Abstract
Biocontrol microorganisms are important tools for the control of root knot nematodes (Meloidogyne spp.). Endophytic fungi have shown great potential as biocontrol agents in such applications. We here isolated an endophytic fungus from tomato root galls infected with M. incognita and identified the isolate as Acremonium sclerotigenum based on morphology and the internal transcribed spacer sequence. The biocontrol potential of this fungus was evaluated both in vitro and in vivo. Specifically, in vitro analyses were conducted to determine the potential of A. sclerotigenum to increase Meloidogyne incognita juvenile (J2 stage) mortality and decrease M. incognita egg hatching rates. The results revealed that A. sclerotigenum culture filtrates caused high J2 mortality rates (up to 95.5%) and significantly inhibited egg hatching (by up to ~ 43%). Furthermore, eggs treated with the culture filtrate were disaggregated and could not develop into nematodes. An in vivo experiment showed that treatment of tomato plants with A. sclerotigenum suppressed root knot nematode populations and significantly reduced the galling index. Both A. sclerotigenum treatment and exposure to the nematicide abamectin had good control effects, with efficacy rates of 55.43% and 70.58%, respectively. In summary, the endophytic fungus A. sclerotigenum here showed excellent potential for biocontrol of M. incognita. Further studies should be conducted to identify the nematicidal compounds produced by this fungus and to establish the molecular mechanism of action associated with the observed biocontrol effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the genus Meloidogyne are referred to as sedentary endoparasitic root knot nematodes (RKNs). These pests are economically important throughout the world due to the enormous financial losses they cause as a result of extensive crop damage (Abad et al. 2008). The RKN host range encompasses more than 3000 plant species, including nearly all cultivated crops, such as potatoes, carrots, and tomatoes (Przybylska et al., 2020). There are more than 100 species in the genus Meloidogyne (Tariq-Khan et al. 2017), the most important of which are Meloidogyne incognita, M. javanica, M. arenaria, M. hapla, and M. enterolobii (Čepulytė et al. 2018; Collett et al. 2021; Rashidifard et al. 2018). M. incognita is a highly invasive species that has spread to at least 143 countries (Bebber et al. 2014).
Due to the heavy economic losses caused by RKNs, various measures have been tested and applied to control these species. However, RKNs are difficult to control due to their high multiplication rates, short life cycles, and broad host range. The most common control methods are chemical nematicide application, crop rotation, and the use of resistant cultivars (Atia et al. 2020; Bhat et al. 2023). The latter use of resistant cultivars method is the most effective and economical approach to controlling nematodes. RKN-resistant cultivars of some crops, including tomato (Jacquet et al. 2005) and pepper (Fazari et al. 2012), are commercially available. Although breeders have undertaken research to identify and characterize RKN-resistant cultivars, such efforts have been hampered by a lack of resistant genetic resources. Chemical treatments are generally effective in combatting RKN, but their detrimental effects on the environment and human health, and instances of resistance developing among nematodes, have caused global restrictions on nematicidal chemicals. Although crop rotation is a common method of controlling RKN, their broad host range minimizes the efficacy of this approach as a nematode management method (Salem et al. 2019a).
Several other approaches have been tested to prevent RKN damage to plants, such as grafting (Salem et al. 2019b), application of organic amendments (Abdeldaym et al. 2014) or plant extracts (Ismail et al. 2020; Ahmad 2020), and biological control methods (Atia et al. 2016; Daneshkhah et al. 2013; Haque et al. 2018). Over the past few decades, there has been an increasing focus on the use of microorganisms as biocontrol agents (Atia et al. 2020). Fungal RKN antagonists can play crucial roles in nematode management; such species possess nematophagous or nematicidal activities (Bhat et al. 2023). These fungi are commonly classified into four types: endoparasites, nematode-trapping fungi, egg/female parasites, and toxin producers (Moosavi and Zare 2012; Anjum et al. 2019; Zhang et al. 2020). Nematophagous fungi grow slowly in soil, and a large fungal biomass is required for nematophagous activity in the field. Thus, these species do not have high control efficiency.
Endophytic fungi, which colonize the plant tissue, lack the shortcomings of nematophagous fungi and provide benefits to the host plant, such as enhanced growth (Mehmood et al. 2019), improved stress tolerance (Bastias et al. 2017), or more robust defense responses (De Silva et al. 2019; Hu et al. 2023). Endophytic fungi that are capable of producing nematotoxic metabolites are the most promising as potential biocontrol agents. These fungi can effectively inhibit and suppress RKNs by reducing their penetration, delaying their development, or decreasing their reproductive capacities (Grabka et al. 2022). Many strains of the endophytic fungus Fusarium oxysporum can efficiently reduce M. incognita and Radopholus similis population densities and reproductive rates (Grabka et al. 2022; Kisaakye et al. 2022). Culture filtrate and purified Chaetoglobosin A (ChA) isolated from the endophyte Chaetomium globosum NK 102 both have nematicidal activity, increasing mortality and reducing J2 penetration into cucumber roots (Natori 1983). In tall fescue (Festuca arundinacea), application of Neotyphodium coenophialum enhances host nematode resistance (Choi et al. 2022).
Notably, Acremonium spp. have been shown to secrete nematicidal metabolites (Lozano et al. 2022), suppress nematode egg hatching, and reduce nematode populations (Tian et al. 2014). Acremonium spp. are also known to produce a large number of bioactive metabolites such as phenols (Lu et al. 2022), alkaloids (Januar and Molinski 2013), peptides (Chen et al. 2012), polyketides (Wicklow and Poling 2009), and terpenes (Suciati et al., 2013). Several studies have examined the effects of Acremonium-derived metabolites on insects or nematodes. Furthermore, some Acremonium species have been found to enhance host plant resistance to diseases caused by soil-borne pathogens (Bogner et al. 2016).
Endophytes and nematodes occupy a similar ecological niche, giving endophytes distinct advantages as biocontrol agents compared to nematophagous fungi (Tian et al. 2014; Chen et al. 2022). Indeed, endophytes are well-suited to competition with soil-borne pathogens (whether bacterial, fungal, or nematodal) to protect their niche (Schouten 2016). Previously, fungal endophytes with biocontrol potential have predominantly been isolated from healthy rather than from diseased plants. However, it is feasible to obtain endophytic fungi with latent biocontrol capacities from diseased tissues. In the present study, we therefore aimed to isolate and identify endophytic fungi from M. incognita-infected tomato roots and to evaluate the potential of these isolates to control RKNs.
Materials and methods
This research was carried out in 2022 at the Tianjin Academy of Agricultural Sciences, Institute of Plant Protection, Vegetable Disease Laboratory (39°6’14” N, 117°3’32” E). Infected tomato roots were obtained from the agricultural fields of a village in the North China Plain in which RKN disease was a serious problem in tomato production (Xiawuqi village, Wuqing district, Tianjin; 39°7′5″ N, 116°46′43″ E).
Isolation and identification of endophytic fungi
Endophytic fungi were isolated from the galls of tomatoes infected with RKN. Root galls were washed to remove soil particles, disinfected in 0.5% NaClO solution for 1 min, then washed three times with sterile water. Root knots were cut into pieces (0.5 × 0.5 cm), which were placed on potato dextrose agar (PDA) media containing 100 µg/mL ampicillin, then incubated at 25 °C for 7 d in the dark. Isolates were purified using the single spore method (Constantinescu 1988) and stored at 4 °C.
To identify the isolated fungal species, Petri dishes containing root knots as described above were incubated for 7 d at 25 °C in the laboratory. Fungal cultures were then identified based on microscopic and macroscopic features as described by Wei (1979). Isolates were maintained on PDA plates for further experiments.
To genetically confirm the identities of the fungal isolates, selected fungi were cultured on PDA media for 7 d at 25 °C, then mycelia were transferred to 1.5-mL centrifuge tubes. Fungal DNA was extracted using a fungal genome extraction kit (B511375; Sangon Biotech, Shanghai, China). The nuclear ribosomal internal transcribed spacer (ITS) sequence of each fungus was amplified via PCR with the IST1/IST4 primer pair (Van Nguyen et al., 2007). Each 50-µL reaction contained 100 ng genomic DNA, 0.5 µM each of the ITS1 and ITS4 primers, and 2× EsTaq Master Mix (Dye) (CW0690H, CWBIO, Jiangsu, China). The thermocycling program consisted of denaturation for 4 min at 95 °C; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 60 s; and a final elongation step at 72 °C for 10 min. ITS amplicons were then sequenced by Azenta Life Sciences. The NCBI database was searched using the resulting sequences as queries to identify similar sequences. The ITS sequences of several species in the genus Acremonium were downloaded from GenBank. These sequences were aligned and a phylogenetic tree was constructed in MEGA7.0 (Mega Limited, Auckland, New Zealand) using the neighbor-joining algorithm with 1000 bootstrap replicates.
Nematode culture preparation
A pure culture of the RKN M. incognita has been maintained on pepper (Capsicum annuum L. cv. ‘Qiemen’) plants in the greenhouse at the Tianjin Academy of Agricultural Sciences. For this study, two months after inoculation, galled pepper roots were hand-picked and washed to remove soil particles. Egg masses were collected and sterilized in 0.5% NaClO solution for 1 min, then rinsed twice in sterile distilled water. The final concentration of the egg suspension was adjusted to ~ 1000 eggs/mL. To obtain active M. incognita juveniles at the J2 stage (J2s), eggs were incubated at 25 °C in a Petri dish containing sterilized water to promote hatching. The resulting nematode suspension contained ~ 1000 J2s/mL.
In vitro nematicidal bioassays
Nematicidal bioassays were conducted in vitro to screen 81 fungal isolates for activity against M. incognita. Each isolate was grown on PDA for 5 d, then agar blocks of 5 mm in diameter were removed from the plate and placed in 500-mL flasks containing 200 mL of potato dextrose broth (PDB). After incubation at 25 °C for 5 d with 120 rpm shaking, cultures were centrifuged at 1000 × g for 5 min. The supernatant was passed through a 0.22-µm micropore filter (Pall Life Sciences, Ann Arbor, MI, USA) to remove the conidia and mycelia. Undiluted culture filtrate (100% concentration) was diluted in sterile deionized water to generate 25%, 50%, and 75% culture filtrate solutions. Each well of a sterile 24-microwell plate (Costar® 3526, Corning Incorporated, China) was filled with 100 µL of M. incognita J2 suspension (~ 1000 J2s/mL) and 1 mL of fungal culture filtrate (25%, 50%, 75%, or 100%) or PDB as a control. Microwell plates were then incubated at 25 °C in the dark for 24, 48, and 72 h. Plates were examined under a stereomicroscope at 10× magnification to count the number of dead J2s at each concentration of culture filtrate. Individuals were classified as dead based on their shape and the absence of mobility as described by Duong et al. (2021). The percentage of dead nematodes was calculated to estimate the toxicity of each concentration of fungal filtrate. This experiment was repeated five times independently. Based on the results of this screening, one promising fungal endophyte (A. sclerotigenum) was selected for further testing.
In vitro egg hatching activity assay
Egg hatching bioassays were conducted in vitro following the protocol described above for the nematicidal assays, with the substitution of eggs for J2s. The numbers of hatched nematodes were counted at 3, 4, and 5 d of treatment, then the hatching inhibition rate for each treatment was calculated at each timepoint. The experiment was repeated five times independently.
In vivo experiments under greenhouse conditions
Seeds of the susceptible Solanum lycopersicum variety ‘Jinpeng No. 1’ (produced by Xi’an Jinpeng Seedlings Co., LTD) were obtained from a market. Tomato seeds were sown in a seedbed containing sterilized soil mix (1:1:1 clay:sand:loam [v/v/v]) and grown at 25 °C under a 16/8 h light/dark photoperiod. When tomato seedlings reached the trifolate stage, a suspension of 5-d-old A. sclerotigenum conidia was prepared, containing 106 conidia as measured with a hemocytometer under an optical microscope. After the seedlings were gently removed from the soil, the roots were drenched in the conidia suspension for 10 s, then replanted in 140 × 125 × 100 mm plastic pots. A 1% microcapsule suspension of the nematicide abamectin (Jiangsu Yancheng Shuangning Agrochemical Co. LTD, China) at a dosage of 5.52 kg/hm2 was used as the positive control, and sterilized water was used as the negative control. After 7 d, each pot was inoculated with 1000 fresh M. incognita J2s at three locations surrounding the stem. Each treatment group contained 10 seedlings. After 2 months, roots were harvested from the soil and infected lateral roots were examined to evaluate the control effects of the selected fungal isolate (A. sclerotigenum) and abamectin. Control effects were determined based on the galling index (GI) scores and the reproduction factor (Rf) values. GI scores were assessed for each plant using a scale from 0 to 4 as follows: 0, no galls on root; 1, 1–25% of the root area covered in galls; 2, 26–50% of the root area covered in galls; 3, 51–75% of the root area covered in galls; and 4, 75–100% of the root area covered in galls (Ma et al. 2017). Disease index (DI) values were calculated with the following formula:
The control efficacy was calculated as follows:
Rf was calculated as described by Naz et al. (2021):
Where Pf and Pi are the final and initial nematode populations, respectively. This experiment was repeated three times independently.
Statistical analysis
Data were analyzed using SPSS v21.0 (IBM, Chicago, IL, USA). Analysis of variance (ANOVA) was conducted to analyze differences in the mean mortality rate, egg hatching rate, RKN population, GI, and DI between treatment groups. Where ANOVA revealed significant differences, a post-hoc Tukey’s honestly significant difference (HSD) test was applied. Significant differences were called at p ≤ 0.05.
Results
Isolation and identification of endophytic fungi
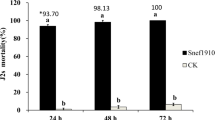
A total of 81 endophytic fungi were obtained in this study, and all strains were screened against M.incognita in laboratory. Base on the results of this screening, on promising fungal endophyte (A. sclerotigenum) was selected for further testing, which showed over 90% mortality rates of after exposure for 72 h.
After the selected fungal isolate was grown, morphology was assessed to establish its identity (Fig. 1). When grown on solid medium, colonies were white on the top surface and light yellow on the lower surface. Conidia were hyaline and ellipsoidal and were 4.0–5.1 μm × 1.1–1.9 μm in size.
The ITS region of the isolated fungus was then sequenced (GenBank OP926024). In a phylogenetic analysis of related ITS sequences, the selected fungal isolate was in the same clade as an A. sclerotigenum strain, with which the selected isolate had 100% sequence similarity (Fig. 2). Thus, based on morphological characteristics and the ITS sequence, the isolated fungus was identified as A. sclerotigenum.
Phylogenetic tree showing relationships between the nuclear ribosomal internal transcribed spacer (ITS) regions of the isolated fungus (OP926024) and other Acremonium species and strains, with Fusarium oxysporum serving as the outgroup. The tree was constructed using the neighbor-joining method with 1000 bootstrap replicates. Bootstrap support values are shown at each node
Effects of A. sclerotigenum on M. incognita J2 mortality
All four A. sclerotigenum culture filtrate concentrations (25%, 50%, 75%, and 100%) showed significant (p < 0.05) nematicidal effects against M. incognita J2s after 24, 48, and 72 h of exposure (Fig. 3). The highest concentration of culture filtrate (100%) killed 95.5% of M. incognita J2s after exposure for 72 h. This was significantly higher than the mortality rate in the control group (3.0%), which was treated with PDB.
Effects of A. sclerotigenum on M. incognita egg hatching rates
Treatment with A. sclerotigenum culture filtrate markedly inhibited M. incognita egg hatching after 5 d (Fig. 4). In the control group, 88.75% of the eggs hatched, whereas only 50.75% of eggs in the group treated with 100% culture filtrate hatched. Notably, the egg hatching inhibition rate increased along with the culture filtrate concentration and incubation time. Furthermore, eggs in the control group were smooth and intact and could hatch into nematodes (Fig. 5C). However, treated eggs tended to disaggregate and could not develop into nematodes (Fig. 5A, B).
Effects of A. sclerotigenum treatment on M. incognita in vivo
Data obtained from greenhouse-grown tomato plants infected with M. incognita indicated that treatment with A. sclerotigenum suppressed RKN infection, significantly reducing the GI values of treated plants (p < 0.05) (Fig. 6). Compared with the control treatment, both the A. sclerotigenum and the abamectin treatments had good control effects; the preventive efficacies of the two treatments were 55.43% and 70.58%, respectively (Table 1). The Rf of M. incognita was highest in the control group (3.06), lower in samples treated with the biocontrol agent A. sclerotigenum (1.57), and lowest in samples treated with the nematicide abamectin (1.33) (Table 1).
Discussion
Numerous publications have emphasized the negative impacts of chemical nematicide application on the ecological environment and human health (e.g., Hardoim et al. 2015; Mishra et al. 2021). It is therefore important to identify putative nematicidal biocontrol agents. Studying these fungi will enable researchers to identify potential biocontrol agents and understand their interactions with pathogenic nematodes, ultimately minimizing the necessity of chemical nematicide use.
We here studied application of the endophytic fungus A. sclerotigenum (in the form of culture filtrates or conidia suspensions) for RNK management. In vitro bioassays revealed strong nematicidal activity of the culture filtrate. In a test of varying culture filtrate concentrations, the highest concentration of culture filtrate (100%) caused 95.5% M. incognita J2 morality after 72 h. Moreover, treatment with A. sclerotigenum culture filtrate markedly inhibited M. incognita egg hatching; the hatching inhibition rate increased along with the culture filtrate concentration and the incubation time. Fungal nematicidal activity can usually be attributed to either the production of nematicidal metabolites or to fungal parasitism. In this case, the use of culture filtrate precluded the possibility of parasitism. Consistent with the hypothesis that a nematicidal metabolite was involved, M. incognita responses to treatment with A. sclerotigenum culture filtrate were concentration-dependent. Similar effects have been reported previously. For example, undiluted Trichoderma harzianum culture filtrate causes higher M. incognita hatching inhibition rates than diluted cultures (Babu et al. 2021). Culture filtrates of some non-pathogenic endophytic F. oxysporum strains contain specialized metabolites that are highly toxic to M. incognita (Constantin et al. 2019). C. globosum is known to secrete many types of specialized metabolites with anti-nematode activity (Khan et al. 2019). A study conducted with endophytes isolated from banana roots showed that endophyte treatment caused 100% inhibition of M. incognita egg hatching and resulted in a 81.33–96.33% J2 mortality rate after treatment for 72 h (Ganeshan et al. 2021). The endophytic fungus Acremonium implicatum was previously obtained from the root galls of tomato plants infected with M. incognita; this fungus inhibited M. incognita egg hatching and showed excellent nematicidal effects against J2s (Tian et al. 2014). The filamentous fungus Acremonium strictum, which markedly reduces nematode populations and improves tomato plant health, was isolated from M. incognita egg masses (Oro et al. 2021). A. strictum also parasitizes M. incognita eggs, inhibiting hatching (Bhat et al. 2023).
In the present study, in vivo experiments revealed that A. sclerotigenum significantly suppressed RKN infection and drastically reduced the Rf of nematodes associated with treated compared to untreated plants. Similarly, the endophyte C. globosum significantly inhibits M. incognita J2 penetration and female reproduction, ultimately reducing galling in cotton plants (Zhou et al. 2016). Treatment with the endophytic fungus Fusarium oxysurum has also been shown to reduce the M. incognita populations associated with tomato, squash, and melon (Ganeshan et al. 2021). Endophytic F. oxysporum treatment reduces female M. incognita reproduction and J2 penetration of tomato roots (Bogner et al. 2016).
The benefits of endophytic fungal associations with crops are numerous. However, a key issue with the use of endophytes as biological control agents is the difficulty of detecting and quantifying them within the host plant to assess persistence. Quantitative PCR (qPCR) can be used to quantify microorganism abundance. In a study of tomato roots, qPCR was applied to measure colonization by Pochonia chlamydosporia. This experiment revealed that colonization decreased over time (Escudero and Lopez-Llorca 2012). Another study found that P. chlamydosporia 123 persisted endophytically in banana roots up to 75 d after a single inoculation, and that repeated application of the fungus lengthened the duration of root colonization (Mingot-Ureta et al. 2020). Such results demonstrate that endophytic fungi can persist in the roots over long durations. However, further optimization is required to increase fungal abundance in the roots, which decreased as the plants developed.
We here applied the endophytic fungus A. sclerotigenum to control RKNs, not only colonizing host plant tissue, but also producing a desirable control efficiency rate. These findings validated the strategy of screening infected plant tissues to identify organisms with putative biocontrol effects. However, the nematicidal metabolites produced by A. sclerotigenum, and the molecular genetic mechanism underlying their biosynthesis, remain unknown. Further analyses of this system should be conducted to delineate the relationships between endophytes, nematodes, and plants at the molecular level, specifically addressing the nematicidal effects of specialized metabolites produced by the fungi.
Conclusion
A. sclerotigenum culture filtrates were here shown to significantly increase M. incognita mortality and to decrease egg hatching rates. Treatment with culture filtrate also caused M. incognita eggs to disaggregate and fail to develop into nematodes. Importantly, treatment of tomato plants with A. sclerotigenum suppressed the RKN population and significantly reduced the GI to levels comparable to those of plants treated with the nematicide abamectin. Our study clearly showed that the endophytic fungus A. sclerotigenum isolated from tomato plants has great potential as a biocontrol agent against RKNs, advancing sustainable agricultural methods and promoting food security.
Data Availability
Data analyzed for this study are included in the manuscript.
Abbreviations
- DI:
-
Disease index
- GI:
-
Galling index
- PDA:
-
Potato dextrose agar
- PDB:
-
Potato dextrose broth
- RKN:
-
Root knot nematode
- RF:
-
Reproduction factor
- ITS:
-
Internal transcribed spacer
- qPCR:
-
Quantitative PCR
References
Abad P, Gouzy J, Aury JM et al (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915. https://doi.org/10.1038/nbt.1482
Abdeldaym EA, Erriquens F, Sasanelli N et al (2014) Effects of several amendments on organic melon growth and production, Meloidogyne incognita population and soil properties. Sci Hort 180:156–160. https://doi.org/10.1016/j.scienta.2014.10.032
Ahmad S (2020) Concealed endophytic nematode management in sunflower using plant-based soil amendments. Pure and Applied Biology 9(3):1763–1772. https://doi.org/10.19045/bspab.2020.90187
Anjum R, Afzal M, Baber R et al (2019) Endophytes: as potential biocontrol agent-review and future prospects. J Agric Sci 11:113. https://doi.org/10.5539/jas.v11n4p113
Atia MA, Osman GH, Elmenofy WH (2016) Genome-wide in silico analysis, characterization and identification of microsatellites in Spodoptera littoralis multiple nucleopolyhedrovirus (SpliMNPV). Sci Rep 6:33741. https://doi.org/10.1038/srep33741
Atia MAM, Abdeldaym EA, Abdelsattar M et al (2020) Piriformospora indica promotes cucumber tolerance against Root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J Biol Sci 27(1):279–287. https://doi.org/10.1016/j.sjbs.2019.09.007
Babu GS, Ramesh C, Singh SK et al (2021) Efficacy of Trichoderma viride and T. harzianum culture filtrate on egg hatching and larval mortality of root-knot nematode, Meloidogyne incognita. Annals of Plant Protection Sciences 29(3):241–245. https://doi.org/10.5958/0974-0163.2021.00052.5
Bastias DA, Martínez-Ghersa MA, Ballaré CL et al (2017) Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends Plant Sci 22:939–948. https://doi.org/10.1016/j.tplants.2017.08.005
Bebber DP, Holmes T, Smith D et al (2014) Economic and physical determinants of the global distributions of crop pests and pathogens. New Phytol 202(3):901–910. https://doi.org/10.1111/nph.12722
Bhat AA, Shakeel A, Waqar S et al (2023) Microbes vs. nematodes: insights into biocontrol through antagonistic organisms to control root-knot nematodes. Plants 12(3):451. https://doi.org/10.3390/plants12030451
Bogner CW, Kariuki GM, Elashry A et al (2016) Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycological progress 15(3):30. https://doi.org/10.1007/s11557-016-1169-9
Čepulytė R, Danquah WB, Bruening G et al (2018) Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci Rep 8:10847. https://doi.org/10.1038/s41598-018-29165-4
Chen ZM, Song YX, Chen YC et al (2012) Cyclic heptapeptides, cordyheptapeptides C-E, from the marine-derived fungus acremonium persicinum SCSIO 115 and their cytotoxic activities. J Nat Prod 75(6):1215–1219. https://doi.org/10.1021/np300152d
Chen XL, Sun MC, Chong SL et al (2022) Transcriptomic and metabolomic approaches deepen our knowledge of plant–endophyte interactions. Front Plant Sci 12:3339. https://doi.org/10.3389/fpls.2021.700200
Choi CJ, Valiente J, Schiavon M et al (2022) Bermudagrass cultivars with different tolerance to nematode damage are characterized by distinct fungal but similar bacterial and archaeal microbiomes. Microorganisms 10(2):457. https://doi.org/10.3390/microorganisms10020457
Collett RL, Marais M, Daneel M et al (2021) Meloidogyne enterolobii, a threat to crop production with particular reference to sub-saharan Africa: an extensive, critical and updated review. Nematology 23(3):247–285. https://doi.org/10.1163/15685411-bja10076
Constantin ME, de Lamo FJ, Vlieger BV et al (2019) Endophyte-mediated resistance in tomato to Fusarium oxysporum is independent of ET JA and SA. Front Plant Sci 10:979. https://doi.org/10.3389/fpls.2019.00979
Constantinescu O (1988) An instrument and procedure for single-spore isolation. Trans Br Mycological Soc 91(4):700–702. https://doi.org/10.1016/S0007-1536(88)80049-4
Daneshkhah R, Cabello S, Rozanska E et al (2013) Piriformospora indica antagonizes cyst nematode infection and development in Arabidopsis roots. J Exp Bot 64:3763–3774. https://doi.org/10.1093/jxb/ert213
De Silva NI, Brooks S, Lumyong S et al (2019) Use of endophytes as biocontrol agents. Fungal Biology Reviews 33:133–148. https://doi.org/10.1016/j.fbr.2018.10.001
Duong B, Nguyen XH, Phan VHa et al (2021) Identification and characterization of vietnamese coffee bacterial endophytes displaying in vitro antifungal and nematicidal activities. Microbiol Res 242:126613. https://doi.org/10.1016/j.micres.2020.126613
Escudero N, Lopez-Llorca LV (2012) Effects on plant growth and root-knot nematode infection of an endophytic GFP transformant of the nematophagous fungus Pochonia chlamydosporia. Symbiosis 57:33–42. https://doi.org/10.1007/s13199-012-0173-3
Fazari A, Palloix A, Wang LH et al (2012) The root-knot nematode resistance N-gene colocalizes in the Me-genes cluster on the pepper (Capsicum annuum L.) P9 chromosome. Plant Breeding 131(5):665–673. https://doi.org/10.1111/j.1439-0523.2012.01994.x
Ganeshan K, Vetrivelkalai P, Bhagawati B et al (2021) Endophytic fungi as potential bio-control agents against root kot nematode, Meloidogyne incognita in banana. Curr J Appl Sci Technol 40:7–18. https://doi.org/10.9734/CJAST/2021/V40I2931536
Grabka R, d’Entremont TW, Adams SJ et al (2022) Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants 11:384. https://doi.org/10.3390/plants11030384
Haque Z, Khan MR, Ahamad F (2018) Relative antagonistic potential of some rhizosphere biocontrol agents for the management of rice root-knot nematode, Meloidogyne graminicola. Biol Control 126:109–116. https://doi.org/10.1016/j.biocontrol.2018.07.018
Hardoim PR, Overbeek LV, Berg G et al (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biology Reviews Mmbr 79(3):293–320. https://doi.org/10.1128/MMBR.00050-14
Hu S, Mojahid MS, Bidochka MJ (2023) Root colonization of industrial hemp (Cannabis sativa L.) by the endophytic fungi Metarhizium and Pochonia improves growth. Ind Crops Prod 198:116716. https://doi.org/10.1016/j.indcrop.2023.116716
Ismail M, Fayyaz S, Kowsar A et al (2020) Evaluation of nematocidal effects of some medicinal plant extracts against root-knot nematodes (Meloidogyne incognita). Italian J Agron 15(1):1475. https://doi.org/10.4081/ija.2020.1475
Jacquet M, Bongiovanni M, Martinez M et al (2005) Variation in resistance to the root-knot nematode Meloidogyne incognita in tomato genotypes bearing the Mi gene. Plant Pathol 54(2):93–99. https://doi.org/10.1111/j.1365-3059.2005.01143.x
Januar LA, Molinski TF (2013) Acremolin from Acremonium strictum is N2,3-etheno-2’-isopropyl-1-methylguanine, not a 1H-azirine. Synthesis and structural revision. Org Lett 15(10):2370–2373. https://doi.org/10.1021/ol400752s
Khan B, Yan W, Wei S et al (2019) Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol Lett 366(14):fnz169. https://doi.org/10.1093/femsle/fnz169
Kisaakye J, Fourie H, Haukeland S et al (2022) Endophytic non-pathogenic fusarium xysporum-derived dual benefit for nematode management and improved banana (Musa spp.) productivity. Agriculture 12(2):125. https://doi.org/10.3390/agriculture12020125
Lozano J, Almeida C, Oliveira M et al (2022) Biocontrol of avian gastrointestinal parasites using predatory fungi: current status, challenges, and opportunities. Parasitologia 2:37–44. https://doi.org/10.3390/PARASITOLOGIA2010004
Lu H, Tan Y, Zhang Y et al (2022) Osteoclastogenesis inhibitory phenolic derivatives produced by the Beibu Gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia 159:105201. https://doi.org/10.1016/j.fitote.2022.105201
Ma Y, Li Y, Lai H et al (2017) Effect of two strains of Streptomyces on root-zone microbes and nematodes for biocontrol of root-knot nematode disease in tomato. Appl Soil Ecol 112:34–41. https://doi.org/10.1016/j.apsoil.2017.01.004
Mehmood A, Hussain A, Irshad M et al (2019) Cinnamic acid as an inhibitor of growth, flavonoids exudation and endophytic fungus colonization in maize root. Plant Physiol Biochem 135:61–68. https://doi.org/10.1016/j.plaphy.2018.11.029
Mingot-Ureta C, Lopez-Moya F, Lopez-Llorca LV (2020) Isolates of the nematophagous fungus Pochonia chlamydosporia are endophytic in banana roots and promote plant growth. Agronomy 10(9):1299. https://doi.org/10.3390/agronomy10091299
Mishra S, Bhattacharjee A, Sharma S (2021) An ecological insight into the multifaceted world of plant-endophyte association. CRC Crit Rev Plant Sci 40:127–146. https://doi.org/10.1080/07352689.2021.1901044
Moosavi MR, Zare R (2012) Fungi as biological control agents of plant-parasitic nematodes. In Plant Defence: Biological Control. Neth Springer 12:67–107. https://doi.org/10.1007/978-94-007-1933-0_4
Natori S (1983) Chemical surveys on mycotoxins using cytotoxicity testing with special reference to cytochalasans. Yakugaku Zasshi Journal of the Pharmaceutical Society of Japan 103(11):1109–1128. https://doi.org/10.1248/yakushi1947.103.11_1109
Naz I, Raja AA, TariqBaig A et al (2021) Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol Control 152(1):104429. https://doi.org/10.1016/j.biocontrol.2020.104429
Oro V, Stanisavljevic R, Nikolic B et al (2021) Diversity of mycobiota sssociated with the cereal cyst nematode Heterodera filipjevi originating from some localities of the Pannonian Plain in Serbia. Biology 10(4):283. https://doi.org/10.3390/biology10040283
Przybylska A, Obrępalska-Stęplowska A (2020) Plant defense responses in monocotyledonous and dicotyledonous host plants during root-knot nematode infection. Plant Soil 451(1):239–260. https://doi.org/10.1007/s11104-020-04533-0
Rashidifard M, Fourie H, Véronneau PY et al (2018) Genetic diversity and phylogeny of south african Meloidogyne populations using genotyping by sequencing. Sci Rep 8:13816. https://doi.org/10.1038/s41598-018-31963-9
Salem R, El-Kholy AA, Omar AO et al (2019a) Construction, expression and evaluation of recombinant VP2 protein for serotype-independent detection of FMDV seropositive animals in Egypt. Sci Rep 9:10135. https://doi.org/10.1038/s41598-019-46596-9
Salem R, El-Kholy AA, Ibrahim M (2019b) Eight novel single chain antibody fragments recognising VP2 of foot-and-mouth disease virus serotypes a, o, and SAT 2. Virology 533:145–154. https://doi.org/10.1016/j.virol.2019.05.012
Schouten A (2016) Mechanisms involved in nematode control by endophytic fungi. Annu Rev Phytopathol 54(1):121–142. https://doi.org/10.1146/annurev-phyto-080615-100114
Suciati, Fraser JA, Lambert LK et al (2013) Secondary metabolites of the sponge-derived fungus acremonium persicinum. J Nat Prod 76:1432–1440. https://doi.org/10.1021/np4002114
Tariq-Khan M, Munir A, Mukhtar T et al (2017) Distribution of root-knot nematode species and their virulence on vegetables in northern temperate agro-ecosystems of the pakistani-administered territories of Azad Jammu and Kashmir. J Plant Dis Prot 124:201–212. https://doi.org/10.1007/s41348-016-0045-9
Tian XL, Yao YR, Chen GH et al (2014) Suppression of Meloidogyne incognita by the endophytic fungus acremonium implicatum from tomato root galls. Int J Pest Manage 60:239–245. https://doi.org/10.1080/09670874.2014.958604
Van NN, Kim YJ, Oh KT et al (2007) The role of chitinase from Lecanicillium antillanum B-3 in parasitism to root-knot nematode Meloidogyne incognita eggs. Biocontrol Sci Technol 17:1047–1058. https://doi.org/10.1080/09583150701668658
Wei J (1979) Fungi identification manual book. Shanghai Science and Technology Press, Shanghai
Wicklow DT, Poling SM (2009) Antimicrobial activity of pyrrocidines from Acremonium zeae against endophytes and pathogens of maize. Phytopathology 99:109–115. https://doi.org/10.1094/PHYTO-99-1-0109
Zhang Y, Yang G, Fang M et al (2020) Comparative analyses of mitochondrial genomes provide evolutionary insights into nematode-trapping Fungi. Front Microbiol 11:617. https://doi.org/10.3389/fmicb.2020.00617
Zhou W, Starr JL, Krumm JL, Sword GA (2016) The fungal endophyte Chaetomium globosum negatively affects both above- and belowground herbivores in cotton. FEMS Microbiol Ecol 92:fiw158. https://doi.org/10.1093/femsec/fiw158
Acknowledgements
Not applicable.
Funding
This project was supported by the Innovative Research for Young Scientists of Tianjin Academy of Agricultural Sciences (grant number 2021004).
Author information
Authors and Affiliations
Contributions
This study was designed and conducted by Yurong Yao, Jianfei Huo, Haiyan Ben, Wei Gao, and Yongjuan Hao. Data were analyzed by Wanli Wang and Jingyang Xu. The draft manuscript was prepared by Yurong Yao. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, Y., Huo, J., Ben, H. et al. Biocontrol efficacy of endophytic fungus, Acremonium sclerotigenum, against Meloidogyne incognita under in vitro and in vivo conditions. Biologia 78, 3305–3313 (2023). https://doi.org/10.1007/s11756-023-01505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01505-4