Abstract
Petroleum hydrocarbons are a major concern due to their widespread distribution in the environment, and their harmful effects on humans. The use of biodegradation methods to clean soil that has been contaminated by spilt petroleum can be an effective remediation strategy. Therefore, in this study, the presence of indigenous microorganisms in soil contaminated with crude oil was investigated and their ability to degrade petroleum in vitro was studied. Three methods were used to detect crude oil and pure hydrocarbon degradation by bacterial and fungal isolates. Spectrophotometric method, 2,6-Dichlorophenol indophenol (DCPIP) method and hydrocarbon analysis was performed by GC–MS. Furthermore, PCR was carried out to detect the genes AlkB and PMO in the microbial communities. Seventeen bacterial isolates and 5 fungal isolates were recovered from 19 soil culture samples obtained from three different oil-contaminated sites at Taq-taq (TTOPCO) oil field and from a control site without oil contamination. The bacterial samples were identified culturally and morphologically, and the results were validated by genetic analysis. The results of this study revealed that the indigenous bacterial and fungal isolates had a high capacity to degrade crude oil and pure hydrocarbon fractions. Our results provide insight into the great abilities of Bacillus anthracis, Bacillus cereus, Achromobacter sp., Aspergillus lentulus and Rhizopus arrhizus to degrade crude oil and pure hydrocarbon fractions, and these data are supported by the presence of the PMO and AlkB genes in the isolated microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Kurdistan Region of Iraq (KRI) is located north and northeast of the Arabian plate. The region is one of the oil-rich areas in Iraq (Shlimon et al. 2020) Koya city is one of the oil-rich areas in the Kurdistan Region with many reservoirs, among which the Taq-taq (TTOPCO) reservoir is one. The intensive use of petroleum, however, results in environmental disruption (Xue et al. 2015). Spills that occur during and/or as a result of petroleum extraction, storage, refining, manufacturing, shipping, oilfield development, and leakage from oil pipelines or tankers, and the discharges of petroleum hydrocarbons are also major concerns due to their widespread distribution into the environment, such as soil and water, which affects human health (Chen et al. 2015; Wang et al. 2018).
Managing hydrocarbon contamination has become easier due to the development of several new technologies in recent years. Biological processes are utilized in the bioremediation process, which are a collection of technologies that either aid in the elimination of contaminants or make them minimally hazardous (Janssen et al. 2002). These procedures are cost-effective and can be applied in their entirety to areas that are contaminated. Consequently, microbial degradation is a promising method for the complete mineralisation of hydrocarbons into carbon dioxide and water (Wang et al. 2015).
Bacteria, fungi, and yeast biodegrade hydrocarbons in the environment. Some bacterial species can metabolize specific alkanes, while others break down the aromatic or resin fractions of hydrocarbons in many different manners depending on the particular oxygenase (Xu et al. 2018). Although several fungi can grow in soil, few species can survive in contaminated soils with biodegradation efficiencies ranging from 6 to 82% (Juhasz and Naidu 2000). The aims of this study were to isolate and identify indigenous bacterial and fungal species that were found in the soil of the Taq-taq (TTOPCO) oil field. Then, we investigated the existence of genes that encode monooxygenase enzymes in the isolated microbial species and their ability to degrade crude oil and pure hydrocarbons fractions using spectrophotometry and 2,6-dichlorophenol indophenol (DCPIP) methods. in addition, radial growth was measured, and hydrocarbon analysis was performed by GC–MS.
Materials and methods
Sample and sampling locations
All the samples were collected from the Koya City Taq-taq asphalt seep (TTOPCO), which is in the Kurdistan Region of Iraq (KRI). Samples of contaminated soil were collected throughout the month of October at a depth of 5 to 10 cm from each of the four separate locations shown in Table 1.
All samples were placed into sterilized polyethylene bags. Approximately 2 L of oil samples collected from operating oil wells were stored in bottles that had been carefully sealed. The bottles and polyethylene bags were placed in a container packed with ice for transport to the laboratory and then stored at 4 °C. The physicochemical characteristics of the crude oil and soil are shown in Table 2 (Schofield 2017; Karim et al. 2017).
Morphological and molecular identification of isolated bacteria and fungi
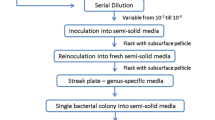
Pure culture techniques were applied to isolate pure single colonies from each soil sample using nutrient agar, McConkey agar, cetrimide agar and mannitol salt agar (Alsayegh et al. 2021). Then, biochemical tests and Gram staining were conducted with all isolated single colonies (Riedel et al. 2019). Fungi were cultivated on potatoes dextrose agar (PDA) and identified using morphological and taxonomic keys found in mycological keys (Watanabe 2018). The genomic DNA of the isolated bacteria and fungi was extracted by a QIAwave DNA Blood and Tissue Kit (Germany, Cat. no. 69556) according to the manufacturer’s protocol. A Nanodrop spectrophotometer (NanoDrop Spectro 117 432-UK) was used to determine the quality and quantity of the genomic DNA. The 16S rRNA gene of bacteria, which contains a highly variable region, was amplified by PCR (Polymerase chain reaction) using the universal primer pair EubA/EubB. On the other hand, the internal transcribed spacer (ITS) region of the fungal isolates was amplified by PCR using the universal primer pair LROR/LR6 (Macrogen Inc., a South Korean) Table 3. Bacterial PCR was performed in a final volume of 25 μL using Prime Q5 Hot Start High-Fidelity 2X Master Mix (cat. no.M0494S). PCR included 12.5 μl of 2 × Master Mix, 10 pmol (1 μL) of each of forwards (Eub A) and reverse (Eub B) primer, 100 ng (1 μL) of template DNA, and 9.5 μL nuclease-free water. PCR was performed using a BIO-RAD T100TM Thermal Cycler (UK) as follows: 2 min of initial denaturation at 98 °C followed by 25 cycles of reaction with the 50 s of denaturing at 98 °C, 50 s of annealing at (64.3, 61, 59, 58, and 56) °C, and 50 s of extension at 72 °C, and the final extension was performed for 4 min at 72 °C.
To amplify the ITS region of the fungal isolates, PCR was performed in a final volume of 25 μL, which included 12.5μL of 2 × Master Mix, 10 pmol (1.5 μL) of each forwards (LROR) and reverse (LR6) primer, 3 μL template DNA, and 6.5 μL of nuclease-free water. PCR was carried out as previously described for bacterial isolates with an annealing temperature of 56 °C and 34 cycles. The efficiency of DNA extraction was evaluated using electrophoresis on a 1% agarose gel (w/v) that was stained with ethidium bromide (10 g/L) and had a 100 bp DNA marker (DENA 100 bp plus DNA size marker II S-5091). The gel was run at 80 V for 1.5 h in a 1X TBE buffer. Then, ultraviolet light was used to visualize the DNA bands, and photographs were taken using a UV gel imager (SynGene 1409) (Lee et al. 2012). All PCR amplicons were sent out for sequencing by Macrogen Inc., a South Korean company, using primer EubA F for the bacterial origin PCR amplicon and primer LROR from the fungal PCR source Table 3. Sequence quality, analysis, and editing were carried out using the DNA base assembly tool. The 16S rDNA sequence was compared to previously identified bacterial DNA sequences using the BLASTN (http://www.ncbi.nih.gov/BLAST) to classify the bacterial isolates independently.
Degradation of crude oil and pure hydrocarbon fractions by the isolated bacteria and fungi
Minimal salt medium (MSM) was used for bacterial and fungal isolates (Sigma-Aldrich). This medium is composed of the following ingredients: 0.2 g/L MgSO4, 0.02 g/L CaCl2, 1 g/L KH2PO4, 1 g/L K2HPO4, 1 g/L NH4NO3, and 0.05 g/L FeCl3. Trace elements (1 mL/L) were added to the mixture (4.77 g of CaCl2.2H2O/100 mL, 0.37 g of FeSO4.7H2O/ 100 mL,0.10 g of MnCl2.4H2O/100 mL, and 0.02 g of sodium molybdate/ 100 mL). The pH was adjusted to 8. Then 1 mL/L vitamin mixture solution was added (10.0 mg of Pyridoxine–HCl, 5.0 mg of p-aminobenzoic acid, 5.0 mg of lipoic acid, 5.0 mg of nicotinic acid, 5.0 mg of riboflavin, 5.0 mg of thiamine-Hcl, 5.0 mg of calcium D1-Pantothenate, 2.0 mg biotin, 2.0 mg of folic acid, and 0.1 mg of vitamin B12 in 100 mL). Finally, 0.1 g/L yeast extract was added to the medium.
Crude oil was distillated using simple distillation. First, the crude oil was poured into a round bottom flask. Then, the fractionation column was adjusted and a thermometer with a conical flask was placed on one side and the condenser was placed on the other. The temperature was measured with a thermometer, The fractional distillation method carried out with a distillation tower separates crude oil into different, more valuable hydrocarbon products based on their relative molecular weights. Gasoline was extracted in -1 to 200 ºC and naphtha was extracted from -1 to 205 ºC (Ristinen et al. 2022; Wolfson 2011; Logeshwaran et al 2018).
Biodegradation experiments were carried out in glass screw cup tubes containing 10 mL of MSM and 1% crude oil as the sole carbon source. Prior to adding the crude oil, both the media and the crude oil were sterilized separately by autoclaving at 121 °C for 15 min. A single colony of bacterial isolate was inoculated into 10 mL nutrient broth and incubated overnight at 30 °C at 150 rpm. Following incubation, the culture was centrifuged for 10 min at 10,000 rpm. The bacterial suspension was ready for use after the cell pellets were washed to remove all nutrients and resuspended in normal saline until the OD at 600 nm was equivalent to 1. One percent of the bacterial suspension was transferred to the MSM supplemented with crude oil. Uninoculated media was used as a control. All tests and controls were performed in triplicate. The same procedure was followed for pure hydrocarbon fraction.
Crude oil and pure hydrocarbon fraction degradation
To detect the extent of Crude oil and pure hydrocarbon degradation by the isolated microbes, the following methods were used:
-
1-
spectrophotometric method: The absorbence was measured at 600 nm. First, DW and the control samples were used as blanks to set the spectrophotometer to 0% (baseline). The absorbance of the samples was measured at time zero (0), 5 days, 10 days, 15 days, 20 days and 30 days to determine bacterial growth.
-
2
–2,6-Dichlorophenol indophenol (DCPIP) method: One millilitre of bacterial suspension with an OD600 nm of 1, 1% V/V crude oil and 1% (0.6 mg/L) of redox indicator (DCPIP) were added to 10 mL of MSM, and the mixture was incubated for seven days at 30 °C with shaking at 150 rpm. Then, every 24 h, the colour changes were monitored. Uninoculated media was utilized as a control, which consisted of 10 mL of MSM, 1% crude oil, and 1% DCPIP (Selvakumar et al. 2014; Balogun et al. 2015). For fungal growth, a glass screw cup container with 10 mL of MSM supplemented with 1% crude oil and 1% 2,6-Dichlorophenol indophenol (DCPIP) redox indicator was used. The inoculated containers were incubated for 7 days at 30 °C with shaking at 150 rpm (Barnes et al. 2018). Moreover, the results from another experimental method were recorded and supported by measuring the radial mycelial growth by cultivating all of the fungal isolates on solid MSM supplemented with varying concentrations of crude oil (5, 10, 15, and 20) %. The control group consisted of solid MSM without crude oil. All plates were incubated for 7 days at 30 °C. The radial mycelial growth (mm/day) was measured after 7 days of incubation at 30 °C (Reyes-César et al. 2014).
-
3-
Hydrocarbon analysis by gas chromatography-mass spectrometry (GC–MS):
Cultures were set up as follows: Prepared MSM for bacteria and fungi were dispensed in aliquots of 10 mL in sterile 50 mL glass bottles, which were inoculated separately with 1% washed microorganisms (17 bacterial isolates and 5 fungal isolates). Then, 1% sterile crude oil or 1% of pure hydrocarbon fraction were added as the carbon source. To facilitate contact between microorganisms and hydrocarbons, small pieces of sterile sponges were added to the cultures. The bottles were closed with aluminum screw caps and Teflon stoppers to avoid evaporation of hydrocarbons. For each isolated microorganism, triplicate cultures were prepared, and each was incubated for 10 days. Triplicate controls for each incubation period were prepared in the same way as the cultures but were not inoculated with microorganisms. The cultures and controls were incubated in a shaker incubator at 150 rpm and 30 ºC. After a 10-day incubation period, the cultures and controls were stored in a -20 ºC freezer until analysis. The purpose of having controls with the incubation batch was to determine the accuracy of hydrocarbon biodegradation and calculate the percent biodegradation.
Gas chromatography-mass spectrometry (GC–MS) analyses: The n-alkanes and other hydrocarbons were extracted from the cultures and controls with dichloromethane (DCM) by using the shaking flask method as described earlier (Schwab et al. 1999). By using sterile glass Pasteur pipettes, twenty millilitres of DCM was transferred to the cultures, and the bottles were closed and shaken on a waver shaker (Waver, VWR Scientific products) at 130 rpm for six hours. Then, the contents of the culture and control bottles were transferred into 100 mL burettes, and the DCM phase that contained hydrocarbons was separated from the water phase, i.e., the medium that contained microorganisms. The water phase was collected in a culture bottle and stored for DNA extraction. The DCM phase was drained through a funnel containing ten grams of Na2SO4 into a sterile 50 mL volumetric flask. Na2So4 was used to absorb any water present in the DCM. Each of the collected hydrocarbon solutions were evaporated in a vacuum evaporator to a volume of 5mL. The hydrocarbons were purified by filtration using SEP columns (Hawach SPE silica cartridges, China). The columns were washed with 5 mL of a mixture of pentane and DCM (1:1). Then 1mL of the hydrocarbon solution was added to the column, and the filtrate was collected in 10 mL sterile volumetric flasks. Before removing the flask, the columns were washed with 5 mL of the same mixture of pentane and DCM to remove any hydrocarbons from the column. Each of the filtered solutions were evaporated to a volume of 2 mL. Prior to analysing the samples on a GC-mass spectrometer (Agilent GC–MS 7890 A), they were further diluted by a factor of 10 to be close to the concentration of the n-alkane standards. The conditions of the GC–MS instrument are given in Table 4. The concentration of n-alkanes was calculated by integrating the peak areas and comparing these areas to those used to determine the linear regression equation obtained with the standard solutions. The percent biodegradation of the n-alkanes was calculated as described by (Deng et al. 2014; Singh et al. 2015).
Amplification of bacterial gene alkB and fungal gene PMO
PCR was carried out using primers that had been specifically designed to target the alkane hydroxylase and putative monooxygenase genes in isolated microbes Table 3. PCR was carried out in the same manner as the reaction mixtures for the 16S rRNA gene and ITS region of the 18S RNA gene, except that 1 μL of 5 × bovine serum albumin (Promega) was added to the PCR mixture to enhance the yield (Farell and Alexandre 2012). Adjustments were made to the annealing temperature because each primer set has a unique annealing temperature, as shown in Table 3. The total number of cycles was raised to 34. The PCR products were detected with 2% agarose ethidium bromide gels in 1x TBE buffer. A 100 bp DNA marker (DENA 100 bp plus DNA size marker II S-5091) was used as the molecular marker.
Results
Phenotypic and molecular characterization of bacterial and fungal isolates
Seventeen bacterial isolates out of 19 soil culture samples were recognized by utilizing Bergey's Manual of Systematic Bacteriology for phenotypic and colony identification (Bilen Ozyurek and Seyis Bilkay 2017), while the other two samples showed no signs of growth. To confirm the identities of the samples, total DNA was extracted from all the seventeen bacterial isolates and then amplified using EubA F and EubB R primer pair, which binds specifically to the 16S rRNA gene, as mentioned in Section "Morphological and molecular identification of isolated bacteria and fungi". The expected size of the DNA fragment (~ 1534) confirmed that amplification was successful. No PCR products were detected in the negative control, as shown in Fig. 1.
Partial amplification of 16S rDNA using primers EubA and EubB. Lanes 1 and 2 are the 100 bp DNA marker (DENA 100 bp plus DNA size marker II S-5091) and a negative control that was run without a DNA template, respectively. Lanes 3 through 19 shows ~ 1534 bp of PCR amplicons from bacterial sample numbers 1 through 17, respectively, generated using a DNA template
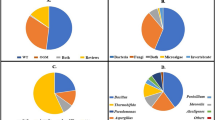
To determine the species of each bacterial isolate, the 16S rRNA gene PCR amplicons that were produced from each isolate using primers EubA F and EubB R were sequenced using the EubA F primer (Table 3). To specify the taxonomic origin of the 17 16S rDNA sequences, independent computerized alignments were performed versus accessible prokaryotic sequences of 16S rDNA employing NCBI BLAST. All 17 individual sequences were found to be identical to previously cultivated bacteria as follows. The identified bacterial genera from Group A soil samples were Caldibacillus thermoamylovorans strain SSBM chromosome (accession number CP023704), Bacillus pumilus strain mv49b (accession number KU230016), Bacillus tropicus strain NP_ 2. (accession number OP048825.1), and Pseudomonas aeruginosa strain DM Bust3A (accession number MF599526.1). Group B soil samples included Aneurinibacillus migulanus strain DSM 2895 (accession number NR 112214.1), Achromobacter sp. MT093185.1, Bacillus anthracis strain FDAARGOS 695 (accession number CP054816.1), Bacillus cereus strain T11-12 (accession number HQ333011.1), and Lysinibacillus sp. 381(accession number KT034471.1). Group C soil samples included Paenibacillus dentritiformis strain PV3-16 (accession number MH472941.1), Aneurinibacillus migulanus strain RD (accession number KX083693.1), Brevibacillus borstelensis strain ML13. (accession number MN604049.1), and Bacillus paramycoides strain EFBC 17 (accession number MN793201.1). Group D soil samples contained Bacillus anthracis strain FDAARGOS_695 (accession number CP054816.1), Pseudomonas stutzeri strain HA549 (accession number KJ535356)., Bacillus paramycoid 2883 (accession number MT611845.1), and Lysinibacillus capsici strain anQ-h6 (accession number CP084108.1).
The predicted size of the DNA fragment (~ 1200 bp) was effectively amplified from the five fungal isolate template DNA samples, and no PCR products were observed in the negative controls (Fig. 2). The PCR amplicons were sequenced using the forwards primer LROR (Table 3), and then the resulting nucleated sequences were submitted to the NCBI to determine the species of each fungal isolate. The following fungal genera were identified. Group A included Aspergillus lentulus 28S ribosomal RNA (accession number XR 004500616.1). Group B included Aspergillus fellis strain FM324 chromosome 3 (accession number CP066505.1), Aspergillus luteonubrus strain MST FP2246 (accession number MT196912.1) and Aspergillus arizonicus isolate CCF 5341 (accession number OK321187.1). Group C included Rhizopus arrhizus Strain SC49B03 (accession number MW113537.1).
Partial amplification of the ITS using the primers LROR/LR6. Lanes 1 and 2 are the 100 bp DNA marker (DENA 100 bp plus DNA size marker II S-5091) and a negative control that was run without a DNA template, respectively. Lanes 3 through 7 shows ~ 1200 bp PCR amplicons from fungal sample numbers 1 through 5, respectively, generated using a DNA template
Degradation of crude oil and pure hydrocarbon fractions by the bacterial isolates
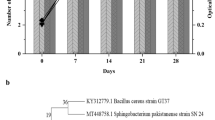
The OD readings of all of the bacterial suspensions was between 0.798 and 0.900 at the beginning of the experiment, which corresponds to the point at which the experiment started (zero time). After five days, the OD values for isolates 6, 7, 8, 10, 11, 12, 14, and 16 remained unchanged or increased slightly, but those for other isolates declined. After a period of 10 days, the OD readings of the isolates 6, 8, 11, 12, and 14 remained the same or increased. These results showed an exponential growth phase between Days 5 and 10 days, during which time the OD readings of other isolates declined. After 15 days, the OD reading for isolate numbers 6, 8 and 14 showed only a minor decline. After 20 days, the optical density (OD) values of the previously mentioned isolates declined further but could still be measured. However, after a period of 30 days, the OD reading demonstrated a significant drop for each of the isolates, as shown in (Table 5). This was due to a decline in the availability of carbon sources and nutrients. The most resistant isolates to crude oil and those that grew well in minimal media supplemented with crude oil were Achromobacter sp., Bacillus cereus, Pseudomonas aeruginosa and Bacillus anthracis (Fig. 3).
Bacterial isolates 6, 7, 8, 10, 11, 12, and 14 which showed good growth in the crude oil solution and had a high OD value, were taken for further testing in degrading pure hydrocarbon fractions. These isolates grew successfully, and the OD reading of isolates 6, 7, 8,12, and 14 was 0.788, 0.724, 0.923, 0.787and 1.124, respectively.
2,6-Dichlorophenol indophenol (DCPIP) method: The suspension for the experiment was set up in accordance with the procedure described in Section "Morphological and molecular identification of isolated bacteria and fungi". According to the findings, sample numbers 7, 8, 11, 12, and 14 had a significant capacity to turn the coloured solution colourless over a period of 4 days. In addition, sample numbers 4, 6, and 16 had the capacity to reduce the colour, although their efficacy was noticeably compared with that of the isolates mentioned above (Fig. 4). No change in colour was observed for the pure hydrocarbon fractions when they were examined separately.
Degradation of crude oil and pure hydrocarbon fractions by fungal isolates
The 2,6-dichlorophenol indophenol (DCPIP) method was used to assess the potential of selected fungal strains to degrade crude oil and pure hydrocarbon fractions. According to the findings, all of the fungal isolates had a strong ability to degrade crude oil and change the colour of the solution from deep blue to colourless, while the pure hydrocarbon fractions were degraded by only Rhizopus arrhizus and Aspergillus lentulus. Furthermore, none of the additional fungal isolates that were introduced to the 1% pure hydrocarbon fractions were successful in changing the colour from blue to colorless.
According to the radial mycelia growth measurement, all isolated fungi had a good ability to tolerate a broad range of concentrations of crude oil. Rhizopus arrhizus had the fastest growth rate, which was 6.8 mm/day on the control medium. It also exhibited growth rates of 6.2, 6.0, 5.8, and 5.2 mm/day when exposed to crude oil concentrations of 5, 10, 15, and 20%, respectively. On control media, the Aspergillus lentulus strain exhibited the second highest growth rate, which was 5.6 mm/day for the control sample. However, it displayed growth rates of 5.5, 5.2, 4.8 and 4.3 mm/day for concentrations of crude oil of 5, 10, 15, and 20%, respectively. It is worth mentioning that the morphology of the isolated fungus on MSM containing crude oil was different than that of the control group (Fig. 5).
GC–MS technique for hydrocarbon analysis
The abilities of the bacteria and fungi to degrade the crude oil in the crude oil contaminated sites was assessed in pure cultures of the microorganisms isolated from these sites. Sterile crude oil was added to the cultures as the sole source of carbon and energy for 10 days of incubation. Generally, all n-alkanes were degraded by the bacterial and fungal cultures. However, the short chain n-alkanes, i.e., C11-C18, were more degraded (45- 88%) than those with longer chains (C19- C28), of which the percent removed was less than 53% (Table 6). The cultures that were added to the pure hydrocarbon fraction showed less degradation in both types of bacterial and fungal cultures. The percent biodegradation was calculated by comparing the alkane concentration in each culture with that in the controls (Table S1). The three bacterial cultures tested in this study were Achromobacter sp., Bacillus cereus, and Bacillus anthracis. All three of these bacterial species have been reported to be able to degrade both aliphatic and aromatic crude oil hydrocarbons (Deng et al. 2014; Dou et al. 2010; Jiang et al. 2019; Kim et al. 2019). Both the Aspergillus lentulus and Rhizopus arrhizus fungal cultures removed slightly less of the pure hydrocarbon than bacterial cultures. These two species of fungi are known for their ability to degrade crude oil (Wemedo et al. 2018).
A variety of biospecific indicators, such as the reduction in ratios of n-C17 to pristane and n-C18 to phytane, were utilized to separate the process of biodegradation from the process of degradation that occurs by abiotic weathering (Head et al. 2010). The branched alkanes pristane and phytane in the tested samples were degraded in all cultures by of 1–20% to 2–30%, respectively. The removal of these branched alkanes could be due to evaporation because theoretically, bacteria prefer saturated alkanes as carbon and energy source over branched alkanes (Head et al. 2010). Thus, when saturated alkanes are available, the branched alkanes are not utilized. Evaporation of the other n-alkanes may also have occurred in the cultures as well as in the controls. However, as mentioned above, the percent biodegradation is calculated by comparing the degradation in the cultures with that in the controls; therefore, the evaporation percent does not affect the biodegradation results. The ratios of C17/pristane and C18/phytane in the bacterial and fungal cultures decreased in comparison to the controls (Table 7). This is indicative of biodegradation, as pristane and phytane have the same molar masses as C17 and C18, respectively; thus, any loss due to evaporation should have an equal effect on them (Head et al. 2010; Horel, et al. 2015).
For all of the strains that were tested, the rate of degradation was approximately 1—30%, as shown in (Table 6). Analysis of the GC–MS results from the crude oil and hydrocarbon fractions revealed that the most proficient bacterial isolates were Achromobacter sp., Bacillus cereus, and Bacillus anthracis and the most proficient fungal isolates were Aspergillus lentulus and Rhizopus arrhizus, as shown in (Table 6 and Table S1); all chromatograms are shown in (Figure S1a, S1b, S1c, S1d, S1e, S1f, S1g, S1h, S1i, S1j and S1k, respectively). The bacteria that most effectively degraded n-alkanes (C11-C28) were Achromobacter sp and Bacillus anthracis, which had rates of degradation that ranged between 87- 33% and 88- 38% respectively. The rates of degradation of the pure hydrocarbon fractions were lower than those of total petroleum by both bacterial isolates and the rate of n-alkane (C11-C24) degradation, which were approximately 78–47% and 74–48%, respectively. Many biological factors, such as adaptability to environmental pressures, new niches, and the pH of the mixture, have an effect on the enzymes that are involved in the biodegradation of hydrocarbons (Daccò et al. 2020). The rates of degradation of the n-alkanes (C11–C18) that included pristane and phytane were 87–52% for Rhizopus arrhizus and 86–52% for Aspergillus lentulus. In addition, these microorganisms are capable of degrading long-chain hydrocarbons at rates of 50–39% and 50–34% respectively. Similarly, they are also capable of degrading pure hydrocarbon fractions in the same way as they degrade crude oil, as shown in (Table 6 and Table S1). The hyphal network of filamentous fungi has an apical growth mode that enables these fungi to penetrate the composite and soil matrix. The mechanical force applied by the hyphae allows the fungi to spread into inaccessible interstices where contaminants are difficult to remove (Dix and Webster 1995).
Enzymes involved in the degradation of crude oil and hydrocarbon fractions
Molecular analysis detected AlkB genes in three bacterial isolates (Achromobacter sp., Bacillus cereus and Bacillus anthrax). Moreover, PMO genes were detected in two fungal isolates (Aspergillus lentulus and Aspergillus fellis), as shown in (Fig. 6 a and b), respectively. Detection of the genes that encode alkane hydroxylase and putative monooxygenase in the current study highlights the fact that the breakdown of crude oil and pure hydrocarbon fractions was most likely carried out by the bacterial isolates and the fungal isolates.
a PCR detection of AlkB genes and b PCR detection of PMO genes. For both gels, Lanes 1 and 2 are the 100 bp DNA marker (DENA 100 bp plus DNA size marker II S-5091) and a negative control that was run without a DNA template, respectively a- Lane 3: Achromobacter sp., Lane 4: Bacillus cereus, and Lane 5: Bacillus anthracis b-Lane 3: Aspergillus lentulus, and Lane 4: Aspergillus fellis
Using three separate methods, the findings of this study indicate that crude oil and pure hydrocarbon fractions was degraded by indigenous bacterial and fungal isolates. The approaches used here are in accordance with the capabilities of Achromobacter sp., Bacillus cereus, and Bacillus anthracis among the bacterial isolates and Aspergillus lentulus and Rhizopus arrhizus among the fungal isolates. These are the most useful microorganisms for the process of bioremediation.
Discussion
Environmental pollution caused by oil spillage is one of the major issues of the century that must be resolved. Both physicochemical and biological approaches are viable options for remediating oil spill sites and bringing them back into usable conditions. As a result of the unfavorable effects of physicochemical methods, a greater emphasis has been placed on the utilization of biological alternatives such as bioremediation (Santisi et al. 2015). Based on our results, the microorganisms Bacillus cereus, Bacillus anthracis and Achromobacter sp (Alcaligenaceae) were the petroleum hydrocarbon degraders that were isolated from oil-contaminated soil, which is consistent with the findings of (Chen et al. 2017). Bacillus sp. and Alcaligenes sp were the petroleum hydrocarbon-degrading bacteria that were isolated from the area that was contaminated with petroleum. A study by (Deng et al. 2020) demonstrated that strain A-8 is a member of the genus Achromobacter. Moreover, they used this strain under a variety condition with different pH values and salt concentrations. According to their findings, strain A-8 is an excellent candidate for the removal of crude oil pollution from high-salinity environments.
The subsequent part of the current study involved the isolation of five different fungal strains. All of the fungal isolates were able to use crude oil and tolerate different concentration of it. Aspergillus lentulus and Rhizopus arrhizus were highly degrading species compared with the others. These data are in agreement with the findings of the study by (Ugboma et al. 2020), which determined that the fungal species isolated from the polluted soil prior to amendment showed the presence of three species: Aspergillus sp., Penicillium sp., and Mucor sp. However, treatment with the various organic amendments gave rise to nine (9) fungal species, seven of which were hydrocarbon utilizers. These species are as follows: Penicillium namely, Penicillium sp., Aspergillus fumigatus, Aspergillus lentulus, Aspergillus flavus, Fusarium sp., Rhizopus sp., Mucor sp., Cryptococcus sp. and Rhodotorula sp. In addition, the findings of the study carried out by (Wemedo et al. 2018) demonstrated that the fungal species Aspergillus niger and Rhizopus arrhizus have a high capacity to degrade crude oil. The findings demonstrated a slow but consistent decline in the concentration of hydrogen ions, as determined by measuring the pH, across all of the experimental conditions. This decrease in pH may be linked to the high concentration of metabolites and the increased metabolism by fungal species that are utilizing the hydrocarbons present as their only source of carbon and energy.
The results of the current study are supported by a number of other studies that investigated the biodegradation of crude oil in vitro using a variety of techniques and varying amounts of crude oil (Wang et al. 2015; Janssen et al. 2002: Xu et al. 2018). According to (Ramdass and Rampersad 2021), bacteria, fungi, and yeast were responsible for the degradation of crude oil. They mixed 2% crude oil with two different types of media. The findings suggested that most of the microorganisms that had been isolated were capable of utilizing crude oil on both kinds of medium in vitro. including Aspergillus sp. (a filamentous fungus). (Rizi et al. 2012) reported gram-positive bacteria such as Bacillus cereus and Bacillus subtilis were predominant in degraders of crude oil, which is consistent with the present study, as Bacillus cereus and Bacillus anthracis were the most predominant crude oil degraders. The findings of the current investigation are consistent with the study of (Al-Zaban, et al. 2021), who isolated four fungal isolates and measured radial mycelial growth on minimal medium (MM) supplied with crude oil at various concentrations. All isolates grew faster in the control treatment (without crude oil) than in the other treatments supplemented with varying concentrations of crude oil. However, they observed remarkable adaptation, and the isolates were able to survive in the presence of high concentrations of crude oil (up to 20%). (Al-Hawash et al. 2018) demonstrated that two strains of Penicillium sp. isolated from the Rumaila oil field, RMA1 and RMA2, can grow and reduce the colour of the DCPIP mixture gradually from deep blue to colourless. This change in colour suggested that Penicillium sp. RMA1 and RMA2 could degrade crude oil. Thus, the current study successfully used the same method for different bacterial and fungal species. (Tanase et al. 2013) effectively detected AlkB (alkane hydroxylase) in Achromobacter xylosoxidans and demonstrated that this bacterial species has been previously described as being a part of the microbial communities found in oil contaminated environments. Moreover, numerous bacterial strains were recovered from the enrichment cultures and these strains were later grown on mineral salt medium containing n-alkanes as the only source of carbon. Achromobacter xylosoxidans one of the bacterial strains that showed the fastest growth on such substrates, was monitored by optical density measurements at 600 nm for 21 days. These results are in accordance with those from the current study in which the AlkB gene was detected in Achromobacter sp. This bacterial species has a high optical density at 600 nm at 10 to 15 days of incubation. Moreover, alkane hydroxylases (AHs) are important enzymes in aerobic degradation of alkanes by bacteria (Nie et al. 2014). Putative monooxygenase in Apergillus sp. has the capacity to degrade aliphatic compounds (D'Annibale et al. 2006; Maddela et al. 2016). Degradation rates are influenced by the amount of oxygen present. In fact, under aerobic conditions, biodegradation happens more rapidly and effectively because oxygen is the terminal electron acceptor (Nagkirti et al 2017; Logeshwaran et al 2018; Varjani 2017). (Borah and Yadav 2014) showed after a period of 28 days, it was found that the Bacillus cereus strain DRDU1 degraded diesel at a rate of 67%, crude oil at a rate of 72%, kerosene at a rate of 42%, and used engine oil at a rate of 42%, respectively. (Alsayegh et al. 2021) discovered three indigenous bacteria in a location where liquid and solid oily wastes were dumped in the industrial district of Dukhan in Qatar. These bacteria were Bacillus sonorensis D11, Bacillus cereus D12, and Pseudomonas stutzeri D13. Accelerated solvent extraction (ASE, U.S. EPA Methods 3540) with certain modifications were employed in this research. This technique makes it possible to extract the hydrocarbons known as GRO (gasoline range organics in the C6-C10 range), DRO (diesel range organics in the C10-C28 range), and ORO (Oil Range Organics up to C35) for the purpose of soil self-purification. At low TPH concentrations, Bacillus sonorensis strain D11 grew the most compared to the other three strains. The slowest rate of development was observed for strain D13 (Pseudomonas stutzeri). D12 was less effective than D11 in removing TPH from the body. In the current study, Bacillus cereus showed a percent degradation between (45–78) % for short-chain n-alkanes (C11- C17) and 22–42% for long-chain n-alkane hydrocarbons (C19-C28). (Horel, et al. 2015) showed that the most dominant hydrocarbon degraders were most similar to Achromobacter sp., Microbulbifer sp., Ruegeria sp., and Pseudomonas sp., The ratios of n-heptadecane (C17) to pristane, n-octadecane (C18) to phytane (Ph), and pristane (Pr) to phytane Continuously declined after all treatments. The preferential degradation of linear over comparable branched alkanes was demonstrated declines in the C17/Pr and C18/Ph alkane ratios in the treatment groups over the course of the investigation. it is comparable to the present study in that the C17 to pristane and C18 to phytane (Ph) ratio of the crude oil and pure hydrocarbon fraction were decreased by all the isolates, including Achromobacter sp.
Conclusion
In this study, various indigenous microorganisms were isolated from three distinct oil-contaminated soil samples from the Taq-taq oil field (TTOPCO) as well as a control (clean) soil sample. Next, whether or not these microbes are capable of degrading crude oil and pure hydrocarbon fractions was determined by carrying out a number of investigations (spectrophotometric method or absorbance measurements; redox reactions with the indicator 2,6-dichlorophenol indophenol (DCPIP); measuring radial mycelial growth on solid MSM; and analysing hydrocarbons by GC–MS). Furthermore, detecting genes that encode alkane hydroxylase and putative monooxygenase, which degrade crude oil and hydrocarbon fractions, it was determined that degradation was most likely carried out by the bacterial isolates and fungal isolates. The analysis gave Bacillus anthracis, Bacillus cereus, and Achromobacter sp. as bacterial isolates. Moreover, Rhizopus arrhizus and Aspergillus lentulus among the fungal isolates have a strong ability to degrade crude oil. Detection of the AlkB and PMO genes emphasizes the fact that the degradation of crude oil and hydrocarbon fractions was most likely carried out by the bacterial isolates and the fungal isolates. However, longitudinal investigation will isolate mRNA and detect whether the genes stated above are expressed. More studies are needed to identify other microorganisms in the Kurdistan Region with the ability of degrading crude oil. Moreover, a mixed culture of bacteria and fungi can be used to observe their synergistic ability to degrade hydrocarbons.
Data availability statement
N/A
Abbreviations
- TTOPCO:
-
Taq-taq operating company
- KRI:
-
Kurdistan Region of Iraq
- DCPIP:
-
2,6-Dichlorophenol indophenol
- PCR:
-
Polymerase Chain Reaction
- ITS:
-
Internal transcribed spacer
- rRNA:
-
Ribosomal ribonucleic acid
- bp:
-
Base pair
- F:
-
Forwards primer
- R:
-
Reverse primer
- NCBI:
-
National Centre for Biotechnology Information
- BLASTN:
-
Basic Local Alignment Search Tool-NCBI
- MSM:
-
Minimal salt medium
- OD:
-
Optical density
- F:
-
Pure hydrocarbon fractions extracted from crude oil by simple fractional distillation
- A.P.I.:
-
American Petroleum Institute
- TQ1:
-
Taq-taq oil field number one
- PAH:
-
Polycyclic aromatic hydrocarbon
- DW:
-
Distilled water
- PDA:
-
Potatoes dextrose agar
References
Al-Hawash AB, Alkooranee JT, Abbood HA, Zhang J, Sun J, Zhang X, Ma F (2018) Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field. Iraq Biotechnol Rep 1(17):104–109. https://doi.org/10.1016/j.btre.2017.12.006
Alsayegh SY, Al-Ghouti MA, Zouari N (2021) Study of bacterial interactions in reconstituted hydrocarbon-degrading bacterial consortia from a local collection for the bioremediation of weathered oily-soils. Biotechnol Rep 29:0598. https://doi.org/10.1016/j.btre.2021.e00598
Al-Zaban MI, AlHarbi MA, Mahmoud MA (2021) Hydrocarbon biodegradation and transcriptome responses of cellulase, peroxidase, and laccase encoding genes inhabiting rhizospheric fungal isolates. Saudi J Biol Sci 28(4):2083–2090. https://doi.org/10.1016/j.sjbs.2021.01.009
Balogun SA, Shofola TC, Okedeji AO, Ayangbenro AS (2015) Screening of hydrocarbonoclastic bacteria using Redox indicator 2, 6-dichlorophenol indophenol. Glob Nest J 17(3):565–73
Barnes NM, Khodse VB, Lotlikar NP, Meena RM, Damare SR (2018) Bioremediation potential of hydrocarbonutilizing fungi from select marine niches of India. 3 Biotech 8:1–10. https://doi.org/10.1007/s13205-017-1043-8
Bilen Ozyurek S, Seyis Bilkay I (2017) Determination of petroleum biodegradation by bacteria isolated from drilling fluid, waste mud pit and crude oil. Turk J Biochem 1 42(6):609–616. https://doi.org/10.1515/tjb-2017-0087
Borah D, Yadav RNS (2014) Biodegradation of diesel, crude oil, kerosene and used engine oil by a newly isolated Bacillus cereus strain DRDU1 from an automobile engine in liquid culture. Arab J Sci Eng 39:5337–5345. https://doi.org/10.1007/s13369-014-1118-3
Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J (2015) Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv 33(6):745–755. https://doi.org/10.1016/j.biotechadv.2015.05.003
Chen Q, Li J, Liu M, Sun H, Bao M (2017) Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS One 12(3):e0174445. https://doi.org/10.1371/journal.pone.0174445
Daccò C, Girometta C, Asemoloye MD, Carpani G, Picco AM, Tosi S (2020) Key fungal degradation patterns enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int Biodeterior Biodegrad 147:104866. https://doi.org/10.1016/j.ibiod.2019.104866
D’Annibale A, Rosetto F, Leonardi V, Federici F, Petruccioli M (2006) Role of autochthonous filamentous fungi in bioremediation of a soil historically contaminated with aromatic hydrocarbons. Appl Environ Microbiol 72:28–36. https://doi.org/10.1128/AEM.72.1.28-36.2006
Deng MC, Li J, Liang FR, Yi M, Xu XM, Yuan JP, Peng J, Wu CF, Wang JH (2014) Isolation and characterization of a novel hydrocarbon-degrading bacterium Achromobacter sp. HZ01 from the crude oil-contaminated seawater at the Daya Bay, southern China. Mar Pollut Bull 83(1):79–86. https://doi.org/10.1016/j.marpolbul.2014.04.018
Deng Z, Jiang Y, Chen K, Li J, Zheng C, Gao F, Liu X (2020) One biosurfactant-producing bacteria Achromobacter sp. A-8 and its potential use in microbial enhanced oil recovery and bioremediation. Front Microbiol 11:247. https://doi.org/10.3389/fmicb.2020.00247
Dix NJ, Webster J (1995) Fungi of extreme environments. Fungal Ecol 322–340. https://doi.org/10.1007/978-94-011-0693-1_12
Dou J, Ding A, Liu X, Du Y, Deng D, Wang J (2010) Anaerobic benzene biodegradation by a pure bacterial culture of Bacillus cereus under nitrate reducing conditions. J Environ Sci 22(5):709–715. https://doi.org/10.1016/s1001-0742(09)60167-4
Farell EM, Alexandre G (2012) Bovine serum albumin further enhances the effects of organic solvents on increased yield of polymerase chain reaction of GC-rich templates. BMC Res Notes 5(1):1–8. https://doi.org/10.1186/1756-0500-5-257
Head I, Larter S, Gray N, Sherry A, Adams J, Aitken C, Jones D, Rowan A, Huang H, Röling W (2010) Hydrocarbon degradation in petroleum reservoirs. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin
Horel A, Mortazavi B, Sobecky PA (2015) Input of organic matter enhances degradation of weathered diesel fuel in sub-tropical sediments. Sci Total Environ 533:82–90. https://doi.org/10.1016/j.scitotenv.2015.06.102
Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M (2002) Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68(5):2391–2396. https://doi.org/10.1128/AEM.68.5.2391-2396.2002
Jiang J, Tang M, Chen J, Yang Y (2019) Identification and degradation characteristics of Bacillus cereus strain WD-2 isolated from prochloraz-manganese-contaminated soils. PLoS One 14(8):e0220975. https://doi.org/10.1371/journal.pone.0220975
Juhasz AL, Naidu R (2000) Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: a review of the microbial degradation of benzo [a] pyrene. Int Biodeterior Biodegrad 45(1–2):57–88. https://doi.org/10.1016/S0964-8305(00)00052-4
Karim AR, Khanaqa P, Shukur DA (2017) Kurdistan crude oils as feedstock for production of aromatics. Arab J Chem 10:S2601–S2607. https://doi.org/10.1016/j.arabjc.2013.09.038
Kim HS, Dong K, Kim J, Lee SS (2019) Characteristics of crude oil-degrading bacteria Gordonia iterans isolated from marine coastal in Taean sediment. Microbiologyopen 8(6):e00754. https://doi.org/10.1002/mbo3.754
Lane DJ (1991) 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic Acid Techniques in Bacterial Systematic. John Wiley and Sons, New York, pp 115–175
Lee PY, Costumbrado J, Hsu CY, Kim YH (2012) Agarose gel electrophoresis for the separation of DNA fragments. J vis Exp 62:3923. https://doi.org/10.3791/3923
Logeshwaran P, Megharaj M, Chadalavada S, Bowman M, Naidu R (2018) Petroleum hydrocarbons (PH) in groundwater aquifers; An overview of environmental fate, toxicity, microbial degradation and risk-based remediation approaches. Environ Technol Innov 10:175–193
Maddela NR, Burgos R, Kadiyala V, Carrion AR, Bangeppagari M (2016) Removal of petroleum hydrocarbons from crude oil in solid and slurry phase by mixed soil microorganisms isolated from Ecuadorian oil fields. Int Biodeterior Biodegrad 108:85–90. https://doi.org/10.1016/j.ibiod.2015.12.015
Nagkirti P, Shaikh A, Vasudevan G, Paliwal V, Dhakephalkar P (2017) Bioremediation of terrestrial oil spills: Feasibility Assessment. Optimization and Applicability of Bioprocess 141–173. https://doi.org/10.1007/978-981-108663-8_7
Nie Y, Chi CQ, Fang H, Liang JL, Lu SL, Lai GL, Tang YQ, Wu XL (2014) Diverse alkane hydroxylase genes in microorganisms and environments. Sci Rep 4(1):1–11. https://doi.org/10.1038/srep04968
Raja HA, Miller AN, Pearce CJ, Oberlies NH (2017) Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod 80(3):756–770. https://doi.org/10.1021/acs.jnatproud.6b01085
Ramdass AC, Rampersad SN (2021) Diversity and oil degradation potential of culturable microbes isolated from chronically contaminated soils in Trinidad. Microorganisms 9(6):1167. https://doi.org/10.3390/microorganisms9061167
Reyes-César A, Absalon AE, Fernández FJ, González JM, Cortés-Espinosa DV (2014) Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J Microbiol Biotechnol 30:999–1009. https://doi.org/10.1007/s11274-013-1518-7
Riedel S, Hobden JA, Miller Steve, Morse SA et al (2019) Jawetz, Melnick, & Adelberg’s medical microbiology, 28th edn. McGraw-Hill, UK, pp 155–193
Ristinen RA, Kraushaar JJ, Brack JT (2022) Energy and the Environment. John Wiley & Sons
Rizi MS, Sepahi AA, Tabatabaee MS (2012) Crude oil biodegradation by a soil indigenous Bacillus sp. isolated from Lavan Island. Bioremediat J 16(4):218–224. https://doi.org/10.1080/10889868.2012.687416
Santisi S, Cappello S, Catalfamo M, Mancini G, Hassanshahian M, Genovese L, Giuliano L, Yakimov MM (2015) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium. Braz J Microbiol 46:377–387. https://doi.org/10.1590/S1517-838246120131276
Schwab AP, Su J, Wetzel S, Pekarek S, Banks MK (1999) Extraction of petroleum hydrocarbons from soil by mechanical shaking. Environ Sci Technol 1 33(11):1940–1945
Schofield P (2017) Taq-taq field, genel energy international Ltd. Competent Person’s Report. (A Report)
Selvakumar S, Sekar P, Rajakumar S, Ayyasamy PM (2014) Rapid screening of crude oil degrading bacteria isolated from oil contaminated areas. Journal of Sci-Tech Research 1:24–27
Shlimon AG, Mansurbeg H, Othman RS, Gittel A, Aitken CM, Head IM, Finster KW, Kjeldsen KU (2020) Microbial community composition in crude oils and asphalts from the Kurdistan Region of Iraq. Geomicrobiol J 37(7):635–652. https://doi.org/10.1080/01490451.2020.1753131
Singh P, Parmar D, Pandya A (2015) Comparative study of crude oil degradation efficiency of microbes isolated from crude oil contaminated site. Bull Env Pharmacol Life Sci 4(2):91–94
Tanase AM, Ionescu R, Chiciudean I, Vassu T, Stoica I (2013) Characterization of hydrocarbon-degrading bacterial strains isolated from oil-polluted soil. Int Biodeterior Biodegradation 84:150–154. https://doi.org/10.1016/j.ibiod.2012.05.022
Ugboma CJ, Ibietela DS, Onwukwe JO (2020) Effect of Different Organic Amendments on Fungi Degrading Hydrocarbons from an Artisanal Crude Oil Refining Polluted Soil in Ngia Kiri, Degema, Rivers State. J Biol Res 6:1–12
Varjani SJ (2017) Microbial degradation of petroleum hydrocarbons. Bioresour Technol Rep 223:277–286. https://doi.org/10.1016/j.biortech.2016.10.037
Wang X, Wang X, Liu M, Bu Y, Zhang J, Chen J, Zhao J (2015) Adsorption–synergic biodegradation of diesel oil in synthetic seawater by acclimated strains immobilized on multifunctional materials. Mar Pollut Bull 92(1–2):195–200. https://doi.org/10.1016/j.marpolbul.2014.12.033
Wang C, Liu X, Guo J, Lv Y, Li Y (2018) Biodegradation of marine oil spill residues using aboriginal bacterial consortium based on Penglai 19–3 oil spill accident, China. Ecotoxicol Environ Saf 159:20–27. https://doi.org/10.1016/j.ecoenv.2018.04.059
Watanabe T (2018) Pictorial atlas of soilborne fungal plant pathogens and diseases. Can J Res 11:18–31. https://doi.org/10.1201/b22340
Wemedo SA, Nrior RR, Ike AA (2018) Biodegradation potential of Aspergillus Niger and Rhizopus arrhizus isolated from crude oil spilled site in Rivers State. J Environ Sci Toxicol Food Technol 12:49–57. https://doi.org/10.9790/2402-1212014957
Wolfson R (2011) Energy, environment, and climate, 2nd ed. WW Norton & Company
Xu X, Liu W, Tian S, Wang W, Qi Q, Jiang P, Gao X, Li F, Li H, Yu H (2018) Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front Microbiol 9:2885. https://doi.org/10.3389/fmicb.2018.02885
Xue J, Yu Y, Bai Y, Wang L, Wu Y (2015) Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: a review. Curr Microbiol 71(2):220–228. https://doi.org/10.1007/s00284-015-0825-7
Funding
This scientific paper was conducted and completed without any external funding or financial support. The research and analysis presented herein were solely carried out by the authors' own resources and efforts.
Financial or non-financial interests: I will upgrade my account to gold access.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
N/A
Informed consent
N/A
Conflict of interest
The authors declare that there are no conflicts of interest associated with this scientific paper. The research conducted and the results presented herein were not influenced by any personal or financial relationships that could be perceived as having biased the findings or interpretation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11756_2023_1513_MOESM2_ESM.svg
Supplementary file2 Fig. S1 GC-MS chromatograms documenting the absence of n-alkanes in the bacterial and fungal suspension: Fig. S1a Control sample followed by samples were treated with crude oil Fig. S1b Achromobacter sp. Fig. S1c Bacillus cereus Fig. S1d Bacillus anthracis Fig. S1e Aspergillus lentulus and Fig. S1f Rhizopus arrhizus. Samples were treated with pure hydrocarbon fractions (F) Fig. S1g Control F Fig. S1h Achromobacter sp. Fig. S1i Bacillus anthracis Fig. S1j Aspergillus lentulus, and Fig. S1k Rhizopus arrhizus (SVG 979 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed, S.A., Omar Zrary, T.J. & Hasan, A.H. Degradation of crude oil and the pure hydrocarbon fractions by indigenous soil microorganisms. Biologia 78, 3637–3651 (2023). https://doi.org/10.1007/s11756-023-01513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01513-4