Abstract
In this work, we focus on the identification of novel fungal peroxygenase gene belonging to the peroxidase-peroxygenase superfamily. We applied a metagenomic approach on soil samples from primeval forest and appropriate bioinformatics tools for analysis of obtained genomic DNA sequence. Peroxidases are ubiquitous metalloenzymes that are able to reduce reactive peroxides, mainly hydrogen peroxide, into water, whereas several substrates can be concomitantly oxidized during their catalytic reaction. Our purpose was to collect unique peroxygenase sequence data originating from a preserved biotope for a robust phylogenetic reconstruction of a particular gene family coding for highly versatile heme-thiolate peroxidases that has peculiar yet undiscovered representatives among ectomycorrhizal fungi. We identified unique DNA sequence, 812 bp long, from ectomycorrhizal Suillus species coding for a heme-thiolate peroxidase with 1 typical intron that appears distinctive for Carpathian forests. After translation in corresponding protein sequence 251 amino acids long we could identify typical signatures of this peroxygenase. On the proximal side of heme we found the conserved P-C-P triad responsible for efficient ligation of heme iron thus influencing the reactivity of this peroxidase. On the distal side we recognized the E-H-D-X-S-L motif for interaction with a stabilizing magnesium ion. Maximum likelihood reconstruction of protein phylogeny revealed with a high bootstrap support the presence of a monophyletic HTP4 clade originating in numerous Suillus representatives. Together with sister clades of edible Boletus and poisonous Paxillus containing diverse peroxygenases these newly discovered heme catalyst can be considered for application of oxyfunctionalization of organic molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peroxidases are ubiquitous enzymes that are able to reduce peroxides, including mainly hydrogen peroxide, into water, whereas several substrates can be concomitantly oxidized during this reaction (Bindoli and Rigobello 2013). This class of oxidoreductases can be basically divided into heme and nonheme peroxidases. Heme peroxidases contain catalytic center with a prosthetic heme b group. Nonheme peroxidases have mainly a selenol or reactive thiol at their active sites. The classification of all known peroxidases into several independent superfamilies and families reflects their supposed reaction mechanisms of peroxide bond cleavage in their peculiar active sites (Savelli et al. 2019).
During a long-term evolution in prokaryotes and eukaryotes four superfamilies of heme containing peroxidases have emerged. They are classified as superfamilies of peroxidase-catalases, peroxidase–chlorite dismutases, peroxidase–cyclooxygenases and peroxidase–peroxygenases, respectively. Enzymes from these divergent groups differ in architecture of their active site or substrate specificity and a typical protein fold (Zámocký et al. 2015). Probably the most versatile known heme protein biocatalysts belong to the peroxidase-peroxygenase superfamily because they reveal very flexible catalytic properties typical for several further classes of oxidoreductases containing heme, namely monofunctional plant and fungal peroxidases, cytochrome P450 monooxygenases, and even catalases (Hofrichter and Ullrich 2006; Zámocký and Harichová, 2022).
Certain heme peroxidases belong to key lignolytic enzymes in microbes and fungi. Many peroxidases have significant potential in converting lignin into suitable biomass product which can be then potentially converted into biofuels. Another prospective use is for the bioremediation because they are able to degrade many xenobiotics (Chowdhary et al. 2019). They are able to transform dangerous organic compounds like pesticides, dioxins, phenols, polychlorinated biphenyls in harmless substances. They can reduce their toxic effects by decrease of their bioavailability or removal from aqueous phase (Bansal and Kanwar 2013). These enzymes, in free or immobilized form, are promising in biotransformations of different synthetic dyes in reactors (Svetozarević et al. 2022). Peroxidases are also important components of biosensors. Use of heme peroxidases in electrochemical biosensors as a biological recognition element is spread for detection of phenolic compounds or hydrogen peroxide (Neumann and Wollenberger 2020). Horseradish peroxidase, as a classical type, was used as an efficient catalyst in reaction with luminol serving as an oxidizable substrate to quantify H2O2 as the oxidizing molecule (Bocanegra-Rodríguez, 2020).
A specific bioinformatic database focused mainly on genomics and proteomics of enzymes peroxidases and related catalases was called PeroxiBase (http://peroxibase.toulouse.inra.fr/ ) and recently it was renamed to RedoxiBase (Savelli et al. 2019). Its main scope is focused in the classification of obtained sequences (from various genomic projects and from metagenomics) in particular peroxidase families and superfamilies. Recently, new functions and tools for analysis have been added to this unique database (Fawal et al. 2013) and it is constantly updated. A detailed molecular phylogeny focused on bifunctional heme peroxidases was published previously (Zámocký et al. 2012) and molecular evolution of heme peroxygenase superfamily, known to contain numerous very versatile biocatalysts, has been reconstructed recently (Zámocký and Harichová, 2022).
Ectomycorrhizal fungi usually form extraradical mycelium. It is a collection of filamentous fungal hyphae surrounding the root. This symbiosis is based on the reciprocal exchange of natural resources. It can positively influence the host plant water relations and response to drought (Cahanovitc et al. 2022). Ectomycorrhizal fungi fix carbon from the host tree, but they retain the ability to express enzymes that break down woody substrates as an alternative source of carbon. There is a hypothesis that an ectomycorrhizal fungus, Suillus granulatus, in a pure pine system retains the ability to produce enzymes that break down woody substrates. This ability is inducible by reduction of host photosynthetic potential via partial defoliation. Results presented in that work indicate that after defoliation of host plant, fungus expressed all tested enzymes: glucosidase, laccase, phosphatase, protease, manganese and lignin peroxidase (Cullings et al. 2008). In addition, in boreal forests, where nitrogen source is limited, ectomycorrhizal fungi are considered to play a vital role for trees in nitrogen processing and supplying (Näsholm et al. 2013).
The main aim of this study was the identification and in silico characterization of a novel peroxidase gene, the translated product of which would have a promising biotechnological application. We were able to identify a peculiar gene coding for a heme-thiolate peroxidase within prepared fungal soil metagenomic DNA libraries. This gene belongs to the peroxidase–peroxygenase superfamily (Zámocký a kol., 2015). In the older literature and in some sequence databases we can also encounter the names aromatic peroxygenase (APO, Pecina et al. 2009) or chloroperoxidase (CPO, Ayala et al. 2011; Sundaramoorthy et al. 1995) but the nomenclature of these enzymes shall reflect their biochemical reactivity and involved ligands as will be obvious from a multiple sequence alignment that was used for identification of essential functional parts of newly discovered peroxidase from a primeval forest.
Materials and methods
Fungal material
In this work, new fungal peroxidase genes were identified from DNA libraries of ectomycorrhizal fungi that can potentially serve as a reservoir of yet unknown oxidoreductase genes. Specific types of fungi are preferentially found in forests with a high degree of naturalness (Kunca et al. 2022). The Badín primeval forest includes primeval climax forest communities of the beech vegetation stage, where all three basic development stages of primeval forests on the territory of Europe are represented. The occurrence of specific mycoflora, typical for the specific ecological and climatic conditions of the Carpathian forests, is contingent on the Badín forest national nature reservation (Mihál 2013).
Therefore, we have chosen Badín primeval forest as the main source of ectomycorrhizal fungi. We picked soil samples from different locations of Badín primeval forest, which contained metagenomic DNA with genomes from many microorganisms occurring in a specific part of this primeval forest (Fig. 1). Soil samples with fruiting bodies of mushrooms identified as Suillus sp. were picked in Vtáčnik mountains (geographical locations are shown in Fig. 1).
Chemicals
In this work we used following laboratory kits: Thermo Scientific® GeneJET Plant Genomic DNA Purification Mini Kit (Cat. No. K0702, Thermo Fisher Scientific®, Litva), DNeasy® PowerMax® Soil Kit (10), (Cat. No. 12988-10, Qiagen GmbH®, Germany), Thermo Scientific® Maxima First Strand cDNA Synthesis Kit with dsDNase (Ref. No. K1671, Thermo Fisher Scientific®, Lithuania), RNeasy® PowerSoil® Total RNA Kit (25) (Cat. No. 12866-25, Qiagen GmbH®, Germany). Monarch® DNA Gel Extraction Kit, (Cat. No. #T1020S, New England Biolabs® Inc., USA), Zero Blunt® TOPO® PCR cloning Kit for Sequencing, (Cat. No. 450,031, InvitrogenTM, USA), Plasmid Miniprep Kit (Cat. No. #T1010L, New England Biolabs® Inc., USA) and Thermo Scientific GeneJET Plant RNA Purification Mini Kit (Cat. No. K0801, Thermo Fisher Scientific®, Lithuania).
Chemicals used in this work were Chelaton III p.a. (Cat. No. 000149, Slavus s.r.o, Slovakia), Acetic Acid (Mikrochem®, Slovakia), Agarose SERVA Wide Range, (Cat. No. 11406.02, Serva Electrophoresis GmbH, Germany), Agar Powder, Bacteriological (Cat. No. GRM026-500G, ČADERSKÝ-ENVITEK, spol. s.r.o, Czech Republic), Tryptone Type-1(Casitose Type-I) (Cat. No. RM014-500G, HiMedia Laboratories Pvt. Ltd., India), Sodium chloride p.a. (Cat. No. L01876-1KG, Centralchem®, Slovakia), Yeast extract, SERVABACTER® powder (Cat. No. 24540.02, Serva Electrophoresis GmbH, Germany), Tris ultrapure (Cat. No. A1086,1000, Panreac AppliChem ITW Reagents Spain), Quick-Load® Purple 1 kb DNA Ladder (Cat. No. N0552S, New England Biolabs® Inc., USA), GeneRuler 100 bp DNA Ladder (Cat. No. SM0241, Thermo Fisher Scientific®, Lithuania), GelRed® Nucleic Acid Gel Stain (Cat. No. 41,003, Biotium, Inc., Canada), Q5® HotStart High-Fidelity 2x Master Mix (Cat. No. M0494S, New England Biolabs® Inc., USA).
DNA primers used in this work are presented in Supplementary Table S1.
Competent cells used in this work were E.coli One Shot® TOP10 (Cat. No. 450,031, Invitrogen, USA).
Barcodes for DNA sequencing were provided by Eurofins Genomics (Germany).
Methods for obtaining peroxidase genes
The soil samples, down to 30 cm depth, were collected from Badín primeval forest. They were transported in 50 mL sterile centrifugation test tubes and frozen in liquid nitrogen. Fruiting bodies were collected manually with sterile gloves and were placed in sterile bags. They were transported on ice in a thermobox. After transport to the laboratory, both types of samples, soils and fruiting bodies, were frozen at -80 °C until use.
Genomic DNA was isolated from fungal fruiting bodies by Thermo Scientific® GeneJET Plant Genomic DNA Purification Mini Kit (Cat. No. K0702, Thermo Fisher Scientific®, Litva) and metagenomic DNA was isolated from soil samples using the DNeasy® PowerMax® Soil Kit (10), (Cat. No. 12988-10, Qiagen GmbH®, Germany).
Genomic or metagenomic DNA was used as a template for PCR amplification with appropriate primers. In all cases, the PCR reaction consisted of Q5® HotStart High-Fidelity 2x Master Mix, 0.5 µM Forward primer, 0.5 µM Reverse primer, genomic DNA or cDNA in concentration 0.8–1.6 ng.µL-1and the rest of the volume was filled with sterile water. PCR thermocycles depended on the length of the PCR product and melting temperature (TM) of the used primers (Suppl. Table S1). ITS (White et al. 1990) and 28 S rRNA (Kurtzman and Robnett 1997) primers were used for fungal species identification (Suppl. Table S1). In order to amplify the peroxidase genes, various primers designed according to the known peroxidase gene of Suillus luteus were used. The primers SlutHTP4aFwd and SlutHTP4aRev (Suppl. Table S1) were used to amplify the conserved segment encoding the catalytic core of the enzyme. In order to amplify the complete peroxidase genes, primers SlutHTP4cFwd and SlutHTP4cRev (Suppl. Table S1) were used. To amplify the gene without introns, we used the same primers and PCR procedure, but cDNA obtained by transcription of total RNA using the Thermo Scientific® Maxima First Strand cDNA Synthesis Kit with dsDNase (Ref. No. K1671, Thermo Fisher Scientific®, Lithuania) was used as a template.
Total RNA was obtained using the RNeasy® PowerSoil® Total RNA Kit (25) (Cat. No. 12866-25, Qiagen GmbH®, Germany) and Thermo Scientific GeneJET Plant RNA Purification Mini Kit (Cat. No. K0801) (Thermo Fisher Scientific®, Lithuania). PCR products were electrophoretically separated and subsequently extracted from an 1% agarose gel using the Monarch® DNA Gel Extraction Kit, (Cat. No. #T1020S, New England Biolabs® Inc., USA). Agarose gel was prepared in TAE solution (Tris 2 M, acetic acid 1 M, Chelaton III 50 mM). Then was added 0,01 %Gel red.
Purified PCR products of the appropriate size were cloned into a plasmid pCR®-Blunt II-TOPO® that is part of the Zero Blunt® TOPO® PCR cloning Kit for Sequencing, (Cat. No. 450,031, InvitrogenTM, USA) and subsequently were chemically competent Escherichia coli One Shot® cells TOP10 (Cat. No. 450,031, Invitrogen™, USA)transformed with the recombinant plasmids, which were seeded on Petri dishes with solid LB medium (1% Trypton, 0,5% Yeast extract, 0,5% NaCl) with kanamycin sulphate (50 mg.mL− 1). We incubated them overnight at 37 °C. Recombinants were distinguished from transformants without an insert based on blue-white screening, when IPTG and X-gal were applied to solid LB soil in Petri dishes. White colonies were picked and subsequently spread over a larger area on solid LB medium with kanamycin in a new Petri dish. They were also incubated overnight at 37 °C. From the grown recombinants, we inoculated 5 mL of liquid LB medium with kanamycin, which was incubated overnight at 37 °C. Subsequently, the overnight culture was centrifuged and the recombinant plasmid was isolated from it, using the Monarch® Plasmid Miniprep Kit (Cat. No. #T1010L, New England Biolabs® Inc., USA).
The isolated recombinant plasmid was sent for sequencing by Eurofins Genomics (Germany). We pipetted 7.5 µL recombinant plasmid and 2.5 µL of primer M13 Forward or M13 Reverse (10 pmol.µL− 1) into a centrifuge microtube marked with a barcode (Table 1).
We processed the resulting data from DNA sequencing in the BioEdit program, where we removed the sequence parts containing plasmid DNA based on the restriction of the target sequence by the primers used in the PCR reaction. The sequences were subsequently aligned using the Pairwise Alignment function found in the BioEdit program. We also managed to put together the sequences of phylogenetically related genes we were looking for.
Bioinformatic analyses
In the bioinformatics tool BLAST (NCBI), we determined the most similar output to DNA sequences discovered by us. The BLAST suite is an essential bioinformatics tool (Altschul et al. 1990). It includes more programs, where Nucleotide BLAST (NCBI) includes standard blastn, megablast or discontiguous megablast. These serve as alternative algorithms for searching nucleotide databases (Johnson et al. 2008). On the other hand, BlastX can be used instead of nucleotide blast to generate more precise hits via searching, thanks to better conserved protein sequences than nucleotide sequences (Nieminen et al. 2012). UniProt is a comprehensive protein online database which includes three main tools. The BLAST of UniProt serves for alternative searching of sequence conjunction and similarity (Pundir et al. 2016). Website of UniProt offers an interface to find the protein of interest and then investigate corresponding data. It is possible to use the search bar in the UniProt banners to enable simultaneous searches in different UniProt datasets (Pundir et al. 2017). BioEdit is program offering the largest and most adaptable pallet of such tools (Tippmann 2004). BioEdit was originally developed as a biological sequence alignment editor. Its basic components are easy manual alignment, split window view, auto integration with other programs such as ClustalW and Blast (Hall et al. 2011).
Obtained ITS and 28SrDNA sequences from fungal soil samples were evaluated using Nucleotide BLAST (NCBI). Further DNA sequences obtained from PCR amplification with SlutHTP4a and SlutHTP4c primer sets were evaluated using the blastX tool. If the sequence could not be identified using the BLAST (NCBI) and blastX (NCBI) tools, or if it was necessary to verify the sequence in another database, we used the BLAST bioinformatics tool in the UniProt database. However, here we copied the amino acid sequence. Such a sequence was generated for us by the Sorted six frame translation tool in the BioEdit program. This tool also allows us to set preferences related to the first codon in the nucleotide sequence or the preferred minimum length of the open reading frame.
Multiple sequence alignment of 201 collected heme-thiolate protein sequences was performed with Muscle algorithm implemented in MEGA X with up to 1000 iterations (Kumar et al. 2018). Optimized alignment parameters were gap open − 0.8, gap extend − 0.05, and hydrophobicity multiplier 0.9. The output was inspected and manually refined with GeneDoc software (version 2.7.000 https://nrbsc.org/gfx/genedoc/). Selected sequences from this alignment were used for detection of conserved amino acid residues responsible for the specific catalytic turnover within newly discovered sequence of SuilspHTP4.
Phylogenetic reconstruction was performed with MEGA X software package (Kumar et al. 2018) on the optimized protein alignment of 201 heme thiolate protein sequences obtained with Muscle algorithm. The maximum likelihood method was chosen with the application of Le Gascuel model of amino acid substitutions. This was proven as the model revealing the lowest Bayesian Information Criterion score among 56 available models in MEGA X. Following optimized parameters were used for the final phylogeny reconstruction: 2000 bootstrap replicates, gamma distributed substitution rates with invariant sites and seven discrete categories (+ G, parameter = 1.1076), partial deletion of gaps with a cut off at 95%, a nearest-neighbor-interchange heuristic method, initial tree constructed with BioNJ, branch swap filter set at very strong and number of threads set to 5. In total, 201 protein sequences with 187 alignment positions were used for the presentation of the final evolutionary tree.
For comparison the phylogeny of HTPs was also performed with Bayesian approach implemented in MrBayes version 3.2.6 located at www.phylogeny.fr (Dereeper et al. 2008). WAG model of amino acid substitutions and gamma rates of variation across sites were applied in this case. Four Markov Chain Monte Carlo (MCMC) chains were run for 100,000 generations with the first 500 sampled trees discarded as “burn in”. Finally, a 50% majority consensus tree was constructed.
Results and discussion
Our research is focused on the investigation of fungal biodiversity in various samples collected from Carpathian forests to compare them with related biotopes worldwide. We focus mainly on large group of enzymes known as peroxidases and fungi are a rich reservoir of their genes as documented in the international sequence database called RedoxiBase (Fawal et al. 2013; Savelli et al. 2019). Mainly lignolytic fungi were demonstrated to contain large variety of lignin, manganese and versatile peroxidases that all contain heme as a prosthetic group. Ectomycorrhizal fungi are less investigated in this respect. Therefore, we primarily attempted our metagenomic searches on potential peroxidase genes from soil ectomycorrhizal fungi. Among 4 known heme peroxidase superfamilies that are already well described (Zámocký et al. 2015) the most attractive appears the fungal peroxidase-peroxygenase superfamily which reveals the highest reaction versatility with respect to potential biotechnological applications (Hofrichter and Ulrich, 2006, Hofrichter et al. 2015). Members of this superfamily are frequently described as unspecific peroxygenases. Most of these peroxygenases are spread among various fungal species (Zámocký 2022) so it is interesting to compare the diversity of fungi with the appearance of paralogs of unspecific peroxygenases. Out of 10 samples collected from the area of Badín primeval forest we succeeded in PCR amplifications of a particular peroxidase-peroxygenase gene in the sample number 6 taken from a location with an altitude of 794 m over sea level from the northern part of a hill. The GPS coordinates for this location are 48°14 × 18´´N and 19°03 × 06´´E and it is dominated by very old beech trees. A similar fungal sample was collected also from a mixed forest located in Vtáčnik mountains with GPS coordinates 48°40’45"N 18°38’52"E (Fig. 1). Based on the ITS and 28 S rDNA sequences (not shown) from these two soil samples, we identified fungi belonging to the genus Suillus that were further subjected to PCR searches for the presence of peroxidase genes of interest.
Output from gel electrophoretic separation of PCR products in 1% agarose gel. a (left gel) - PCR products from reaction with SlutHTP4aRev and SlutHTP4aFwd - L1: DNA molecular weight standard 1kB, L2: DNA molecular weight standard 100 bp, Lane 1: PCR product from cDNA of Suillelus luridus, Lane 2: PCR product from cDNA of Suillus sp., Lane 3: PCR product from genomic DNA of Suillelus luridus, Lane 4: PCR product from genomic DNA of Suillus sp. b (right gel) - PCR products from reaction with SlutHTP4cFwd and SlutHTP4cRev from genomic DNA - L1: DNA molecular weight standard 1kB, L2: DNA molecular weight standard 100 bp, Lane 1 and 2: PCR product from genomic DNA of Suillelus luridus, Lane 3 and 4: PCR products from genomic DNA of Suillus sp, Lane 5: Negative control. Axis x– lanes with ladders and samples, axis y– sizes of PCR products and DNA fragments of ladder
We were able to first amplify the highly conserved regions of fungal HTP peroxidase genes in the PCR screening from genomic DNA libraries for diverse species of Suillus (Fig. 2a). We further decided to apply the RT-PCR method to discover such conserved parts of spliced peroxidase genes from transcriptomes isolated from total RNA of fungal fruiting bodies subsequently reverse transcribed (Fig. 2, a (left) gel lanes 1–2). They were amplified using specific primers SlutHTP4aFwd and SlutHTP4aRev. We obtained corresponding gene parts with introns by using the same set of primers on the corresponding genomic DNA of fruiting bodies (Fig. 2, a (left) gel lines 3–4). DNA sequencing of obtained clones revealed a small difference between genomic DNA and cDNA corresponding to the presence of a single intron with the size of 56 bp. This intron contains the typical highly conserved motif of dinucleotides GT/AG at intron 5´and 3´boundaries, respectively, as obvious from the comparison of the genomic and cDNA sequence (Suppl. Figure S1). Such conserved motif occurs as a rule in numerous fungal introns (e.g. Sakuradani et al. 1999).
Using primers SlutHTP4cFwd and SlutHTP4cRev, we were able to amplify the entire heme-thiolate peroxidase gene 4 from the genome of Suillus sp. isolated in Vtáčnik mountains (Fig. 2, b (right) gel, lanes 3,4). Obtained PCR product revealed after DNA sequencing the size of 812 bp corresponding to the protein with the size of 251 amino acids which fits well to the average size of heme-thiolate peroxidases of typical Basidiomycota. This gene from the sequenced clone GNF513 with the abbreviation SuilspHTP4 was submitted to GenBank and it got the accession code OR148270.1. A similar PCR product was also obtained from Badin primeval forest sample Nr.6 revealing after complete DNA sequencing a 100% identity with OR148270.1. In the case of Suillelus luridus, we were unable to amplify the complete gene with mentioned SlutHTP4c primer pair (Fig. 2, b (right) gel lanes 1,2). This is probably due to the high variability at the 3ʹ end of the corresponding DNA between the peroxidase genes even among members of the same fungal genus.
We managed to amplify and identify further partial sequences of this important peroxidase type spanning the conserved region of presented HTP4 gene from metagenome isolated from already above mentioned 10 soil samples from Badín primeval forest. These were sequences revealing a complete identity with a chloroperoxidase isolated from Suillus bovinus (XP_041303695), chloroperoxidase from Suillus fuscotomentosus (XP_041219546), chloroperoxidase from Suillus plorans (XP_041162229), chloroperoxidase from Suillus subalutaceus (XP_041244917), and also a chloroperoxidase from Boletus edulis BED1 (KAF8439949.1). It needs to be mentioned at this place that “chloroperoxidase” is the old name frequently used by automatic annotation of new genomes. The new and more appropriate name heme-thiolate peroxidase better describes its catalytic properties (Zámocký and Harichová, 2022). However, our newly discovered gene with the accession code OR148270.1 is unique and not present in any sequence database before. With BLAST comparison it revealed a 94.82% sequence identity with a hypothetical protein from Suillus luteus (KIK41958.1), a 64.98%, sequence identity with a chloroperoxidase of Butyriboletus roseoflavus (KAG8215460.1) and even a 61.97% sequence identity with a peroxidase part of a mitochondrial carrier domain-containing_fusion protein from Boletus reticuloceps (KAG6377757.1) that underlines the rather high level of conservation of the enzyme core. It also demonstrates the versatility of primer pair HTP4aFWD & REV (Suppl. Figure S2) that can be used for screening of HTP4 genes even beyond the genus Suillus. This means that corresponding PCR amplifies directly the DNA region coding for the highly conserved enzyme core. In the sequence databases, our newly identified complete gene labelled as SuilspHTP4 (accession code OR148270.1) has the highest similarity to the chloroperoxidase (heme-thiolate peroxidase) from the ectomycorrhizal fungus Suillus luteus (genomic scaffold KN835252) with 94.82% overall sequence identity and to Suillus brevipes (KAG2744134) with 94.42% overall sequence identity. A direct comparison of two HTP4 genes revealing the highest overall sequence similarity translated in their corresponding amino acid sequences is presented in Fig. 3.
Moreover, in the multiple sequence alignment shown in Fig. 4 (and in full length shown in Suppl. Figure S3) we have analyzed the presence of conserved amino acid residues responsible for typical peroxidase catalytic turnover that are already known from selected sequences of other heme-thiolate peroxidases. From comparison of Figs. 3 and 4 (and Suppl. Figure S3) we can conclude that all 13 observed amino acid substitutions of newly discovered SuilspHTP4 against already known SlutHTP4 variant occur in regions that are not essential for the catalytic activity because they are positioned in locations with a low overall sequence similarity. No insertions or deletions throughout the whole sequence can be found in Fig. 3. This means that the overall fold of this peroxidase superfamily is probably well preserved in SuilspHTP4. Presented details in highly conserved positions of the alignment clearly show that in newly discovered sequence of SuilspHTP4 all essential amino acid residues on the proximal (Fig. 4A) and the distal site of heme (Fig. 4B) are present in their correct positions. Among them the peculiar amino acid triad P-C-P is obviously invariant. It was demonstrated that this short proximal heme motif is unique for all characterized unspecific peroxygenases from the HTP superfamily (Zámocký et al. 2015). Two embedding proline residues are holding an exposed cysteine residue in optimal position for perfectly ligating the heme iron (Hofrichter and Ulrich 2006, Hofrichter et al. 2015). This determines the reactivity of the whole prosthetic heme group. Further, on the distal side of heme the second conserved motif E-H-D-X-S-L is also present as highly conserved with just one exception for Agrocybe aegerita enzyme known previously as aromatic peroxygenase but later it was confirmed as unspecific heme-thiolate peroxygenase (Hofrichter et al. 2015). Most important fact is that this distal motif is identical for all HTP sequences from the genus Suillus and it shows identity with 3 other representatives that already have well resolved X-ray structures (Fig. 4B). Within this motif mainly the distal histidine can interact with heme iron and the nearby conserved serine probably interacts with a magnesium ion. Thus, it can be expected that this novel unspecific heme-thiolate protein from a mushroom present in primeval Carpathian forests is able to perform all diverse catalyzed reactions typical for the peroxidase-peroxygenase superfamily. Such reactions mainly include epoxidations, dealkylations and hydroxylations in which one- or two-electron oxidations with oxygen transfer are involved. In common they can be described as oxyfunctionalization of organic molecules (Hofrichter et al. 2015; Zámocký and Harichová, 2022).
Multiple sequence alignment of ten selected protein sequences coding for heme-thiolate peroxidases. Demonstrated are only two highly conserved regions around the heme catalytic center responsible for the versatile enzyme activity: (a) sequence motif around the proximal ligand of heme, (b) sequence motif around the distal site of heme. Color scheme: blue > 95%, green > 80% and yellow > 60% of overall sequence conservation. Abbreviations of presented sequences: HTP– heme thiolate peroxidase, number at the end represents paralogous form according to RedoxiBase, APO– aromatic peroxygenase. Abbreviations of fungal species as sources of presented HTP genes: Suilsp– Suillus species from Vtáčnik primeval forest, Slut– Suillus luteus, Sbre– Suillus brevipes, Socci - Suillus occidentalis, Sampl– Suillus ampliporus, Bedu– Boletus edulis, Aae– Agrocybe aegerita, Mrot– Marasmius rotula, Cfumag– Caldariomyces fumago, Hsp– Hypoxylon sp. Numbers at the end of each sequence represent the amino acid position of presented sequence part in the whole protein molecule. Complete sequence alignment of these 10 sequences is presented in Suppl. Figure S3
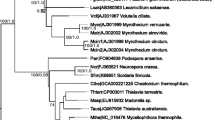
We have further analyzed the phylogenetic relationships of our newly discovered heme thiolate peroxidase gene SuilspHTP4 that we have obtained in two local forms: HTP4a was from Vtáčnik mountains whereas HTP4b originated from Badín primeval forest as sample number 6. After the translation of DNA from both obtained gene variants in corresponding protein sequences they revealed 100% identity. We compared these two sequences with numerous related heme-thiolate peroxidase genes of the large peroxidase-peroxygenase superfamily to demonstrate the exact evolutionary position of SuilspHTP4 paralog in the reconstructed evolutionary tree rendered with the maximum likelihood method. Results obtained after 2000 bootstrap replications are presented in Fig. 5. For comparison this phylogenetic reconstruction was perforemd also with Mr. Bayes approach in the online server. It revealed a tree topology that was very similar to the maximum likelihood tree output of MEGA X package (Kumar et al. 2018) as obvious from statistical values presented in Fig. 5.
The phylogenetic position of newly discovered heme-thiolate peroxidase from Suillussp. is obvious from this robust reconstruction. Although BLAST searches first indicated relative high level of protein sequence conservation along the whole analyzed genomic region for basidiomycetous fungi this evolutionary tree obtained with the maximum likelihood method of the MEGA X package using Le Gascuel model of amino acid substitutions (with lowest Bayesian information criterion) revealed a well-defined and separate clade for numerous HTP genes from the genus of Suillus that form a monophyletic clade with a very high bootstrap support (values of 99/93 for maximum likelihood/Bayesian reconstruction, respectively). Another interesting fusion protein from Boletus reticuloceps containing a similar peroxidase domain fused with a mitochondrial carrier domain is only distantly related to the Suillus peroxidase clade. Together with several other HTP sequences from the otherwise rather closely related genus Boletus this unique protein forms another distinct clade but all other Boletus sequences of peroxidases do not contain such a gene fusion that needs to be verified at the transcriptional and translational level. Another small sister clade to Boletus HTPs are sequences represented by genus Paxillus (Fig. 5) well known as poisonous mushrooms. This is in contrast with mostly edible representatives of the genera Suillus and Boletus. However, the contribution of peroxidases to this phenomenon does not appear to be significant as closely related peroxidase genes exist in both edible and poisonous mushrooms of the Boletaceae family. Important fact is that this unique SuilspHTP4 gene was discovered in a soil fungus originating from a primeval forest where it would be expected that a typical biotope containing diverse trees and ectomycorrhizal fungi is preserved for its natural function for a long time period.
Reconstructed maximum likelihood phylogenetic tree of the HTP (peroxidase-peroxygenase) superfamily obtained with MEGA X (Kumar et al. 2018). Color labels: green: new HTP4 peroxidases from Suillus sp., identified in primeval forests of Vtáčnik (a) and Badín (b), respectively, blue: peroxidase from S. luteus - the most closely related one to the newly discovered sequences, red HTP from well know edible fungus Boletus reticuloceps with a putative gene fusion including a mitochondrial carrier domain. A very similar tree topology was obtained also with MrBayes version 3.2.6. Numbers in nodes represent bootstrap values for 2000 replications and posterior probabilities, respectively. Only values above 30 are shown
Conclusion
Ectomycorrhizal fungi have a large impact on the forest ecology by enhancing nutrient uptake for trees and their contribution to carbon cycling. As we have demonstrated in this contribution basidiomycetous ectomycorrhizal fungi are a rich source of various types of peroxidases, including mainly heme-thiolate peroxidases. We decided to amplify and clone one selected variant named SuilspHTP4 that was subjected to various in silico bioinformatic analyses. In this connection it is interesting to note that in the genome of Cortinarius glaucopus considered also as an ectomycorrhizal fungus up to 11 peroxidases were found, a number comparable to many wood decay fungi (Bödeker et al. 2014). In the case of Suillus luteus 5 diverse heme-thiolate peroxidases were described in the sequence databases but our newly discovered HTP4 variant appears to be unique worldwide. Its origin is in the primeval forest of Badin that can serve as a reservoir of many interesting genes coding for enzymes applicable in modern biotechnologies. We will therefore continue to identify novel peroxidase genes, amplify them with various PCR methods, determine their primary structure and demonstrate their phylogenetic relationships. We will continue to identify various peroxygenases from divergent protein families for oxyfunctionalization which means enzymatic incorporation of oxygen atoms in numerous organic compounds leading to a green chemical technology. For this purpose, we will also attempt the heterologous expression of a construct without intron in suitable hosts. In the next step we will optimize the heterologous production of here identified gene SuilspHTP4 (OR148270.1) for biotechnological purposes.
Data availability
Analysed data are available on request from the corresponding author.
Abbreviations
- AaeAPO:
-
Aromatic peroxygenase from Agrocybe Aegerita
- APO:
-
Aromatic peroxygenase
- BeduHTP4:
-
Heme-thiolate peroxidase 4 from Boletus edulis
- CfumagHTP:
-
Heme-thiolate peroxidase from Caldariomyces fumago
- HspHTP1:
-
Heme-thiolate Peroxidase 1 from Hypoxylon sp
- HTP:
-
Heme-thiolate peroxidase
- HTP4:
-
Heme-thiolate peroxidase 4
- IPTG:
-
Isopropyl β- d-1-thiogalactopyranoside
- ITS:
-
Internal transcribed spacer
- MrotHTP1:
-
Heme-thiolate peroxidase 1 from Marasmius rotula
- SamplHTP4:
-
Heme-thiolate peroxidase 4 from Suillus ampliporus
- SbreHTP4:
-
Heme-thiolate peroxidase 4 from Suillus brevipes
- SlutHTP4:
-
Heme-thiolate peroxidase 4 from Suillus luteus
- SocciHTP4:
-
Heme-thiolate peroxidase 4 from Suillus occidentalis
- SuilspHTP4:
-
Heme-thiolate peroxidase 4 from Suillus sp
- UPO:
-
Unspecific peroxygenase
- X-gal:
-
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Ayala M, Batista CV, Vazquez-Duhalt R (2011) Heme destruction, the main molecular event during the peroxide-mediated inactivation of chloroperoxidase from Caldariomyces Fumago. J Biol Inorg Chem 16:63–68. https://doi.org/10.1007/s00775-010-0702-6
Bansal N, Kanwar SS (2013) Peroxidase (s) in environment protection. Sci World J 2013:714639. https://doi.org/10.1155/2013/714639
Bindoli A, Rigobello MP (2013) Peroxidase biochemistry and redox signaling In Lennarz WJ, Lane MD, encyclopedia of biological chemistry. Academic Press 407–412. https://doi.org/10.1016/B978-0-12-378630-2.00179-1
Bocanegra-Rodríguez S, Jornet-Martínez N, Molins-Legua C, Campíns-Falcó P (2020) New reusable solid biosensor with covalent immobilization of the horseradish peroxidase enzyme: in situ liberation studies of hydrogen peroxide by portable chemiluminescent determination. ACS Omega 5:2419–2427. https://doi.org/10.1021/acsomega.9b03958
Bödeker IT, Clemmensen KE, de Boer W, Martin F, Olson Å, Lindahl BD (2014) Ectomycorrhizal C ortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol 203:245–256. https://doi.org/10.1111/nph.12791
Cahanovitc R, Livne-Luzon S, Angel R, Klein T (2022) Ectomycorrhizal fungi mediate belowground carbon transfer between pines and oaks. ISME J 16:1420–1429. https://doi.org/10.1038/s41396-022-01193-z
Chowdhary P, Shukla G, Raj G, Ferreira LFR, Bharagava RN (2019) Microbial manganese peroxidase: a ligninolytic enzyme and its ample opportunities in research. SN Appl Sci 1:1–12. https://doi.org/10.1007/s42452-018-0046-3
Cullings K, Ishkhanova G, Henson J (2008) Defoliation effects on enzyme activities of the ectomycorrhizal fungus suillus granulatus in a Pinus contorta (lodgepole pine) stand in Yellowstone National Park. Oecologia 158:77–83. https://doi.org/10.1007/s00442-008-1119-6
Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acid Res 36:465–469. https://doi.org/10.1093/nar/gkn180
Fawal N, Li Q, Savelli B, Brette M, Passaia G, Fabre M, Mathé C, Dunand C (2013) PeroxiBase: a database for large-scale evolutionary analysis of peroxidases. Nucleic Acids Res 41:D441–D444. https://doi.org/10.1093/nar/gks1083
Hall T, Biosciences I, Carlsbad CJGBB (2011) BioEdit: an important software for molecular biology. GERF Bull Biosci 2:60–61
Hofrichter M, Ullrich R (2006) Heme-thiolate haloperoxidases: versatile biocatalysts with biotechnological and environmental significance. Appl Microbiol Biotechnol 71:276–288. https://doi.org/10.1007/s00253-006-0417-3
Hofrichter M, Kellner H, Pecyna MJ, Ullrich R (2015) Fungal unspecific peroxygenases: heme-thiolate proteins that combine peroxidase and cytochrome P450 properties. In Hrycay EG, Bandiera SM (ed) Monooxygenase, peroxidase and peroxygenase properties and mechanisms of cytochrome P450. Springer, Cham 851:341–368. https://doi.org/10.1007/978-3-319-16009-2_13
Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL (2008) NCBI BLAST: a better web interface. Nucleic Acids res 36:5–9. https://doi.org/10.1093/nar/gkn201
Kumar S, Stecher G, Li M, Knyaz C, TamuraK (2018) Mega X: Molecular Evolutionary Genetics Analysis across Computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kunca V, Peiger M, Tomka P, Vampola P (2022) Old-growth forest fungi–new localities and habitat and host preferences in Slovakia (I). Czech Mycol 74:33–55. https://doi.org/10.33585/cmy.74103
Kurtzman CP, Robnett C (1997) Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. https://doi.org/10.1128/JCM.35.5.1216-1223.1997
Mihál I (2013) K poznaniu mykoflóry (Ascomycota, Basidiomycota, Deuteromycota–Fungi imperfecti) Národnej prírodnej rezervácie Badínsky prales. Natura Carpatica 54:7–16
Näsholm T, Högberg P, Franklin O, Metcalfe D, Keel SG, Campbell C, Hurry V, Linder S, Högberg MN (2013) Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests? New Phytol 198:214–221. https://doi.org/10.1111/nph.12139
Neumann B, Wollenberger U (2020) Electrochemical biosensors employing natural and artificial heme peroxidases on semiconductors. Sens 20:3692. https://doi.org/10.3390/s20133692
Nieminen TT, Koskinen K, Laine P, Hultman J, Säde E, Paulin L, Paloranta A, Johansson P, Björkroth J, Auvinen P (2012) Comparison of microbial communities in marinated and unmarinated broiler meat by metagenomics. Int J Food Microbiol 157:142–149. https://doi.org/10.1016/j.ijfoodmicro.2012.04.016
Pecina MJ, Ulrich R, Bittner B, Clemens A, Scheibner K, Schubert R, Hofrichter M (2009) Molecular characterization of aromatic peroxygenase from Agrocybe Aegerita. Appl Microbiol Biotechnol 84:885–897. https://doi.org/10.1007/s00253-009-2000-1
Pundir S, Martin MJ, O’Donovan C, UniProt Consortium (2016) UniProt tools. Curr Protoc Bioinform 53:1–29. https://doi.org/10.1002/0471250953.bi0129s53
Pundir S, Martin MJ, O’Donovan C (2017) UniProt protein knowledgebase. Methods Mol Biol 1558:41–55. https://doi.org/10.1007/978-1-4939-6783-4_2
Sakuradani E, Kobayashi M, Shimiyu S (1999) Identification of an NADH-cytochrome b5 reductase gene from an arachidonic acid-producing fungus, Mortierella alpina 1S-4, by sequencing of the encoding cDNA and heterologous expression in a fungus, aspergillus oryzae. Appl Environ Microbiol 65:3873–3879. https://doi.org/10.1128/aem.65.9.3873-3879.1999
Savelli B, Li Q, Webber M, Jemmat AM, Robitaille A, Zamocky M, Mathé C, Dunand C (2019) RedoxiBase: a database for ROS homeostasis regulated proteins. Redox Biol 26:101247. https://doi.org/10.1016/j.redox.2019.101247
Sundaramoorthy M, Terner J, Poulos TL (1995) The crystal structure of chloroperoxidase: a heme peroxidase–cytochrome P450 functional hybrid. Structure 3:1367–1377. https://doi.org/10.1016/s0969-2126(01)00274-x
Svetozarević M, Šekuljica N, Onjia A, Barać N, Mihajlović M, Knežević-Jugović Z, Mijin D (2022) Biodegradation of synthetic dyes by free and cross-linked peroxidase in microfluidic reactor. Environ Technol Innov 26:102373. https://doi.org/10.1016/j.eti.2022.102373
Tippmann HF (2004) Analysis for free: comparing programs for sequence analysis. Brief Bioinform 5:82–87. https://doi.org/10.1093/bib/5.1.82
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: Guide Methods Appl 18:315–322
Zámocký M (2022) Discovering diverse roles of peroxidases and catalases in photosynthetic and non-photosynthetic eukaryotes. Antioxidants 11:2337. https://doi.org/10.3390/antiox11122337
Zámocký M, Droghetti E, Bellei M, Gasselhuber B, Pabst M, Furtmüller PG, Battistuzzi G, Smulevich G, Obinger C (2012) Eukaryotic extracellular catalase-peroxidase from Magnaporthe grisea– Biophysical/chemical characterization of the first representative from a novel phytopathogenic KatG group. Biochimie 94:673–683. https://doi.org/10.1016/j.biochi.2011.09.020
Zámocký M, Hofbauer S, Schaffner I, Gasselhuber B, Nicolussi A, Soudi M, Pirker KF, Furtmüller PG, Obinger C (2015) Independent evolution of four heme peroxidase superfamilies. Arch Biochem Biophys 574:108–119. https://doi.org/10.1016/j.abb.2014.12.025
Zámocký M, Harichová J, (2022) Evolution of heme peroxygenases: Ancient roots and later evolved branches. Antioxidants 11:1011. https://doi.org/10.3390/antiox11051011
Acknowledgements
Our research was supported by the Slovak Grant Agency with project VEGA 2/0012/22 and by the Slovak Research and Development Agency with project APVV-20-0284.
Funding
Our research was supported by the Slovak Grant Agency with project VEGA 2/0012/22 and by the Slovak Research and Development Agency with project APVV-20-0284.
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Contributions
BK and PF collected data, BK, KC and MZ analyzed data, BK and MZ wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not required.
Informed consent
Not applicable.
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubala, B., Ferianc, P., Chovanová, K. et al. In silico analysis of a heme-thiolate peroxidase gene discovered in an ectomycorrhizal fungus of Carpathian primeval forest: implications for biotechnological applications. Biologia 79, 2253–2264 (2024). https://doi.org/10.1007/s11756-024-01709-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01709-2