabilitation in Australia: a mixed-methods study. Asia Pac J Clin Oncol. 2017;13:e515–27.
O’Connor M, Drummond F, O’Donovan B, Donnelly C, National Cancer Registry Ireland. National cancer survivorship needs assessment: the unmet needs of cancer survivors in Ireland: a scoping review 2019. https://www.hse.ie/eng/services/list/5/cancer/profinfo/survivorshipprogramme/unmet%20needs%20of%20cancer%20survivors.pdf.
Strasser-Weippl K, Chavarri-Guerra Y, Villarreal-Garza C, Bychkovsky B, Debiasi M, Liedke P, et al. Progress and remaining challenges for cancer control in Latin America and the Caribbean. Lancet Oncol 2015;16(1405–1438).
Tan C, Yip S, Chan R, Chew L, Chan A. Investigating how cancer-related symptoms influence work outcomes among cancer survivors: a systematic review. J Cancer Surviv. 2022;16:1065–78.
Syrjala K, Stover A, Yi J, Artherholt S, Abrams J. Measuring social activities and social function in long-term cancer survivors who received hematopoietic stem cell transplantation. . Psycho-Oncol. 2010;19(462–471).
Qaderi S, Ezendam N, Verhoeven R, Custers J, de Wilt J, Mols F. Follow-up practice and healthcare utilisation of colorectal cancer survivors. . Euro J Cancer Care. 2021;30(5).
Heins MJ, Korevaar JC, Rijken PM, Schellevis FG. For which health problems do cancer survivors visit their general practitioner? Eur J Cancer. 2013;49(1):211–8.
Behringer K, Goergen H, Müller H, Thielen I, Brillant C, Kreissl S, et al. Cancer-related fatigue in patients with and survivors of Hodgkin lymphoma: the impact on treatment outcome and social reintegration. J Clin Oncol. 2016;34(36):4329–37.
Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–9.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. JPain SymptomManage. 1997;13(2):63–74.
Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–61.
Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13(4):246–54.
Muzzatti B, Annunziata M. Assessing the social impact of cancer: a review of available tools. Support Care Cancer. 2012;20(10):2249–57.
Wright P, Smith A, Keding A, Velikova G. The Social Difficulties Inventory (SDI): development of subscales and scoring guidance for staff. Psychooncology. 2011;20(1):36.
Smith A, Wright P, Selby P, Velikova G. Measuring social difficulties in routine patient centred assessment: a Rasch analysis of the social difficulties inventory. Qual Life Res. 2007;16:823–31.
Wright P, Marshall L, Smith A, Velikova G, Selby P. Measurement and interpretation of social distress using the social difficulties inventory (SDI). Eur J Cancer. 2008;44:1529–35.
Wright E, Kiely M, Johnston C, Smith A, Cull A, Selby P. Development and evaluation of an instrument to assess social difficulties in routine oncology practice. . Qual Life Res. 2005;14(373–386).
Wright P, Smith A, Roberts K, Selby P, Velikova G. Screening for social difficulties in cancer patients: clinical utility of the social difficulties inventory. Br J Cancer. 2007;97:1063–70.
Kessler R, Barber C, Beck A, Berglund P, Cleary P, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med. 2003;45(2):156–74.
Kessler R, Ames M, Hymel P, Loeppke R, McKenas D, Richling D, et al. Using the WHO Health and Work Performance Questionnaire (HPQ) to evaluate the indirect workplace costs of illness. J Occup Environ Med. 2004;46(Suppl.6):S23–37.
Scuffham P, Vecchio N, Whiteford H. Exploring the validity of HPQ-based presenteeism measures to estimate productivity losses in the health and education sectors. Med Decis Making. 2014;34(1):127–37.
Bernaards C, Proper K, Hildebrandt V. Physical activity, cardiorespiratory fitness, and body mass index in relationship to work productivity and sickness absence in computer workers with preexisting neck and upper limb symptoms. J Occup Environ Med. 2007;49(6):633–40.
Furukawa T, Horikoshi M, Kawakami N, Kadota M, Sasaki M, Sekiya Y, et al. Telephone cognitive-behavioral therapy for subthreshold depression and presenteeism in workplace: a randomized controlled trial. Plos One. 2012;7(4).
Geraedts A, Kleiboer A, Wiezer N, van Mechelen W, Cuijpers P. Short-term effects of a web-based guided self-help intervention for employees with depressive symptoms: randomized controlled trial. J Med Internet Res. 2014;16(5).
Kessler R, Petukhova M, McInnes K, Üstün T. HPQ short form questions and scoring. 2007 [Available from: https://www.hcp.med.harvard.edu/hpq/ftpdir/absenteeism%20presenteeism%20scoring%20050107.pdf.
Browne G, Roberts J, Gafni A, Byrne C, Weir R, Majumdar B, et al. Economic evaluations of community-based care: lessons from twelve studies in Ontario. J Eval Clin Pract. 1999;5(4):380–5.
Browne G, Gafni A, Roberts J, Whittaker S, Wong M, Prica M. Approach to the measurement of costs (expenditures) when evaluating health and social programs (Working-Paper Series 01–03). System-Linked Research Unit on Health and Social Service Utilization. In: University M, editor. Hamilton, Ontario, Canada.2001.
Sussman J, Howell D, Bainbridge D, Brazil K, Pyette N, Whelan T. The impact of specialized oncology nursing on patient supportive care outcomes. J Psychosoc Oncol. 2011;29:286–307.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022 [Available from: https://www.R-project.org/.
Garofalo J, Choppala S, Hamann H, Gjerde J. Uncertainty during the transition from cancer patient to survivor. Cancer Nurs. 2009;32(4):E8–14.
Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437–58.
Holt-Lunstad J, Smith T, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10:227–37.
Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. https://doi.org/10.1371/journal.pmed.1000316. https://journals.plos.org/plosmedicine/article/file?id=10.1371/journal.pmed.1000316&type=printable.
Howick J, Kelly P, Kelly M. Establishing a causal link between social relationships and health using the Bradford Hill guidelines. SSM Popul Health. 2019;8:100402. https://doi.org/10.1016/j.ssmph.2019.100402. Erratum in: SSM Popul Health. 2020;12:100711.
Kroenke C, Michael Y, Poole E, Kwan M, Nechuta S, Leas E, et al. Postdiagnosis social networks and breast cancer mortality in the After Breast Cancer Pooling Project. Cancer. 2017;123(7):1228–37.
Kroenke C, Kubzansky L, Schernhammer E, Holmes M, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24(7):1105–11.
Sarma E, Kawachi I, Poole E, Tworoger S, Giovannucci E, Fuchs C, et al. Social integration and survival after diagnosis of colorectal cancer. Cancer. 2018;124(4):833–40.
Kroenke C, Paskett E, Cené C, Caan B, Luo J, Shadyab A, et al. Prediagnosis social support, social integration, living status, and colorectal cancer mortality in postmenopausal women from the women’s health initiative. Cancer. 2020;126(8):1766–75.
Caravati-Jouvenceaux A, Launoy G, Klein D, Henry-Amar M, Abeilard E, Danzon A, et al. Health-related quality of life among long-term survivors of colorectal cancer: a population-based study. Oncologist. 2011;16(11):1626–36.
Wright E, Kiely M, Lynch P, Cull A, Selby P. Social problems in oncology. Br J Cancer. 2002;87:1099–104.
de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301(7):753–62.
Drolet M, Maunsell E, Brisson J, Brisson C, Mâsse B, Deschênes L. Not working 3 years after breast cancer: predictors in a population-based study. J Clin Oncol. 2005;23(33):8305–12.
Islam T, Dahlui M, Majid H, Nahar A, Taib N, Su T. Factors associated with return to work of breast cancer survivors: a systematic review. BMC Public Health 2014;14(Suppl 3).
Schmidt M, Scherer S, Wiskemann J, Steindorf K. Return to work after breast cancer: the role of treatment-related side effects and potential impact on quality of life. Eur J Cancer Care 2019;28.
Lauzier S, Maunsell E, Drolet M, Coyle D, Hébert-Croteau N, Brisson J, et al. Wage losses in the year after breast cancer: extent and determinants among Canadian women. J Natl Cancer Inst. 2008;100(5):321–32.
Lundh M, Lampic C, Nordin K, Ahlgren J, Bergkvist L, Lambe M, et al. Changes in health-related quality of life by occupational status among women diagnosed with breast cancer–a population-based cohort study. Psychooncology. 2013;22(10):2321–31.
Van Muijen P, Weevers N, Snels I, Duits S, Bruinvels D, Schellart A, et al. Predictors of return to work and employment in cancer survivors: a systematic review. Eur J Cancer Care. 2013;22:144–60.
McKay G, Knott V, Delfabbro P. Return to work and cancer: the Australian experience. J Occup Rehabil. 2013;23(1):93–105.
Bryan M, Bryce A, Roberts J. Dysfunctional presenteeism: effects of physical and mental health on work performance. Manch Sch. 2022;90(4):409–38.
Chan R, Gordon L, Zafar S, Miaskowski C. Financial toxicity and symptom burden: what is the big deal? Support Care Cancer. 2018;26:1357–9.
Garaszczuk R, Yong J, Sun Z, de Oliveira C. The economic burden of cancer in Canada from a societal perspective. Curr Oncol. 2022;29:2735–48.
Ekwueme D, Yabroff K, Guy GJ, Banegas M, JS dM, Li C, et al. Centers for Disease Control and Prevention (CDC) Medical costs and productivity losses of cancer survivors–United States 2008–2011. MMWR Morb Mortal Wkly Rep. 2014;63(23):505–10.
American Cancer Society. Cancer facts & figures 2011. Atlanta: American Cancer Society; 2011. National Home Office: American Cancer Society Inc. 250 Williams Street, NW, Atlanta, GA. file:///C:/Users/csilva/Downloads/cancer-facts-and-figures-2011.pdf
Goetzel R, Long S, Ozminkowski R, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. 2004;46(4):398–412.
Wang P, Beck A, Berglund P, Leutzinger J, Pronk N, Richling D, et al. Chronic medical conditions and work performance in the HPQ calibration surveys. J Occup Environ Med. 2003;45(12):1303–11.
de Boer A, de Wind A, Coenen P, van Ommen F, Greidanus M, Zegers A, et al. Cancer survivors and adverse work outcomes: associated factors and supportive interventions. Br Med Bull. 2023;145(1):60–71.
Stehle L, Hoosain M, van Niekerk L. A systematic review of work-related interventions for breast cancer survivors: potential contribution of occupational therapists. Work. 2022;72(1):59–73.
Short P, Vasey J, Tunceli K. Employment pathways in a large cohort of adult cancer survivors. Cancer. 2005;103(5):1292–301.
Silver J, Gilchrist L. Cancer rehabilitation with a focus on evidence-based outpatient physical and occupational therapy interventions. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S5–15.
Dun L, Xian-Yi W, Xiao-Ying J. Effects of moderate-to-vigorous physical activity on cancer-related fatigue in patients with colorectal cancer: a systematic review and meta-analysis. Arch Med Res. 2020;51(2):173–9.
Thong M, van Noorden C, Steindorf K, Arndt V. Cancer-related fatigue: causes and current treatment options. Curr Treat Options in Oncol. 2020;21(17):1–19.
Thijs K, de Boer A, Vreugdenhil G, van de Wouw A, Houterman S, Schep G. Rehabilitation using high-intensity physical training and long-term return-to-work in cancer survivors. J Occup Rehabil. 2012;22(2):220–9.
Wilson T, Nambiema A, Porro B, Descatha A, Aublet-Cuvelier A, Evanoff B, et al. Effectiveness of physical activity interventions on return to work after a cancer diagnosis: a systematic review and meta-analysis. J Occup Rehabil. 2023;33:4–19.
De Boer A, Taskila T, Tamminga S, Frings-Dresen M, Feuerstein M, Verbeek J. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev. 2011;16(2).
Kuoppala J, Lamminpaa A. Rehabilitation and work ability: a systematic literature review. J Rehabil Med. 2008;40(10):796–804.
Dibble K, Kaur M, Lyu J, Connor A. Evaluation of health perceptions and healthcare utilization among population-based female cancer survivors and cancer-free women. Cancer Causes Control. 2022;33(1):49–62.
van de Poll-Franse L, Mols F, Vingerhoets A, Voogd A, Roumen R, Coebergh J. Increased health care utilisation among 10-year breast cancer survivors. Support Care Cancer. 2006;14:436–43.
Rajotte E, Heron L, Syrjala K, Baker K. Health care utilization among long-term cancer survivors. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2016;34(3):22–3.
Roorda C, Berendsen AJ, Groenhof F, van der Meer K, de Bock GH. Increased primary healthcare utilisation among women with a history of breast cancer. Support Care Cancer. 2013;21(4):941–9.
Fabi A, Bhargava R, Fatigoni S, Guglielmo M, Horneber M, Roila F, et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–23.
Howell D, Keller–Olaman S, Oliver T, Hack T, Broadfield L, Biggs K, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3).
Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am J Prev Med. 2012;43(2):e1-24.
Mustian K, Alfano C, Heckler C, Kleckner A, Kleckner I, Leach C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–8.
Campbell K, Winters-Stone K, Wiskemann J, May A, Schwartz A, Courneya K, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90.
Ng A, Ngo-Huang A, Vidal M, Reyes-Garcia A, Liu D, Williams J, et al. Exercise barriers and adherence to recommendations in patients With cancer. JCO Oncol Pract. 2021;17(7):e972–81.
Park J, Lee J, Oh M, Park H, Chae J, Kim D, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121(16):2740–8.
Stout N, Santa Mina D, Lyons K, Robb K, Silver J. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin. 2021;71(2):149–75.
Elshahat S, Treanor C, Donnelly M. Factors influencing physical activity participation among people living with or beyond cancer: a systematic scoping review. . Int J Behav Nutr Phys Act 2021;18(1).
Schmitz K, Campbell A, Stuiver M, Pinto B, Schwartz A, Morris G, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–84.
Lie H, Rueegg C, Fosså S, Loge J, Ruud E, Kiserud C. Limited evidence of non-response bias despite modest response rate in a nationwide survey of long-term cancer survivors—results from the NOR-CAYACS study. J Cancer Surviv. 2019;13:353–63.
de Rooij B, Oerlemans S, van Deun K, Mols F, de Ligt K, Husson O, et al. Symptom clusters in 1330 survivors of 7 cancer types from the PROFILES registry: a network analysis. Cancer. 2021;127(24):4665–74.
Redman S, Turner J, Davis C. Improving supportive care for women with breast cancer in Australia: the challenge of modifying health systems. Psycho-Oncol: Journal of the Psychological, Social and Behavioral Dimensions of Cancer. 2003;12(6):521–31.
Acknowledgements
The authors would like to thank the individuals who participated in this study. The study sponsor had no role in the design of the study or the collection or analyses of the data nor the writing of the manuscript or decision to submit the manuscript for publication.
Funding
This work was funded through the CCS-Canadian Centre for Applied Research Cancer Control (grant #019789).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; JMJ, DH, KO, PB, CL and EA obtained funding for the project. JMJ provided primary supervision and was responsible for data collection. EA and PB provided support for data acquisition and BC assisted in data collection and collation. JMJ, LA and SZ wrote the first draft of the manuscript. LA conducted the statistical analyses and prepared the tables and figures. All authors contributed to the interpretation of the results and reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the University Health Network and Mount Sinai Hospital Research Ethics Boards. Informed written consent was obtained from all individual participants in the study.
Competing interests
Dr. Jones is an Associate Editor for Journal of Cancer Survivorship. The other authors have no competing financial or non-financial interests that would influence the results or discussion reported in this paper.
Conflict of interest
Dr. Jones is an Associate Editor for Journal of Cancer Survivorship. Dr. Howell reports a consultant and scientific advisory board relationship to Carevive Systems, Inc. and funding from Astra Zeneca unrelated to this study. Philippe Bedard’s conflicts of interests are reported here: https://coi.asco.org/share/PCN-CFSR/Philippe%20Bedard and are unrelated to this study. Eitan Amir's conflicts of interest are reported here: https://coi.asco.org/share/VNV-A5SK/Eitan%20Amir and are unrelated to this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jones, J.M., Howell, D., Longo, C. et al. The association of cancer-related fatigue on the social, vocational and healthcare-related dimensions of cancer survivorship. J Cancer Surviv (2023). https://doi.org/10.1007/s11764-023-01451-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-023-01451-9